Abstract
Background: Cancellous bone from patients with osteoarthritis (OA) has been reported to be undermineralised and that from patients with osteoporosis (OP) is more liable to fracture. Changes in the mineral component might be implicated in these processes.
Objectives: To investigate the thermal stability and the mineral structure of cancellous bone from femoral heads of patients with either OA or OP.
Methods: Powdered bone was prepared from femoral heads of patients with either OA or OP and a control group. Composition and thermal stability were determined using a thermogravimetric analyser coupled to a mass spectrometer. Unit cell dimensions and the crystallite size of the mineral were measured using x ray diffraction.
Results: Thermal stability of the bone matrix, or of the mineral phase alone, was little altered by disease, though OA bone contained less mineral than OP or control bone. In all three groups, x ray diffraction showed that the mineral unit cell dimensions and crystallite sizes were the same. The mean carbonate content in the mineral from all three groups was between 7.2 and 7.6% and is suggested to be located in both the A site (that is, substituting for hydroxyl groups), and the B site (that is, substituting for phosphate groups).
Conclusions: These results confirm that there is a lower mass fraction of mineral in OA bone, and indicate that the nature of the mineral is not a factor in either disease process.
Full Text
The Full Text of this article is available as a PDF (236.0 KB).
Figure 1.
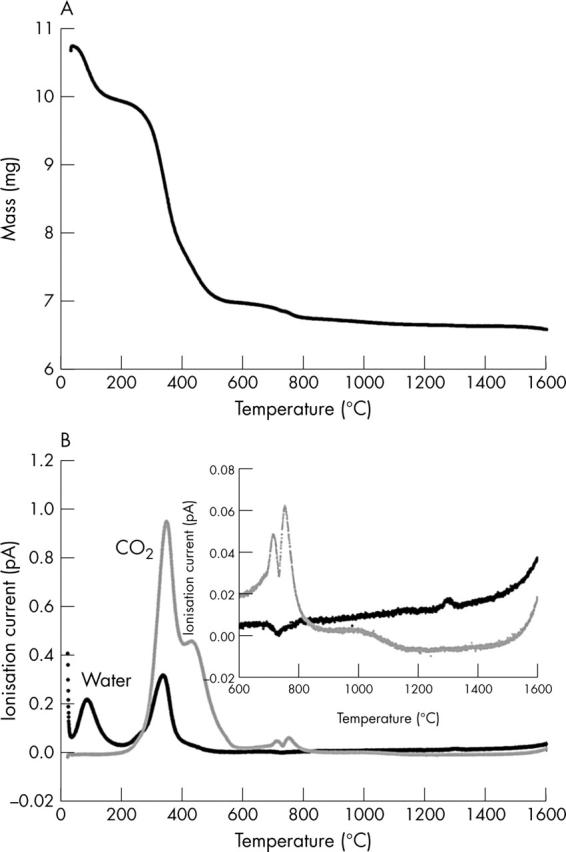
A typical trace, from a control sample, of (a) the mass as a function of temperature, measured using TGA, and (b) the corresponding traces of water and carbon dioxide recorded on the mass spectrometer (the inset is an enlarged version of the traces above 600°C). All samples were air dried, so no account was taken of the first mass lost, which can be explained by loss of residual water. Organic components are lost between 200–550°C. Carbonate was evolved in two stages: a doublet peak between 700–800°C then an extended loss up to 1100°C. Final decomposition of the mineral occurs at about 1300°C.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aspden R. M., Scheven B. A., Hutchison J. D. Osteoarthritis as a systemic disorder including stromal cell differentiation and lipid metabolism. Lancet. 2001 Apr 7;357(9262):1118–1120. doi: 10.1016/S0140-6736(00)04264-1. [DOI] [PubMed] [Google Scholar]
- Bailey A. J., Knott L. Molecular changes in bone collagen in osteoporosis and osteoarthritis in the elderly. Exp Gerontol. 1999 Jun;34(3):337–351. doi: 10.1016/s0531-5565(99)00016-9. [DOI] [PubMed] [Google Scholar]
- Bigi A., Cojazzi G., Panzavolta S., Ripamonti A., Roveri N., Romanello M., Noris Suarez K., Moro L. Chemical and structural characterization of the mineral phase from cortical and trabecular bone. J Inorg Biochem. 1997 Oct;68(1):45–51. doi: 10.1016/s0162-0134(97)00007-x. [DOI] [PubMed] [Google Scholar]
