Abstract
Background: The anti-tumour antibiotic mithramycin is also a potent inhibitor of fibrosis after glaucoma surgery. This drug displays high affinity binding to GC-rich sequences in DNA, including those present in the promoter of the gene encoding the α1 chain of type I collagen (COL1A1).
Objective: To evaluate the effects of mithramycin on COL1A1 expression in systemic sclerosis fibroblasts.
Methods: Confluent cultures of dermal fibroblasts from patients with recent onset diffuse systemic sclerosis were treated with mithramycin in vitro. Cell viability and protein expression were examined by fluorescence and confocal imaging. Type I collagen production was analysed by confocal imaging and metabolic labelling. COL1A1 messenger RNA levels and stability were assessed by northern hybridisation, and COL1A1 transcription was examined by transient transfections.
Results: Treatment of systemic sclerosis fibroblasts with mithramycin (10–100 nmol/l) did not cause significant cytotoxicity. Type I collagen biosynthesis decreased by 33–40% and 50–70% in cells cultured with mithramycin at 10 nmol/l and 100 nmol/l, respectively. Mithramycin at 50 nmol/l decreased COL1A1 mRNA levels by 40–60%. The effects of mithramycin on collagen gene expression were mediated by transcriptional and post-transcriptional mechanisms as shown by the reduction of COL1A1 promoter activity and by a decrease in the stability of these transcripts, respectively.
Conclusions: Mithramycin causes potent inhibition of collagen production and gene expression in systemic sclerosis dermal fibroblasts in vitro in the absence of cytotoxic effects. These results suggest that this drug may be an effective treatment for the fibrotic process which is the hallmark of systemic sclerosis.
Full Text
The Full Text of this article is available as a PDF (351.0 KB).
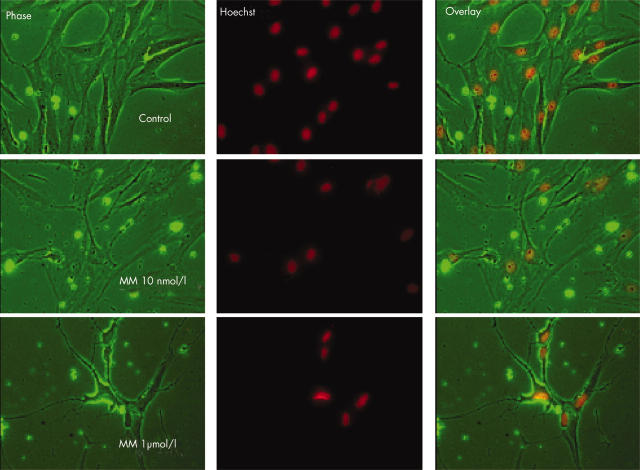
Figure 1.
Effects of mithramycin (MM) on cell viability. Cell viability was examined as described in "Materials and methods" employing various concentrations of mithramycin (10 nmol/l to 1 µmol/l) applied for 48 hours to confluent systemic sclerosis fibroblast cultures in three experiments using three different cell lines, each in duplicate. The results showed that mithramycin at 1 µmol/l decreased the number of cells by over 80%, whereas 10 nmol/l (or 50 nmol/l; not shown) did not cause any significant change in cell viability. The phase contrast image shows that treatment with mithramycin at 1 µmol/l altered the shape of the cells which shrunk and lost their cytoplasm, although the nuclear staining with Hoechst did not indicate apoptotic process. No change in cell shape was seen using mithramycin at 10 nmol/l.
Figure 2.
