Abstract
Objective: To determine whether Bcl-2, p53, and Fas/CD95 help to control cartilage metabolism.
Methods: Six normal and 14 osteoarthritic (OA) cartilage samples were examined, and two zones from each sample showing the least (Min) and most (Max) anatomical damage were selected. Chondrocytes were isolated by sequential enzymatic digestion and freshly processed. Bcl-2, p53, and Fas/CD95 expression was evaluated by immunofluorescence and FACS analysis; the cell cycle was analysed using propidium iodide, and chondrocyte proliferation assessed by [3H]thymidine incorporation.
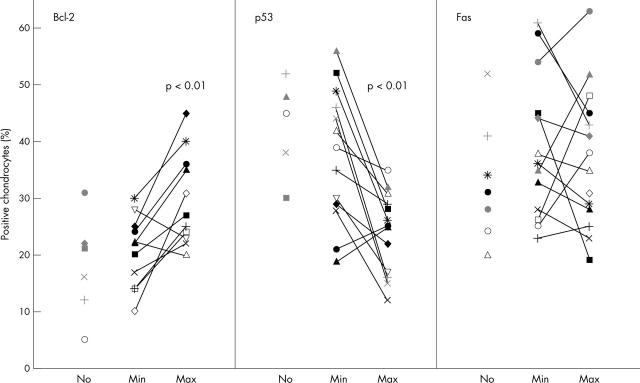
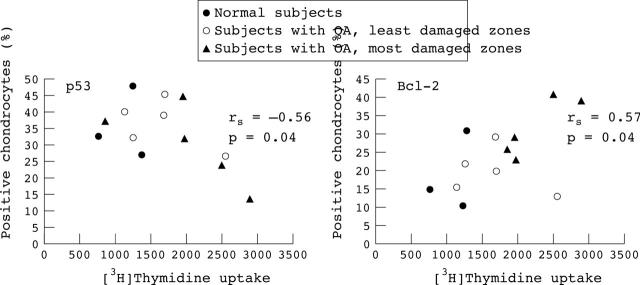
Results: Intracellular levels of Bcl-2 were significantly higher in Max (27.5%) than in Min (21%, p<0.01) OA or normal chondrocytes (18.5%, p<0.01). Intracellular p53 expression was significantly decreased in Max (25.5%) compared with Min (37%, p<0.01) OA or normal cartilage (41.5%, p<0.05). Fas/CD95 receptor expression on surface chondrocytes did not significantly differ between OA and normal cartilage. Cell cycle analysis showed that the proportion of activated chondrocytes in the S phase was significantly higher in Max (69%) than in Min (49%) OA or normal cartilage (43%). The prevalence of proliferating chondrocytes progressively increased according to the degree of OA damage (mean (SEM) Min 1247 (260), Max 2423 (460), p<0.05). Chondrocyte [3H]thymidine uptake correlated positively with Bcl-2 (rs = 0.62, p = 0.009) and correlated inversely with p53 levels (rs = –0.55, p = 0.02).
Conclusions: Bcl-2 and p53 play a part in apoptosis, but also help to regulate chondrocyte growth and differentiation. Whereas Bcl-2 promotes cell survival, p53 can arrest cell cycle. The data confirm that chondrocyte activity is enhanced in OA and suggest that the increased Bcl-2/p53 ratio sustains the metabolic boost of chondrocytes.
Full Text
The Full Text of this article is available as a PDF (337.6 KB).
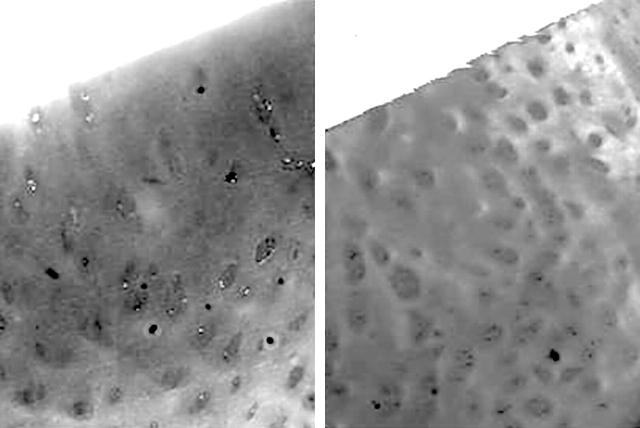
Figure 1.
Photomicrographs of safranin O stained histological sections of zones with macroscopically defined minimum (left) and maximum (right) damage in typical OA cartilage (original magnification x60). A diffuse staining of the cartilage, almost entirely masking the chondrocytes, is present in the minimum zone, while in the maximum zone there is evident hypocellularity together with some clusters of chondrocytes and severe reduction of the safranin O staining.
Figure 2.
Percentage of chondrocytes expressing intracellular p53 and Bcl-2, and cell surface Fas/CD95 in six normal cartilages (No), and in the lowest (Min) and highest (Max) damaged zones of 14 OA cartilages.
