Abstract
Background: Knowledge of the deformational behaviour of articular cartilage in vivo is required to understand the pathogenesis of osteoarthritis and the mechanical target environment of prospective cartilage transplant recipients.
Objectives: To study the in vivo deformational behaviour of patellar and femorotibial cartilage for different types of physiological activities; and to test the hypothesis that in vivo deformation of cartilage is modified by intense physical exercise.
Methods: Magnetic resonance imaging and 3D digital image analysis were used to determine cartilage volume before and after physical activity in the patella of 12 volunteers (knee bends, squatting, normal gait, running, cycling). Deformation of femorotibial cartilage was investigated in 10 subjects (knee bends, static compression, high impact loading). Patellar cartilage deformation after knee bends was compared in seven professional weight lifters, seven sprinters, and 14 untrained volunteers.
Results: Patellar cartilage deformation was –5.9% after knee bends, –4.7% after squatting, –2.8% after normal walking, –5.0% after running, and –4.5% after cycling. The pattern of patellar cartilage deformation corresponded to the range of motion involved in the particular activity. Tibial cartilage deformation was greatest under high impact loading (–7%), but small for other activities. No significant difference was found between athletes and non-athletic controls.
Conclusions: Patellar cartilage deformation shows a "dose dependent" response, where more intense loading leads to greater deformation. Relatively little deformation was observed in the femorotibial joint, except during high impact activities. The findings provide no evidence that adult human cartilage properties are amendable to training effects in vivo.
Full Text
The Full Text of this article is available as a PDF (271.8 KB).
Figure 1.
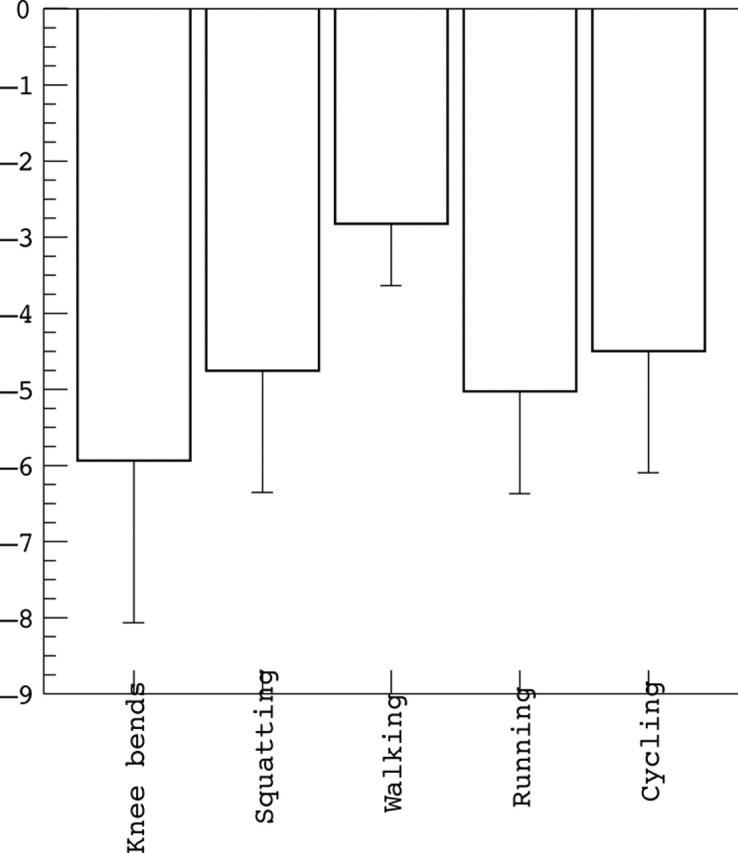
Magnitude of patellar cartilage deformation after knee bends, squatting (20 second static loading at 90° knee flexion), normal walking, running (including steps), and cycling.
Figure 2.