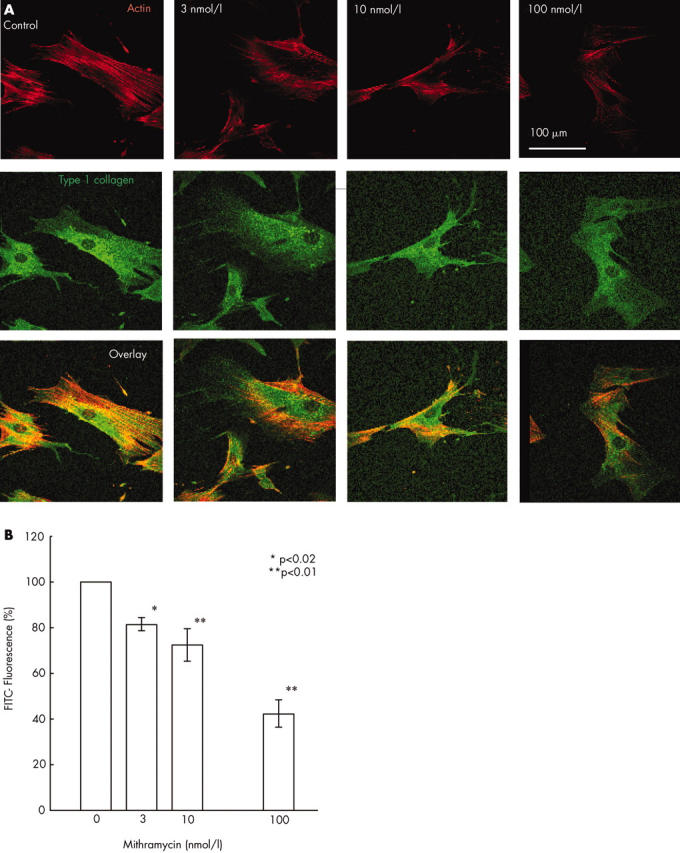
Evaluation of type I collagen production in systemic sclerosis dermal fibroblast monolayer cultures by confocal imaging after mithramycin treatment. Type I collagen present in individual cells of systemic sclerosis fibroblast cultures was examined by immunomicroscopy and confocal microscopy imaging as described in "Materials and methods" in five separate experiments using four cell lines. (A) Fluorescence of the FITC conjugated antirabbit IgG labelling type I collagen is shown in green and that of rhodamine labelled phalloidin labelling actin in red in one illustrative experiment. (B) Quantitative evaluation of the mithramycin effect on type I collagen production at the individual cell level showed that 48 hours of treatment with mithramycin at 10 nmol/l and 100 nmol/l decreased the protein level by about 33% and 50%, respectively. The results show the averages (SD) from five separate experiments with four different cell lines.
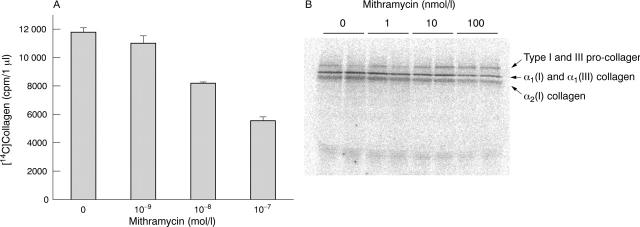
Figure 3.
Evaluation of type I collagen biosynthesis by metabolic labelling after mithramycin treatment. Confluent monolayer cultures of systemic sclerosis fibroblasts were labelled with [14C]proline under control conditions or under treatment with various concentrations of mithramycin, and collagen biosynthesis was determined employing a specific collagenase assay in pooled media plus cell lysate samples as described in "Materials and methods". (A) Collagen biosynthesis was decreased by 30% using mithramycin at 10 nmol/l and by 53% using mithramycin at 100 nmol/l. The results of one experiment are shown. (B) SDS gel electrophoresis of labelled proteins from the medium showed similar results with a 30% decrease in [14C]collagen after 10 nmol/l mithramycin treatment and about a 50% decrease after 100 nmol/l mithramycin administration. Two separate experiments, each in duplicate, were performed with similar results.
