Abstract
Background: Gene therapy of the joint has great potential as a new therapeutic approach for the treatment of rheumatoid arthritis (RA). The vector chosen is of crucial importance for clinical success.
Objective: To investigate the tropism and transduction efficiency in arthritic joints in vivo, and in synovial cells in vitro, using five different serotypes of recombinant adeno-associated virus (rAAV) encoding ß-galactosidase or green fluorescent protein genes.
Methods: rAAV was injected into the ankle joints of rats with adjuvant arthritis after the onset of disease. Synovial tissue was examined at different time points for ß-galactosidase protein and gene expression by in situ staining and polymerase chain reaction (PCR) analysis, respectively. In addition, the ability of rAAV to transduce primary human fibroblast-like synoviocytes from patients with RA was investigated in vitro.
Results: Intra-articular injection of the rAAV5 serotype resulted in the highest synovial transduction, followed by much lower expression using rAAV2. Expression of the transgene was already detectable 7 days after injection and lasted for at least 4 weeks. Only background staining was seen for serotypes 1, 3, and 4. Importantly, there was a minimal humoral immune response to rAAV5 compared with rAAV2. Additionally, it was found that both rAAV2 and rAAV5 can efficiently transduce human fibroblast-like synoviocytes obtained from patients with RA.
Conclusion: Intra-articular rAAV mediated gene therapy in RA might be improved by using rAAV5 rather than other serotypes.
Full Text
The Full Text of this article is available as a PDF (289.4 KB).
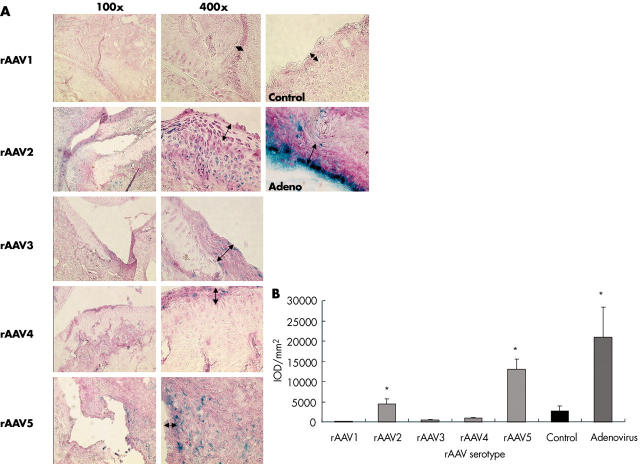
Figure 1.
ß-Gal expression in rat synovial tissue 2 weeks after intra-articular injection of different rAAV serotypes. Joints were snap frozen and sections were cut and stained in situ for ß-Gal activity and counterstained with nuclear red. Representative cryosections of right ankle joints of rats with adjuvant induced arthritis (AIA), injected with 6.1x1010 GC rAAV, Ad.LacZ as a positive control or saline as a negative control are shown (magnification x100, x400). The lining layer is indicated by arrows (A). Tissue sections were quantified for ß-Gal expression by computer assisted digital image analysis (B). Values are expressed as mean (SD) cumulative IOD/mm2. *p<0.05 as compared with the control group, Mann-Whitney U test.
Figure 2.
Viral genomic copies in the right ankle joints of rats injected with different rAAV serotypes. Two weeks after intra-articular rAAV injection, gDNA was isolated from crushed ankle joints and analysed for viral GC by quantitative PCR. Specific primers against the CMV promoter of the transgene were used. No viral DNA was found in control or left joints. Values are expressed as mean (SD) log GC/µg DNA. *p<0.05.
Figure 3.
ß-Gal expression in rat synovial tissue 1, 2, 3, and 4 weeks after intra-articular (IA) injection of 1.14x1010 GC rAAV serotype 2 or 5, quantified by digital image analysis. Injected joints were snap frozen and cryosections were stained in situ for ß-Gal activity. Staining was evaluated by computer assisted analysis and expressed as IODx10/mm2.