- Boyde A., Jones S. J., Aerssens J., Dequeker J. Mineral density quantitation of the human cortical iliac crest by backscattered electron image analysis: variations with age, sex, and degree of osteoarthritis. Bone. 1995 Jun;16(6):619–627. doi: 10.1016/8756-3282(95)00119-x. [DOI] [PubMed] [Google Scholar]
- Burr D. B. The importance of subchondral bone in osteoarthrosis. Curr Opin Rheumatol. 1998 May;10(3):256–262. doi: 10.1097/00002281-199805000-00017. [DOI] [PubMed] [Google Scholar]
- Coats A. M., Zioupos P., Aspden R. M. Material properties of subchondral bone from patients with osteoporosis or osteoarthritis by microindentation testing and electron probe microanalysis. Calcif Tissue Int. 2003 Jul;73(1):66–71. doi: 10.1007/s00223-002-2080-8. [DOI] [PubMed] [Google Scholar]
- Dequeker J., Mokassa L., Aerssens J. Bone density and osteoarthritis. J Rheumatol Suppl. 1995 Feb;43:98–100. [PubMed] [Google Scholar]
- Ding M. Age variations in the properties of human tibial trabecular bone and cartilage. Acta Orthop Scand Suppl. 2000 Jun;292:1–45. doi: 10.1080/000164700753749791. [DOI] [PubMed] [Google Scholar]
- Eckstein F., Milz S., Anetzberger H., Putz R. Thickness of the subchondral mineralised tissue zone (SMZ) in normal male and female and pathological human patellae. J Anat. 1998 Jan;192(Pt 1):81–90. doi: 10.1046/j.1469-7580.1998.19210081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellies L. G., Nelson D. G., Featherstone J. D. Crystallographic structure and surface morphology of sintered carbonated apatites. J Biomed Mater Res. 1988 Jun;22(6):541–553. doi: 10.1002/jbm.820220609. [DOI] [PubMed] [Google Scholar]
- Fazzalari N. L., Darracott J., Vernon-Roberts B. Histomorphometric changes in the trabecular structure of a selected stress region in the femur in patients with osteoarthritis and fracture of the femoral neck. Bone. 1985;6(3):125–133. doi: 10.1016/8756-3282(85)90044-4. [DOI] [PubMed] [Google Scholar]
- Fazzalari N. L., Forwood M. R., Smith K., Manthey B. A., Herreen P. Assessment of cancellous bone quality in severe osteoarthrosis: bone mineral density, mechanics, and microdamage. Bone. 1998 Apr;22(4):381–388. doi: 10.1016/s8756-3282(97)00298-6. [DOI] [PubMed] [Google Scholar]
- Gevers G., Dequeker J., Martens M., Van Audekercke R., Nyssen-Behets C., Dhem A. Biomechanical characteristics of iliac crest bone in elderly women according to osteoarthritis grade at the hand joints. J Rheumatol. 1989 May;16(5):660–663. [PubMed] [Google Scholar]
- Grynpas M. D., Alpert B., Katz I., Lieberman I., Pritzker K. P. Subchondral bone in osteoarthritis. Calcif Tissue Int. 1991 Jul;49(1):20–26. doi: 10.1007/BF02555898. [DOI] [PubMed] [Google Scholar]
- Handschin R. G., Stern W. B. Crystallographic and chemical analysis of human bone apatite (Crista Iliaca). Clin Rheumatol. 1994 Dec;13 (Suppl 1):75–90. [PubMed] [Google Scholar]
- Hasegawa K., Turner C. H., Recker R. R., Wu E., Burr D. B. Elastic properties of osteoporotic bone measured by scanning acoustic microscopy. Bone. 1995 Jan;16(1):85–90. doi: 10.1016/s8756-3282(94)00013-1. [DOI] [PubMed] [Google Scholar]
