Abstract
Objective: To determine the presence of raised titres of anti-serum amyloid P component (SAP) antibodies in patients with systemic lupus erythematosus (SLE) and to evaluate their correlation with clinical disease by the SLEDAI and clinical manifestations.
Methods: 452 samples were screened for raised anti-SAP antibody titres by an ELISA. Clinical measures and SLEDAI scores were independently reviewed from medical records. 21 serial samples from 7 patients with SLE were assessed for a change in anti-SAP antibody titres after treatment.
Results: Raised anti-SAP antibody titres were detected in 145/328 (44%) SLE samples. In 112 randomly selected samples, 69/112 (62%) patients had raised anti-SAP antibodies and anti-dsDNA antibody titres, whereas only 32/112 (28%) had raised anti-dsDNA antibody titres without raised anti-SAP antibody titres. The mean titre of anti-SAP antibodies in patients with active disease was higher than in patients with inactive disease and controls. SLEDAI scores, assessed in 54 patients, were raised in 26/31 (84%) patients with raised anti-SAP antibody titres. A SLEDAI score ⩾8 was found in 16/31 (52%) patients with raised anti-SAP antibody titres but in only 5/23 (22%) patients without raised titres. No specific pattern of disease was detected in patients with or without raised titres of anti-SAP antibodies. Serial sampling from patients with active SLE and raised anti-SAP antibody titres showed that anti-SAP antibody titres decreased after treatment and correlated with clinical improvement.
Conclusion: Raised anti-SAP antibody titres detected in patients with SLE correlate with disease activity and decrease with improvement of clinical disease, and thus may serve as an additional prognostic marker.
Full Text
The Full Text of this article is available as a PDF (110.0 KB).
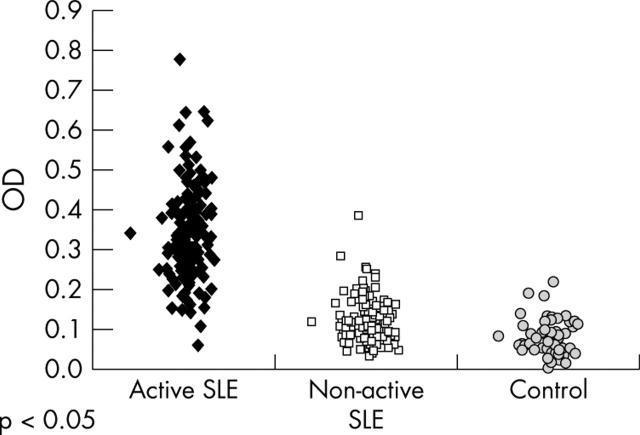
Figure 1.
Titres of anti-SAP antibodies in 189 patients with SLE; 54 patients with SLE with clinically active disease (group I—Israel), 135 patients with SLE with clinically inactive disease (group II—Germany), and in 54 healthy controls.
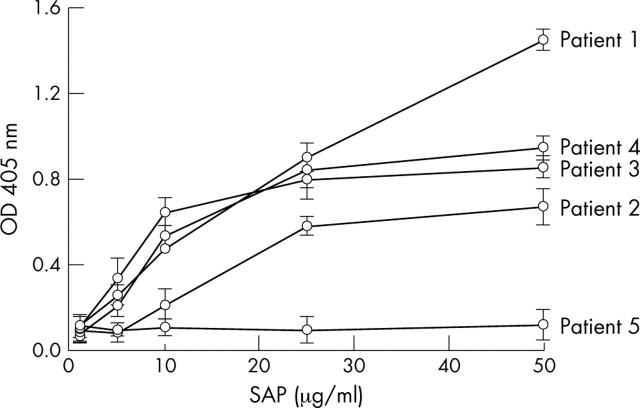
Figure 2.
SAP dose dependent binding of protein G and dsDNA-cellulose affinity purified total IgG from four lupus patients positive for SAP (patients 1–4) and one lupus patient negative for SAP recognition (patient 5). The binding was assayed by ELISA. Data presented as OD at 405 nm, mean (SD) of three experiments.