Figure 4.
LacZ mRNA in rat ankle joints after intra-articular injection of rAAV2 and rAAV5. Total mRNA was extracted from crushed ankle joints and cDNA was synthesised. PCR was performed using specific primers for the LacZ gene. GAPDH was used as internal control. To correct for differences in GAPDH mRNA, LacZ/GAPDH ratios are displayed as measured by computer assisted gel quantification. The uninjected left joints as well as the control joints did not show LacZ expression.
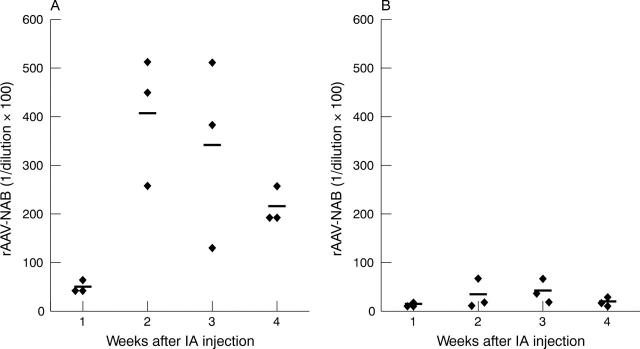
Figure 5.
Neutralising antibody (NAB) titres in serum after intra-articular (IA) injection of rAAV2 or rAAV5. Arthritic rats were injected with 1.14x1010 GC rAAV2 (A) or rAAV5 (B) into the right ankle joints. Serum samples were obtained 1, 2, 3, and 4 weeks after injection. Titres were calculated as the highest dilution which shows no decrease in number of ß-Gal positive cells compared with wells incubated with rAAV.LacZ alone.
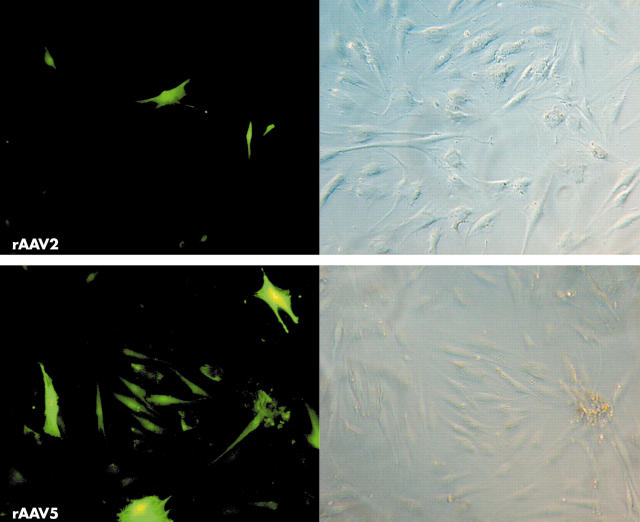
Figure 6.
Transduction of FLS with rAAV in vitro. Human FLS isolated from synovial biopsy specimens from patients with RA were incubated with rAAV2.GFP or rAAV5.GFP. After 48 hours of incubation, the cells were fixed and transgene expression was determined by fluorescent microscopy. Representative pictures of three independent experiments using cells from three different patients are shown. Left: fluorescent microscopy, right: phase contrast microscopy.
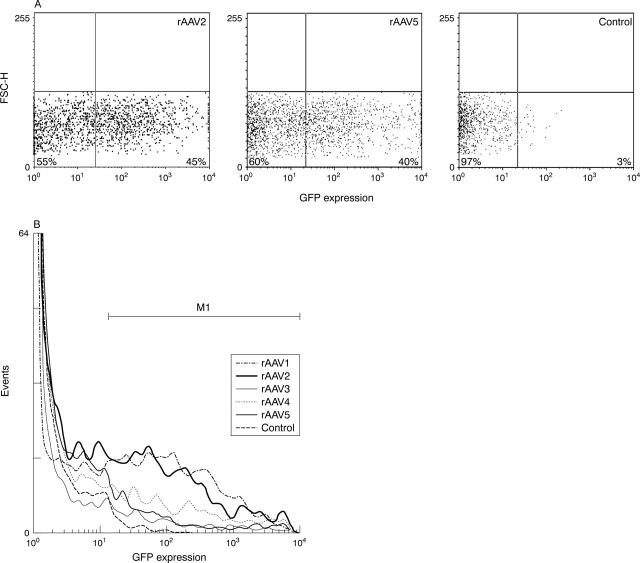
Figure 7.