- Layani J. D., Mayer I., Cuisinier F. J. Carbonated hydroxyapatites precipitated in the presence of Ti. J Inorg Biochem. 2000 Jul 15;81(1-2):57–63. doi: 10.1016/s0162-0134(00)00115-x. [DOI] [PubMed] [Google Scholar]
- Lereim P., Goldie I. F. Relationship between morphologic features and hardness of the subchondral bone of the medial tibial condyle in the normal state and in osteoarthritis and rheumatoid arthritis. Arch Orthop Unfallchir. 1975;81(1):1–11. doi: 10.1007/BF00417022. [DOI] [PubMed] [Google Scholar]
- Li B., Aspden R. M. Composition and mechanical properties of cancellous bone from the femoral head of patients with osteoporosis or osteoarthritis. J Bone Miner Res. 1997 Apr;12(4):641–651. doi: 10.1359/jbmr.1997.12.4.641. [DOI] [PubMed] [Google Scholar]
- Li B., Aspden R. M. Material properties of bone from the femoral neck and calcar femorale of patients with osteoporosis or osteoarthritis. Osteoporos Int. 1997;7(5):450–456. doi: 10.1007/s001980050032. [DOI] [PubMed] [Google Scholar]
- Li B., Aspden R. M. Mechanical and material properties of the subchondral bone plate from the femoral head of patients with osteoarthritis or osteoporosis. Ann Rheum Dis. 1997 Apr;56(4):247–254. doi: 10.1136/ard.56.4.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Marshall D., Roe M., Aspden R. M. The electron microscope appearance of the subchondral bone plate in the human femoral head in osteoarthritis and osteoporosis. J Anat. 1999 Jul;195(Pt 1):101–110. doi: 10.1046/j.1469-7580.1999.19510101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppen Cynthia A., Smith Elisheva, Spevak Lyudmila, Boskey Adele L., Frenkel Baruch. Bone morphogenetic protein-2 restores mineralization in glucocorticoid-inhibited MC3T3-E1 osteoblast cultures. J Bone Miner Res. 2003 Jul;18(7):1186–1197. doi: 10.1359/jbmr.2003.18.7.1186. [DOI] [PubMed] [Google Scholar]
- Paschalis E. P., Betts F., DiCarlo E., Mendelsohn R., Boskey A. L. FTIR microspectroscopic analysis of human iliac crest biopsies from untreated osteoporotic bone. Calcif Tissue Int. 1997 Dec;61(6):487–492. doi: 10.1007/s002239900372. [DOI] [PubMed] [Google Scholar]
- Raymaekers G., Aerssens J., Van den Eynde R., Peeters J., Geusens P., Devos P., Dequeker J. Alterations of the mineralization profile and osteocalcin concentrations in osteoarthritic cortical iliac crest bone. Calcif Tissue Int. 1992 Oct;51(4):269–275. doi: 10.1007/BF00334486. [DOI] [PubMed] [Google Scholar]
- Rey C., Beshah K., Griffin R., Glimcher M. J. Structural studies of the mineral phase of calcifying cartilage. J Bone Miner Res. 1991 May;6(5):515–525. doi: 10.1002/jbmr.5650060514. [DOI] [PubMed] [Google Scholar]
- Suetsugu Y., Tanaka J. Crystal growth of carbonate apatite using a CaCO3 flux. J Mater Sci Mater Med. 1999 Sep;10(9):561–566. doi: 10.1023/a:1008972432076. [DOI] [PubMed] [Google Scholar]
- Young R. A., Holcomb D. W. Variability of hydroxyapatite preparations. Calcif Tissue Int. 1982;34 (Suppl 2):S17–S32. [PubMed] [Google Scholar]
- el Feki H., Rey C., Vignoles M. Carbonate ions in apatites: infrared investigations in the upsilon 4 CO3 domain. Calcif Tissue Int. 1991 Oct;49(4):269–274. doi: 10.1007/BF02556216. [DOI] [PubMed] [Google Scholar]