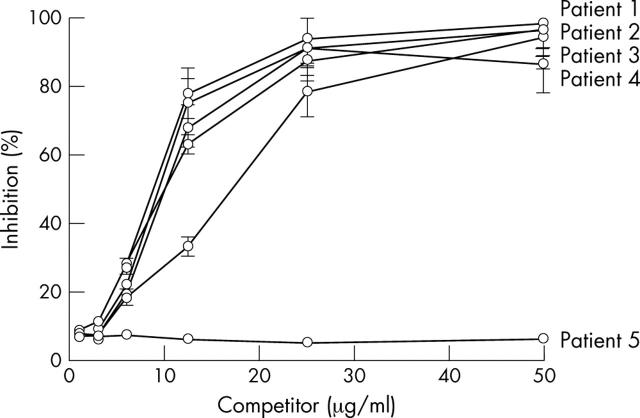
Figure 3.
Inhibition of binding of affinity purified IgG from lupus patients to SAP by SAP. Total IgG affinity purified from five lupus patients incubated with different concentrations of SAP. Patients 1–4 had raised titres of anti-SAP antibodies. but patient 5 did not. The inhibition of the binding was calculated as the percentage inhibition of total IgG to SAP by SAP. The data are presented as mean (SD) of three experiments.
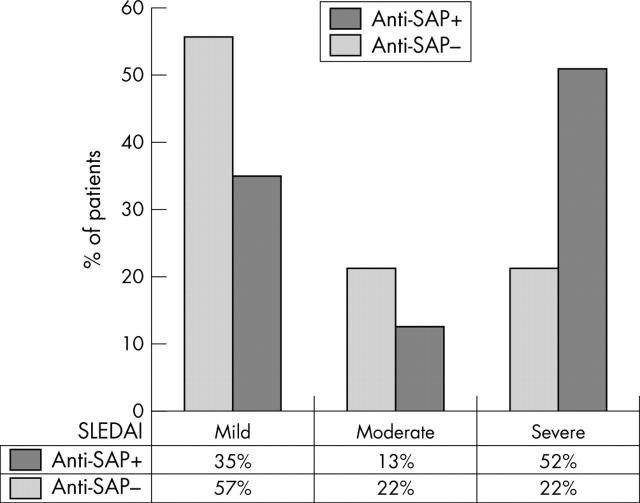
Figure 4.
SLEDAI scores for 54 patients with SLE, comparing the percentage of patients with and without raised anti-SAP antibody titres. Remission and mild disease: SLEDAI 0–4; moderate disease: SLEDAI 5–7; severe disease: SLEDAI ⩾8.
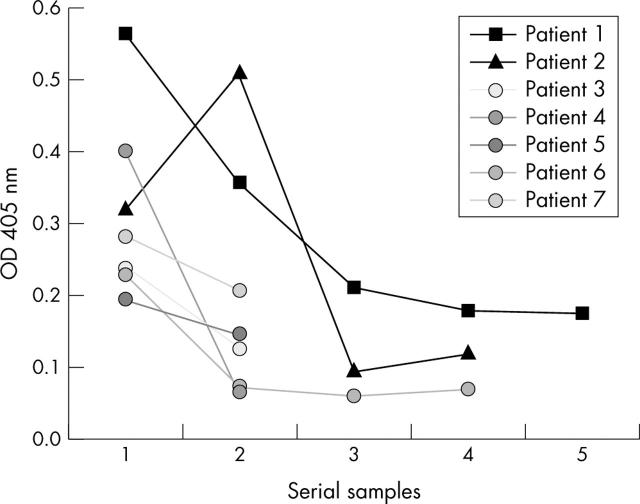
Figure 5.