Flow cytometric analysis of GFP expression in rAAV transduced FLS. Human FLS were incubated with rAAV2.GFP or rAAV5.GFP at a multiplicity of infection of 100. After 72 hours, the cells were washed and analysed for GFP expression by FACS. Flu-1 plotted against FSC-H showed 45% (left panel, rAAV2) and 40% (middle panel, rAAV5) GFP positive cells, compared with 3% in control cells (right panel), p<0.05 (A). Differences between rAAV2 and rAAV5 were not significantly different. In a histogram comparing GFP expression of control cells with all five rAAV serotypes, only differences between rAAV2 or rAAV5 and control or rAAV1, 3, or 4 proved significant using the Mann-Whitney U test (B) (p<0.05). Representative histogram of three independent experiments is shown.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvaro-Gracia J. M., Zvaifler N. J., Brown C. B., Kaushansky K., Firestein G. S. Cytokines in chronic inflammatory arthritis. VI. Analysis of the synovial cells involved in granulocyte-macrophage colony-stimulating factor production and gene expression in rheumatoid arthritis and its regulation by IL-1 and tumor necrosis factor-alpha. J Immunol. 1991 May 15;146(10):3365–3371. [PubMed] [Google Scholar]
- Chan James M. K., Villarreal Gilda, Jin Wen Wen, Stepan Tony, Burstein Haim, Wahl Sharon M. Intraarticular gene transfer of TNFR:Fc suppresses experimental arthritis with reduced systemic distribution of the gene product. Mol Ther. 2002 Dec;6(6):727–736. doi: 10.1006/mthe.2002.0808. [DOI] [PubMed] [Google Scholar]
- Chao H., Liu Y., Rabinowitz J., Li C., Samulski R. J., Walsh C. E. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol Ther. 2000 Dec;2(6):619–623. doi: 10.1006/mthe.2000.0219. [DOI] [PubMed] [Google Scholar]
- Chirmule N., Xiao W., Truneh A., Schnell M. A., Hughes J. V., Zoltick P., Wilson J. M. Humoral immunity to adeno-associated virus type 2 vectors following administration to murine and nonhuman primate muscle. J Virol. 2000 Mar;74(5):2420–2425. doi: 10.1128/jvi.74.5.2420-2425.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton L. A., Henderson B., Bitensky L., Chayen J. DNA synthesis in human rheumatoid and nonrheumatoid synovial lining. Ann Rheum Dis. 1980 Jun;39(3):241–247. doi: 10.1136/ard.39.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pasquale Giovanni, Davidson Beverly L., Stein Colleen S., Martins Inês, Scudiero Dominic, Monks Anne, Chiorini John A. Identification of PDGFR as a receptor for AAV-5 transduction. Nat Med. 2003 Sep 14;9(10):1306–1312. doi: 10.1038/nm929. [DOI] [PubMed] [Google Scholar]
- Duan D., Sharma P., Yang J., Yue Y., Dudus L., Zhang Y., Fisher K. J., Engelhardt J. F. Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long-term episomal persistence in muscle tissue. J Virol. 1998 Nov;72(11):8568–8577. doi: 10.1128/jvi.72.11.8568-8577.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott M. J., Maini R. N., Feldmann M., Kalden J. R., Antoni C., Smolen J. S., Leeb B., Breedveld F. C., Macfarlane J. D., Bijl H. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994 Oct 22;344(8930):1105–1110. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- Erles K., Sebökovà P., Schlehofer J. R. Update on the prevalence of serum antibodies (IgG and IgM) to adeno-associated virus (AAV). J Med Virol. 1999 Nov;59(3):406–411. doi: 10.1002/(sici)1096-9071(199911)59:3<406::aid-jmv22>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Flotte Terence R., Brantly Mark L., Spencer L. Terry, Byrne Barry J., Spencer Carolyn T., Baker Dawn J., Humphries Margaret. Phase I trial of intramuscular injection of a recombinant adeno-associated virus alpha 1-antitrypsin (rAAV2-CB-hAAT) gene vector to AAT-deficient adults. Hum Gene Ther. 2004 Jan;15(1):93–128. doi: 10.1089/10430340460732490. [DOI] [PubMed] [Google Scholar]