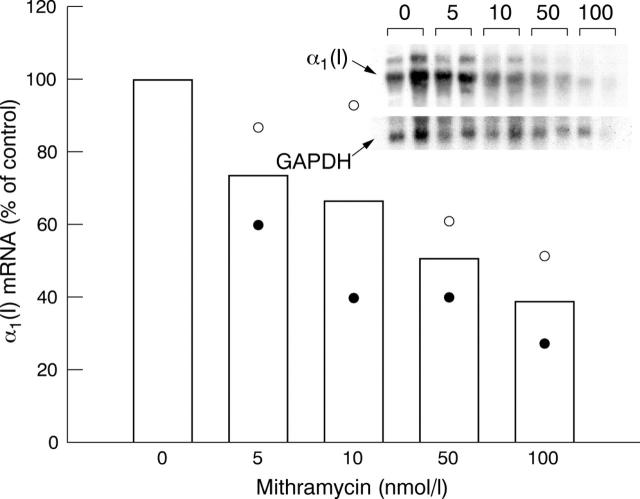
Figure 4.
Analysis of steady state mRNA levels by northern blot hybridisation. Confluent monolayer cultures of two systemic sclerosis dermal fibroblast cell lines were treated with various concentrations of mithramycin and total RNA extracted from the cultures was examined by northern hybridisation with cDNA for COL1A1 and GAPDH as described in "Materials and methods". Inset: northern analysis of cell line 1. Bar graph: open circles, cell line 1; closed circles, cell line 2. Mithramycin (50 nmol/l) decreased the steady state mRNA level of α1(I) by 40–60%.
Figure 5.
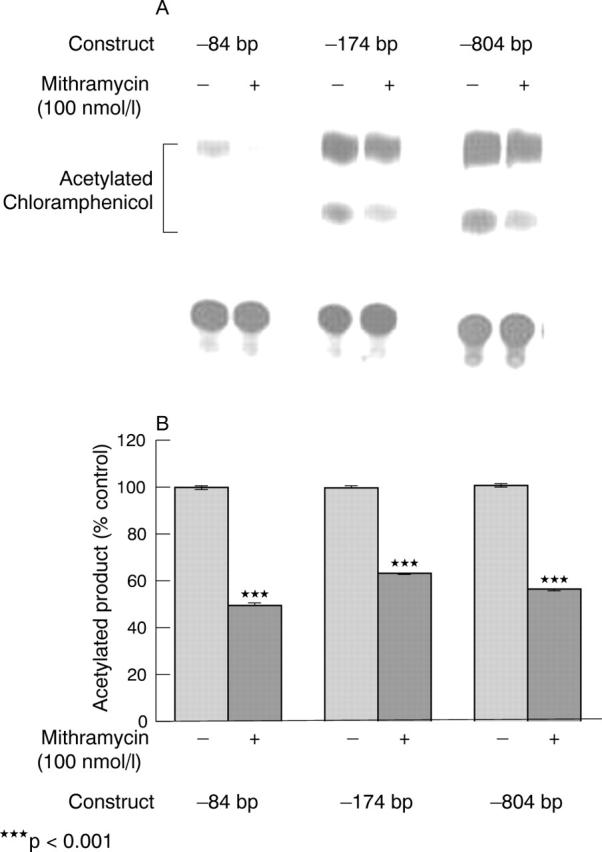
COL1A1 promoter activity after mithramycin treatment. Transient transfection of systemic sclerosis fibroblasts with COL1A1 promoter-CAT constructs followed by mithramycin (50–100 nmol/l) was performed as described in "Materials and methods". (A) The results with –84 bp, –174 bp, and –804 bp constructs are shown. The efficiency of transfection was corrected by assays of ß-galactosidase activity measured by an enzymatic assay. These experiments were repeated three times, each in duplicate. (B) Densitometric analysis of three separate experiments, each performed in duplicate. p Values obtained comparing the transcriptional activity of each of the constructs in cells treated with mithramycin compared with the transcriptional activity of the same constructs in the untreated cells were significant (p<0.001 for each of the constructs).