Serial sampling of anti-SAP antibody titres in seven patients on 2–4 occasions. All patients had been recently diagnosed with SLE or had a flare. The first samples were taken before treatment. The OD for 3SD of the mean controls was 0.154.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann Irith, Kolowos Wasilis, Voll Reinhard E., Manger Bernhard, Gaipl Udo, Neuhuber Winfried L., Kirchner Thomas, Kalden Joachim R., Herrmann Martin. Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum. 2002 Jan;46(1):191–201. doi: 10.1002/1529-0131(200201)46:1<191::AID-ART10027>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Bharadwaj D., Mold C., Markham E., Du Clos T. W. Serum amyloid P component binds to Fc gamma receptors and opsonizes particles for phagocytosis. J Immunol. 2001 Jun 1;166(11):6735–6741. doi: 10.4049/jimmunol.166.11.6735. [DOI] [PubMed] [Google Scholar]
- Bickerstaff M. C., Botto M., Hutchinson W. L., Herbert J., Tennent G. A., Bybee A., Mitchell D. A., Cook H. T., Butler P. J., Walport M. J. Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nat Med. 1999 Jun;5(6):694–697. doi: 10.1038/9544. [DOI] [PubMed] [Google Scholar]
- Bijl M., Bootsma H., Van Der Geld Y., Limburg P. C., Kallenberg C. G. M., Van Rijswijk M. H. Serum amyloid P component levels are not decreased in patients with systemic lupus erythematosus and do not rise during an acute phase reaction. Ann Rheum Dis. 2004 Jul;63(7):831–835. doi: 10.1136/ard.2002.004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijl Marc, Horst Gerda, Bijzet Johan, Bootsma Hendrika, Limburg Pieter C., Kallenberg Cees G. M. Serum amyloid P component binds to late apoptotic cells and mediates their uptake by monocyte-derived macrophages. Arthritis Rheum. 2003 Jan;48(1):248–254. doi: 10.1002/art.10737. [DOI] [PubMed] [Google Scholar]
- Bombardier C., Gladman D. D., Urowitz M. B., Caron D., Chang C. H. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992 Jun;35(6):630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- Breathnach S. M., Kofler H., Sepp N., Ashworth J., Woodrow D., Pepys M. B., Hintner H. Serum amyloid P component binds to cell nuclei in vitro and to in vivo deposits of extracellular chromatin in systemic lupus erythematosus. J Exp Med. 1989 Oct 1;170(4):1433–1438. doi: 10.1084/jem.170.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casciola-Rosen L. A., Anhalt G., Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994 Apr 1;179(4):1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Clos T. W. The interaction of C-reactive protein and serum amyloid P component with nuclear antigens. Mol Biol Rep. 1996;23(3-4):253–260. doi: 10.1007/BF00351177. [DOI] [PubMed] [Google Scholar]
- Emsley J., White H. E., O'Hara B. P., Oliva G., Srinivasan N., Tickle I. J., Blundell T. L., Pepys M. B., Wood S. P. Structure of pentameric human serum amyloid P component. Nature. 1994 Jan 27;367(6461):338–345. doi: 10.1038/367338a0. [DOI] [PubMed] [Google Scholar]
- Familian A., Zwart B., Huisman H. G., Rensink I., Roem D., Hordijk P. L., Aarden L. A., Hack C. E. Chromatin-independent binding of serum amyloid P component to apoptotic cells. J Immunol. 2001 Jul 15;167(2):647–654. doi: 10.4049/jimmunol.167.2.647. [DOI] [PubMed] [Google Scholar]
- Gillmore Julian D., Hutchinson Winston L., Herbert Jeff, Bybee Alison, Mitchell Daniel A., Hasserjian Robert P., Yamamura Ken-Ichi, Suzuki Misao, Sabin Caroline A., Pepys Mark B. Autoimmunity and glomerulonephritis in mice with targeted deletion of the serum amyloid P component gene: SAP deficiency or strain combination? Immunology. 2004 Jun;112(2):255–264. doi: 10.1111/j.1365-2567.2004.01860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladman Dafna D., Hirani Naushad, Ibañez Dominique, Urowitz Murray B. Clinically active serologically quiescent systemic lupus erythematosus. J Rheumatol. 2003 Sep;30(9):1960–1962. [PubMed] [Google Scholar]