- Gao Guangping, Vandenberghe Luk H., Alvira Mauricio R., Lu You, Calcedo Roberto, Zhou Xiangyang, Wilson James M. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004 Jun;78(12):6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goater J., Müller R., Kollias G., Firestein G. S., Sanz I., O'Keefe R. J., Schwarz E. M. Empirical advantages of adeno associated viral vectors in vivo gene therapy for arthritis. J Rheumatol. 2000 Apr;27(4):983–989. [PubMed] [Google Scholar]
- Grimm D., Kay M. A. From virus evolution to vector revolution: use of naturally occurring serotypes of adeno-associated virus (AAV) as novel vectors for human gene therapy. Curr Gene Ther. 2003 Aug;3(4):281–304. doi: 10.2174/1566523034578285. [DOI] [PubMed] [Google Scholar]
- Grimm D., Kern A., Rittner K., Kleinschmidt J. A. Novel tools for production and purification of recombinant adenoassociated virus vectors. Hum Gene Ther. 1998 Dec 10;9(18):2745–2760. doi: 10.1089/hum.1998.9.18-2745. [DOI] [PubMed] [Google Scholar]
- Grimm Dirk, Kay Mark A., Kleinschmidt Juergen A. Helper virus-free, optically controllable, and two-plasmid-based production of adeno-associated virus vectors of serotypes 1 to 6. Mol Ther. 2003 Jun;7(6):839–850. doi: 10.1016/s1525-0016(03)00095-9. [DOI] [PubMed] [Google Scholar]
- Grimm Dirk, Zhou Shangzhen, Nakai Hiroyuki, Thomas Clare E., Storm Theresa A., Fuess Sally, Matsushita Takashi, Allen James, Surosky Richard, Lochrie Michael. Preclinical in vivo evaluation of pseudotyped adeno-associated virus vectors for liver gene therapy. Blood. 2003 Jun 5;102(7):2412–2419. doi: 10.1182/blood-2003-02-0495. [DOI] [PubMed] [Google Scholar]
- Hamilton Henry, Gomos Janette, Berns Kenneth I., Falck-Pedersen Erik. Adeno-associated virus site-specific integration and AAVS1 disruption. J Virol. 2004 Aug;78(15):7874–7882. doi: 10.1128/JVI.78.15.7874-7882.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa A., Muramatsu S., Qiu J., Mizukami H., Brown K. E. Adeno-associated virus (AAV)-3-based vectors transduce haematopoietic cells not susceptible to transduction with AAV-2-based vectors. J Gen Virol. 2000 Aug;81(Pt 8):2077–2084. doi: 10.1099/0022-1317-81-8-2077. [DOI] [PubMed] [Google Scholar]
- High Katherine A. Clinical gene transfer studies for hemophilia B. Semin Thromb Hemost. 2004 Apr;30(2):257–267. doi: 10.1055/s-2004-825639. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Genant H. K., Watt I., Cobby M., Bresnihan B., Aitchison R., McCabe D. A multicenter, double-blind, dose-ranging, randomized, placebo-controlled study of recombinant human interleukin-1 receptor antagonist in patients with rheumatoid arthritis: radiologic progression and correlation of Genant and Larsen scores. Arthritis Rheum. 2000 May;43(5):1001–1009. doi: 10.1002/1529-0131(200005)43:5<1001::AID-ANR7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Kaludov N., Brown K. E., Walters R. W., Zabner J., Chiorini J. A. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J Virol. 2001 Aug;75(15):6884–6893. doi: 10.1128/JVI.75.15.6884-6893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraan M. C., Haringman J. J., Ahern M. J., Breedveld F. C., Smith M. D., Tak P. P. Quantification of the cell infiltrate in synovial tissue by digital image analysis. Rheumatology (Oxford) 2000 Jan;39(1):43–49. doi: 10.1093/rheumatology/39.1.43. [DOI] [PubMed] [Google Scholar]