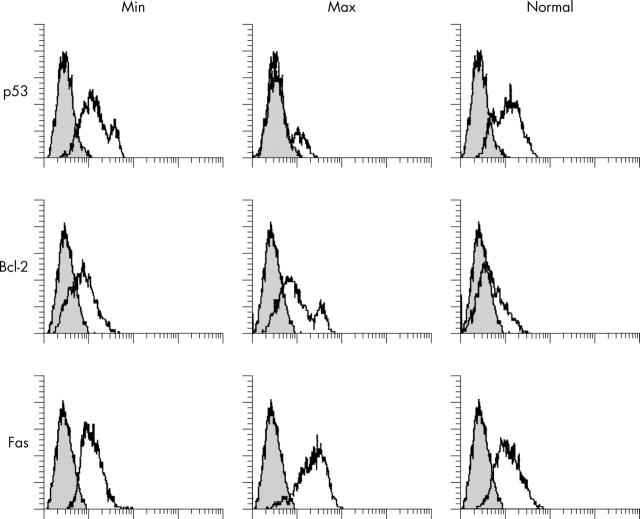
Figure 3.
Fluorescence intensity for intracellular p53 and Bcl-2 and surface CD95/Fas from an OA cartilage with minimal (Min) and maximum (Max) anatomical damage, and from a normal cartilage. Negative controls (a non-binding mAb) are shown as grey overlapping histograms. The y axis shows the percentage of positive chondrocytes and the x axis the mean channel fluorescence. Protein expression of p53 is definitely lower in Max than in Min or normal cartilage, and Bcl-2 was higher in Max than in Min or normal cartilage.
Figure 4.
Global correlation of intracellular levels of p53 and Bcl-2 with chondrocyte proliferation at day 5 assessed in three normal subjects and in the least (open circles) and most (closed triangles) damaged zones of 14 OA cartilage specimens. Spearman correlation test: rs correlation coefficient; p, significance level.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. E., Brandt K. D. Hypertrophic repair of canine articular cartilage in osteoarthritis after anterior cruciate ligament transection. J Rheumatol. 1991 Mar;18(3):428–435. [PubMed] [Google Scholar]
- Aigner T., Hemmel M., Neureiter D., Gebhard P. M., Zeiler G., Kirchner T., McKenna L. Apoptotic cell death is not a widespread phenomenon in normal aging and osteoarthritis human articular knee cartilage: a study of proliferation, programmed cell death (apoptosis), and viability of chondrocytes in normal and osteoarthritic human knee cartilage. Arthritis Rheum. 2001 Jun;44(6):1304–1312. doi: 10.1002/1529-0131(200106)44:6<1304::AID-ART222>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Aigner T., Kim H. A. Apoptosis and cellular vitality: issues in osteoarthritic cartilage degeneration. Arthritis Rheum. 2002 Aug;46(8):1986–1996. doi: 10.1002/art.10554. [DOI] [PubMed] [Google Scholar]
- Blanco F. J., Guitian R., Vázquez-Martul E., de Toro F. J., Galdo F. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998 Feb;41(2):284–289. doi: 10.1002/1529-0131(199802)41:2<284::AID-ART12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Erlacher L., Maier R., Ullrich R., Kiener H., Aringer M., Menschik M., Graninger W. Differential expression of the protooncogene bcl-2 in normal and osteoarthritic human articular cartilage. J Rheumatol. 1995 May;22(5):926–931. [PubMed] [Google Scholar]
- Erlacher L., Ng C. K., Ullrich R., Krieger S., Luyten F. P. Presence of cartilage-derived morphogenetic proteins in articular cartilage and enhancement of matrix replacement in vitro. Arthritis Rheum. 1998 Feb;41(2):263–273. doi: 10.1002/1529-0131(199802)41:2<263::AID-ART10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Feng L., Balakir R., Precht P., Horton W. E., Jr Bcl-2 regulates chondrocyte morphology and aggrecan gene expression independent of caspase activation and full apoptosis. J Cell Biochem. 1999 Sep 15;74(4):576–586. [PubMed] [Google Scholar]
- Hamerman D. The biology of osteoarthritis. N Engl J Med. 1989 May 18;320(20):1322–1330. doi: 10.1056/NEJM198905183202006. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Setareh M., Ochs R. L., Lotz M. Fas/Fas ligand expression and induction of apoptosis in chondrocytes. Arthritis Rheum. 1997 Oct;40(10):1749–1755. doi: 10.1002/art.1780401004. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Takahashi K., Amiel D., Coutts R. D., Lotz M. Chondrocyte apoptosis and nitric oxide production during experimentally induced osteoarthritis. Arthritis Rheum. 1998 Jul;41(7):1266–1274. doi: 10.1002/1529-0131(199807)41:7<1266::AID-ART18>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Hildeman David A., Zhu Yanan, Mitchell Thomas C., Kappler John, Marrack Philippa. Molecular mechanisms of activated T cell death in vivo. Curr Opin Immunol. 2002 Jun;14(3):354–359. doi: 10.1016/s0952-7915(02)00335-7. [DOI] [PubMed] [Google Scholar]