- Levy Y., Sherer Y., Ahmed A., Langevitz P., George J., Fabbrizzi F., Terryberry J., Meissner M., Lorber M., Peter J. B. A study of 20 SLE patients with intravenous immunoglobulin--clinical and serologic response. Lupus. 1999;8(9):705–712. doi: 10.1191/096120399678841007. [DOI] [PubMed] [Google Scholar]
- Mold C., Gresham H. D., Du Clos T. W. Serum amyloid P component and C-reactive protein mediate phagocytosis through murine Fc gamma Rs. J Immunol. 2001 Jan 15;166(2):1200–1205. doi: 10.4049/jimmunol.166.2.1200. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Dash A. C., Markham R. E., Thomas H. C., Williams B. D., Petrie A. Comparative clinical study of protein SAP (amyloid P component) and C-reactive protein in serum. Clin Exp Immunol. 1978 Apr;32(1):119–124. [PMC free article] [PubMed] [Google Scholar]
- Pepys M. B., Herbert J., Hutchinson W. L., Tennent G. A., Lachmann H. J., Gallimore J. R., Lovat L. B., Bartfai T., Alanine A., Hertel C. Targeted pharmacological depletion of serum amyloid P component for treatment of human amyloidosis. Nature. 2002 May 16;417(6886):254–259. doi: 10.1038/417254a. [DOI] [PubMed] [Google Scholar]
- Sen J. W., Recke C., Rahbek L., Skogstrand K., Heegaard N. H. H. Structural, quantitative and functional comparison of amyloid P component in sera from patients with systemic lupus erythematosus and healthy donors. Scand J Immunol. 2002 Dec;56(6):645–651. doi: 10.1046/j.1365-3083.2002.01178.x. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y., Rauch J., Massicotte H., Datta S. K., André-Schwartz J., Stollar B. D., Schwartz R. S. Polyspecificity of monoclonal lupus autoantibodies produced by human-human hybridomas. N Engl J Med. 1983 Feb 24;308(8):414–420. doi: 10.1056/NEJM198302243080802. [DOI] [PubMed] [Google Scholar]
- Soma M., Tamaoki T., Kawano H., Ito S., Sakamoto M., Okada Y., Ozaki Y., Kanba S., Hamada Y., Ishihara T. Mice lacking serum amyloid P component do not necessarily develop severe autoimmune disease. Biochem Biophys Res Commun. 2001 Aug 10;286(1):200–205. doi: 10.1006/bbrc.2001.5364. [DOI] [PubMed] [Google Scholar]
- Szalai A. J., van Ginkel F. W., Wang Y., McGhee J. R., Volanakis J. E. Complement-dependent acute-phase expression of C-reactive protein and serum amyloid P-component. J Immunol. 2000 Jul 15;165(2):1030–1035. doi: 10.4049/jimmunol.165.2.1030. [DOI] [PubMed] [Google Scholar]
- Sørensen I. J., Holm Nielsen E., Schrøder L., Voss A., Horváth L., Svehag S. E. Complexes of serum amyloid P component and DNA in serum from healthy individuals and systemic lupus erythematosus patients. J Clin Immunol. 2000 Nov;20(6):408–415. doi: 10.1023/a:1026478914129. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Tomer Y., Shoenfeld Y. Successful treatment of psychosis secondary to SLE with high dose intravenous immunoglobulin. Clin Exp Rheumatol. 1992 Jul-Aug;10(4):391–393. [PubMed] [Google Scholar]
- Walsh M. T., Divane A., Whitehead A. S. Fine mapping of the human pentraxin gene region on chromosome 1q23. Immunogenetics. 1996;44(1):62–69. doi: 10.1007/BF02602657. [DOI] [PubMed] [Google Scholar]
- Walz LeBlanc B. A., Gladman D. D., Urowitz M. B. Serologically active clinically quiescent systemic lupus erythematosus--predictors of clinical flares. J Rheumatol. 1994 Dec;21(12):2239–2241. [PubMed] [Google Scholar]
- Zahedi K. Characterization of the binding of serum amyloid P to laminin. J Biol Chem. 1997 Jan 24;272(4):2143–2148. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.