- Kraan M. C., Smith M. D., Weedon H., Ahern M. J., Breedveld F. C., Tak P. P. Measurement of cytokine and adhesion molecule expression in synovial tissue by digital image analysis. Ann Rheum Dis. 2001 Mar;60(3):296–298. doi: 10.1136/ard.60.3.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraan Maarten C., Reece Richard J., Smeets Tom J. M., Veale Douglas J., Emery Paul, Tak Paul P. Comparison of synovial tissues from the knee joints and the small joints of rheumatoid arthritis patients: Implications for pathogenesis and evaluation of treatment. Arthritis Rheum. 2002 Aug;46(8):2034–2038. doi: 10.1002/art.10556. [DOI] [PubMed] [Google Scholar]
- Lalor P. A., Mapp P. I., Hall P. A., Revell P. A. Proliferative activity of cells in the synovium as demonstrated by a monoclonal antibody, Ki67. Rheumatol Int. 1987;7(5):183–186. doi: 10.1007/BF00541375. [DOI] [PubMed] [Google Scholar]
- Lo W. D., Qu G., Sferra T. J., Clark R., Chen R., Johnson P. R. Adeno-associated virus-mediated gene transfer to the brain: duration and modulation of expression. Hum Gene Ther. 1999 Jan 20;10(2):201–213. doi: 10.1089/10430349950018995. [DOI] [PubMed] [Google Scholar]
- Maini R. N., Elliott M., Brennan F. M., Williams R. O., Feldmann M. TNF blockade in rheumatoid arthritis: implications for therapy and pathogenesis. APMIS. 1997 Apr;105(4):257–263. doi: 10.1111/j.1699-0463.1997.tb00567.x. [DOI] [PubMed] [Google Scholar]
- Moreland L. W., Baumgartner S. W., Schiff M. H., Tindall E. A., Fleischmann R. M., Weaver A. L., Ettlinger R. E., Cohen S., Koopman W. J., Mohler K. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med. 1997 Jul 17;337(3):141–147. doi: 10.1056/NEJM199707173370301. [DOI] [PubMed] [Google Scholar]
- Moskalenko M., Chen L., van Roey M., Donahue B. A., Snyder R. O., McArthur J. G., Patel S. D. Epitope mapping of human anti-adeno-associated virus type 2 neutralizing antibodies: implications for gene therapy and virus structure. J Virol. 2000 Feb;74(4):1761–1766. doi: 10.1128/jvi.74.4.1761-1766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss Richard B., Rodman David, Spencer L. Terry, Aitken Moira L., Zeitlin Pamela L., Waltz David, Milla Carlos, Brody Alan S., Clancy John P., Ramsey Bonnie. Repeated adeno-associated virus serotype 2 aerosol-mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: a multicenter, double-blind, placebo-controlled trial. Chest. 2004 Feb;125(2):509–521. doi: 10.1378/chest.125.2.509. [DOI] [PubMed] [Google Scholar]
- Nguyen K. H., Boyle D. L., McCormack J. E., Chada S., Jolly D. J., Firestein G. S. Direct synovial gene transfer with retroviral vectors in rat adjuvant arthritis. J Rheumatol. 1998 Jun;25(6):1118–1125. [PubMed] [Google Scholar]