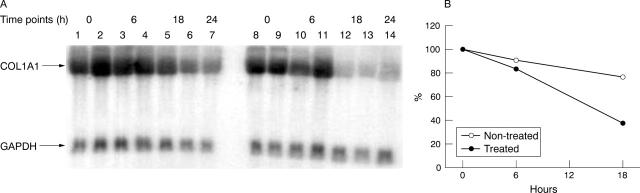
Figure 6.
mRNA stability after mithramycin treatment. The effects of mithramycin on COL1A1 mRNA stability were examined as described in "Materials and methods". (A) Confluent cultures of systemic sclerosis dermal fibroblasts were either kept in culture medium containing 10% FBS and ascorbic acid (50 µg/ml) (lanes 1–7) or were incubated for 4 hours with mithramycin (50 nmol/l; lanes 8–14) before addition of α-amanitin (1 µg/ml). Total RNA was prepared from cells at various intervals after α-amanitin addition and examined by northern analysis. Northern analysis was performed on samples prepared at the time of α-amanitin addition (time point 0): lanes 1, 2, 8, 9; at 6 hours after α-amanitin administration: lanes 3, 4, 10, 11; at 18 hours after α-amanitin treatment: lanes 5, 6, 12, 13; and at 24 hours after α-amanitin: lanes 7 and 14. (B) COL1A1 mRNA stability after mithramycin treatment assessed by densitometric analysis. Values shown were corrected to GAPDH and represent the average of duplicate cultures of different cell lines. The 24 hour value was not included in the graph because it was not performed in duplicate.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell V. W., Davin D., Thomas S., Jones D., Roesel J., Tran-Patterson R., Mayfield C. A., Rodu B., Miller D. M., Hiramoto R. A. The G-C specific DNA binding drug, mithramycin, selectively inhibits transcription of the C-MYC and C-HA-RAS genes in regenerating liver. Am J Med Sci. 1994 Mar;307(3):167–172. doi: 10.1097/00000441-199403000-00002. [DOI] [PubMed] [Google Scholar]
- Chen S. J., Chen Y. F., Miller D. M., Li H., Oparil S. Mithramycin inhibits myointimal proliferation after balloon injury of the rat carotid artery in vivo. Circulation. 1994 Nov;90(5):2468–2473. doi: 10.1161/01.cir.90.5.2468. [DOI] [PubMed] [Google Scholar]
- Chiang S. Y., Azizkhan J. C., Beerman T. A. A comparison of DNA-binding drugs as inhibitors of E2F1- and Sp1-DNA complexes and associated gene expression. Biochemistry. 1998 Mar 3;37(9):3109–3115. doi: 10.1021/bi9721142. [DOI] [PubMed] [Google Scholar]
- Diaz A., Jiménez S. A. Interferon-gamma regulates collagen and fibronectin gene expression by transcriptional and post-transcriptional mechanisms. Int J Biochem Cell Biol. 1997 Jan;29(1):251–260. doi: 10.1016/s1357-2725(96)00112-4. [DOI] [PubMed] [Google Scholar]
- Eckes B., Mauch C., Hüppe G., Krieg T. Differential regulation of transcription and transcript stability of pro-alpha 1(I) collagen and fibronectin in activated fibroblasts derived from patients with systemic scleroderma. Biochem J. 1996 Apr 15;315(Pt 2):549–554. doi: 10.1042/bj3150549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidarova Svetlana, Jiménez Sergio A. Inhibition of basal and transforming growth factor-beta-induced stimulation of COL1A1 transcription by the DNA intercalators, mitoxantrone and WP631, in cultured human dermal fibroblasts. J Biol Chem. 2002 Jul 23;277(41):38737–38745. doi: 10.1074/jbc.M201742200. [DOI] [PubMed] [Google Scholar]
- Hardenbol P., Van Dyke M. W. In vitro inhibition of c-myc transcription by mithramycin. Biochem Biophys Res Commun. 1992 Jun 15;185(2):553–558. doi: 10.1016/0006-291x(92)91660-i. [DOI] [PubMed] [Google Scholar]