- Héraud F., Héraud A., Harmand M. F. Apoptosis in normal and osteoarthritic human articular cartilage. Ann Rheum Dis. 2000 Dec;59(12):959–965. doi: 10.1136/ard.59.12.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannone F., De Bari C., Dell'Accio F., Covelli M., Cantatore F. P., Patella V., Lo Bianco G., Lapadula G. Interleukin-10 and interleukin-10 receptor in human osteoarthritic and healthy chondrocytes. Clin Exp Rheumatol. 2001 Mar-Apr;19(2):139–145. [PubMed] [Google Scholar]
- Iannone F., De Bari C., Dell'Accio F., Covelli M., Patella V., Lo Bianco G., Lapadula G. Increased expression of nerve growth factor (NGF) and high affinity NGF receptor (p140 TrkA) in human osteoarthritic chondrocytes. Rheumatology (Oxford) 2002 Dec;41(12):1413–1418. doi: 10.1093/rheumatology/41.12.1413. [DOI] [PubMed] [Google Scholar]
- Iannone F., Lapadula G. Neuropeptides and human osteoarthritis. J Rheumatol. 1998 Feb;25(2):386–388. [PubMed] [Google Scholar]
- Kim H. A., Lee Y. J., Seong S. C., Choe K. W., Song Y. W. Apoptotic chondrocyte death in human osteoarthritis. J Rheumatol. 2000 Feb;27(2):455–462. [PubMed] [Google Scholar]
- Kim H. A., Song Y. W. Apoptotic chondrocyte death in rheumatoid arthritis. Arthritis Rheum. 1999 Jul;42(7):1528–1537. doi: 10.1002/1529-0131(199907)42:7<1528::AID-ANR28>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Kinkel Mary D., Horton Walter E., Jr Coordinate down-regulation of cartilage matrix gene expression in Bcl-2 deficient chondrocytes is associated with decreased SOX9 expression and decreased mRNA stability. J Cell Biochem. 2003 Apr 1;88(5):941–953. doi: 10.1002/jcb.10442. [DOI] [PubMed] [Google Scholar]
- Kühn K., Lotz M. Regulation of CD95 (Fas/APO-1)-induced apoptosis in human chondrocytes. Arthritis Rheum. 2001 Jul;44(7):1644–1653. doi: 10.1002/1529-0131(200107)44:7<1644::AID-ART287>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Lapadula G., Iannone F., Zuccaro C., Covelli M., Patella V., Lobianco G., Pipitone V. Expression of membrane-bound peptidases (CD10 and CD26) on human articular chondrocytes. Possible role of neuropeptidases in the pathogenesis of osteoarthritis. Clin Exp Rheumatol. 1995 Mar-Apr;13(2):143–148. [PubMed] [Google Scholar]
- Levine A. J. p53, the cellular gatekeeper for growth and division. Cell. 1997 Feb 7;88(3):323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- Lippiello L., Hall D., Mankin H. J. Collagen synthesis in normal and osteoarthritic human cartilage. J Clin Invest. 1977 Apr;59(4):593–600. doi: 10.1172/JCI108676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin H. J., Dorfman H., Lippiello L., Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971 Apr;53(3):523–537. [PubMed] [Google Scholar]
- Mankin H. J., Johnson M. E., Lippiello L. Biochemical and metabolic abnormalities in articular cartilage from osteoarthritic human hips. III. Distribution and metabolism of amino sugar-containing macromolecules. J Bone Joint Surg Am. 1981 Jan;63(1):131–139. [PubMed] [Google Scholar]
- Mankin H. J., Lippiello L. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. J Bone Joint Surg Am. 1970 Apr;52(3):424–434. [PubMed] [Google Scholar]
- Rigg K. M., Shenton B. K., Murray I. A., Givan A. L., Taylor R. M., Lennard T. W. A flow cytometric technique for simultaneous analysis of human mononuclear cell surface antigens and DNA. J Immunol Methods. 1989 Oct 24;123(2):177–184. doi: 10.1016/0022-1759(89)90221-4. [DOI] [PubMed] [Google Scholar]
- Sharif Mohammed, Whitehouse Anne, Sharman Patrick, Perry Mark, Adams Mike. Increased apoptosis in human osteoarthritic cartilage corresponds to reduced cell density and expression of caspase-3. Arthritis Rheum. 2004 Feb;50(2):507–515. doi: 10.1002/art.20020. [DOI] [PubMed] [Google Scholar]
- Trippel S. B. Growth factor actions on articular cartilage. J Rheumatol Suppl. 1995 Feb;43:129–132. [PubMed] [Google Scholar]
- Vaishnaw A. K., McNally J. D., Elkon K. B. Apoptosis in the rheumatic diseases. Arthritis Rheum. 1997 Nov;40(11):1917–1927. doi: 10.1002/art.1780401102. [DOI] [PubMed] [Google Scholar]