- Pan R. Y., Chen S. L., Xiao X., Liu D. W., Peng H. J., Tsao Y. P. Therapy and prevention of arthritis by recombinant adeno-associated virus vector with delivery of interleukin-1 receptor antagonist. Arthritis Rheum. 2000 Feb;43(2):289–297. doi: 10.1002/1529-0131(200002)43:2<289::AID-ANR8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Pan R. Y., Xiao X., Chen S. L., Li J., Lin L. C., Wang H. J., Tsao Y. P. Disease-inducible transgene expression from a recombinant adeno-associated virus vector in a rat arthritis model. J Virol. 1999 Apr;73(4):3410–3417. doi: 10.1128/jvi.73.4.3410-3417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden Carmen S., Burger Corinna, Muzyczka Nicholas, Mandel Ronald J. Circulating anti-wild-type adeno-associated virus type 2 (AAV2) antibodies inhibit recombinant AAV2 (rAAV2)-mediated, but not rAAV5-mediated, gene transfer in the brain. J Virol. 2004 Jun;78(12):6344–6359. doi: 10.1128/JVI.78.12.6344-6359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing K., Mah C., Hansen J., Zhou S., Dwarki V., Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med. 1999 Jan;5(1):71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- Rabinowitz Joseph E., Rolling Fabienne, Li Chengwen, Conrath Hervè, Xiao Weidong, Xiao Xiao, Samulski R. Jude. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol. 2002 Jan;76(2):791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets T. J., Kraan M. C., Versendaal J., Breedveld F. C., Tak P. P. Analysis of serial synovial biopsies in patients with rheumatoid arthritis: description of a control group without clinical improvement after treatment with interleukin 10 or placebo. J Rheumatol. 1999 Oct;26(10):2089–2093. [PubMed] [Google Scholar]
- Snyder R. O. Adeno-associated virus-mediated gene delivery. J Gene Med. 1999 May-Jun;1(3):166–175. doi: 10.1002/(SICI)1521-2254(199905/06)1:3<166::AID-JGM34>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Summerford C., Samulski R. J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998 Feb;72(2):1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerford C., Samulski R. J. Viral receptors and vector purification: new approaches for generating clinical-grade reagents. Nat Med. 1999 May;5(5):587–588. doi: 10.1038/8470. [DOI] [PubMed] [Google Scholar]
- Tak P. P., Gerlag D. M., Aupperle K. R., van de Geest D. A., Overbeek M., Bennett B. L., Boyle D. L., Manning A. M., Firestein G. S. Inhibitor of nuclear factor kappaB kinase beta is a key regulator of synovial inflammation. Arthritis Rheum. 2001 Aug;44(8):1897–1907. doi: 10.1002/1529-0131(200108)44:8<1897::AID-ART328>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Imagawa T., Boivin G. P., Gao G., Wilson J. M., Hirsch R. Adeno-associated virus mediates long-term gene transfer and delivery of chondroprotective IL-4 to murine synovium. Mol Ther. 2000 Aug;2(2):147–152. doi: 10.1006/mthe.2000.0111. [DOI] [PubMed] [Google Scholar]
- Xiao W., Berta S. C., Lu M. M., Moscioni A. D., Tazelaar J., Wilson J. M. Adeno-associated virus as a vector for liver-directed gene therapy. J Virol. 1998 Dec;72(12):10222–10226. doi: 10.1128/jvi.72.12.10222-10226.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. G., Xie J., Yang P., Wang Y., Xu L., Liu D., Hsu H. C., Zhou T., Edwards C. K., 3rd, Mountz J. D. Adeno-associated virus production of soluble tumor necrosis factor receptor neutralizes tumor necrosis factor alpha and reduces arthritis. Hum Gene Ther. 2000 Nov 20;11(17):2431–2442. doi: 10.1089/104303400750038525. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S., Byrne B. J., Mason E., Zolotukhin I., Potter M., Chesnut K., Summerford C., Samulski R. J., Muzyczka N. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999 Jun;6(6):973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]