- Hitraya E. G., Jiménez S. A. Transcriptional activation of the alpha 1(I) procollagen gene in systemic sclerosis dermal fibroblasts. Role of intronic sequences. Arthritis Rheum. 1996 Aug;39(8):1347–1354. doi: 10.1002/art.1780390812. [DOI] [PubMed] [Google Scholar]
- Hitraya E. G., Varga J., Artlett C. M., Jiménez S. A. Identification of elements in the promoter region of the alpha1(I) procollagen gene involved in its up-regulated expression in systemic sclerosis. Arthritis Rheum. 1998 Nov;41(11):2048–2058. doi: 10.1002/1529-0131(199811)41:11<2048::AID-ART21>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Hämäläinen L., Oikarinen J., Kivirikko K. I. Synthesis and degradation of type I procollagen mRNAs in cultured human skin fibroblasts and the effect of cortisol. J Biol Chem. 1985 Jan 25;260(2):720–725. [PubMed] [Google Scholar]
- Ihn H., Tamaki K. Increased phosphorylation of transcription factor Sp1 in scleroderma fibroblasts: association with increased expression of the type I collagen gene. Arthritis Rheum. 2000 Oct;43(10):2240–2247. doi: 10.1002/1529-0131(200010)43:10<2240::AID-ANR11>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Jackson S. P., Tjian R. O-glycosylation of eukaryotic transcription factors: implications for mechanisms of transcriptional regulation. Cell. 1988 Oct 7;55(1):125–133. doi: 10.1016/0092-8674(88)90015-3. [DOI] [PubMed] [Google Scholar]
- Jimenez S. A., Saitta B. Alterations in the regulation of expression of the alpha 1(I) collagen gene (COL1A1) in systemic sclerosis (scleroderma). Springer Semin Immunopathol. 1999;21(4):397–414. doi: 10.1007/BF00870302. [DOI] [PubMed] [Google Scholar]
- Jimenez S. A., Varga J., Olsen A., Li L., Diaz A., Herhal J., Koch J. Functional analysis of human alpha 1(I) procollagen gene promoter. Differential activity in collagen-producing and -nonproducing cells and response to transforming growth factor beta 1. J Biol Chem. 1994 Apr 29;269(17):12684–12691. [PubMed] [Google Scholar]
- Jimenez Sergio A., Derk Chris T. Following the molecular pathways toward an understanding of the pathogenesis of systemic sclerosis. Ann Intern Med. 2004 Jan 6;140(1):37–50. [PubMed] [Google Scholar]
- Nehls M. C., Brenner D. A., Gruss H. J., Dierbach H., Mertelsmann R., Herrmann F. Mithramycin selectively inhibits collagen-alpha 1(I) gene expression in human fibroblast. J Clin Invest. 1993 Dec;92(6):2916–2921. doi: 10.1172/JCI116914. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ray R., Snyder R. C., Thomas S., Koller C. A., Miller D. M. Mithramycin blocks protein binding and function of the SV40 early promoter. J Clin Invest. 1989 Jun;83(6):2003–2007. doi: 10.1172/JCI114110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalai G., Krishnamurthy R., Hajnóczky G. Apoptosis driven by IP(3)-linked mitochondrial calcium signals. EMBO J. 1999 Nov 15;18(22):6349–6361. doi: 10.1093/emboj/18.22.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneswaran N., Mayfield C. A., Rodu B., James R., Kim H. G., Miller D. M. Influence of GC and AT specific DNA minor groove binding drugs on intermolecular triplex formation in the human c-Ki-ras promoter. Biochemistry. 1996 Jan 30;35(4):1106–1114. doi: 10.1021/bi951562b. [DOI] [PubMed] [Google Scholar]