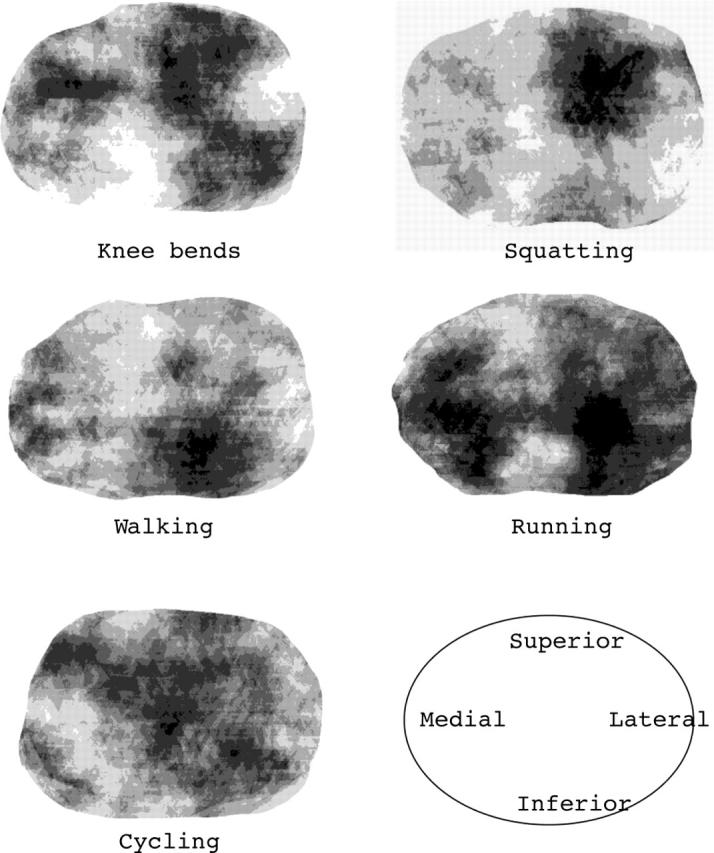
Regional variation of deformation throughout the patella for different types of activities; average over all 12 volunteers. View on the retropatellar surface from posteriorly (lateral facet on the right and medial facet on the left). Dark areas are regions of high deformation and lighter areas are regions of low deformation. The results show that activities with a limited range of knee motion (squatting and walking) lead to deformation of some confined areas, whereas activities that involve a larger range of motion lead to more widespread deformation of patellar cartilage.
Figure 3.
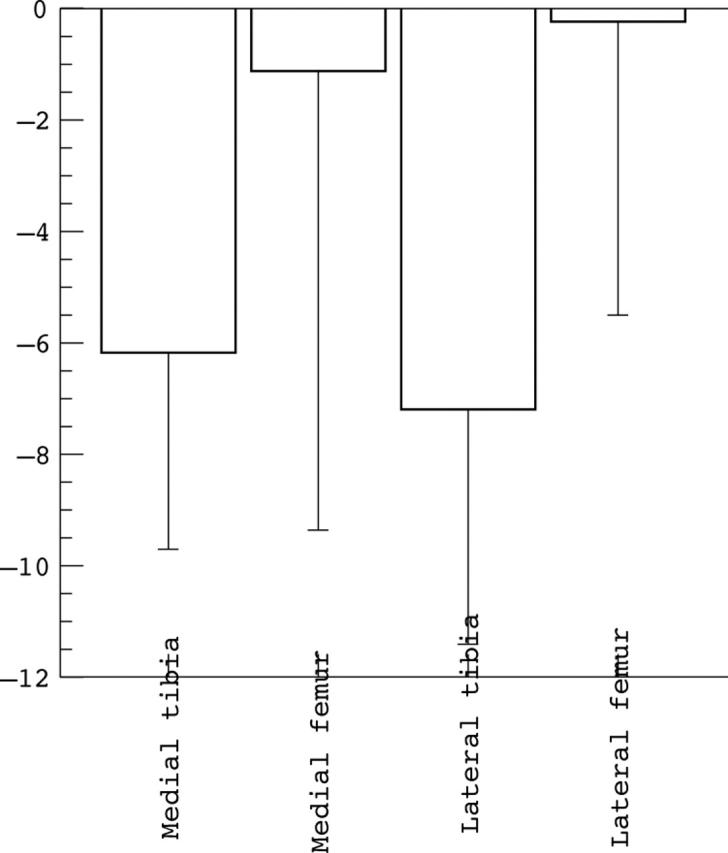
Magnitude of femorotibial cartilage deformation after high impact loading for the medial tibia, lateral tibia, medial femoral condyle, and lateral femoral condyle.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. G., Mow V. C. Variations in the intrinsic mechanical properties of human articular cartilage with age, degeneration, and water content. J Bone Joint Surg Am. 1982 Jan;64(1):88–94. [PubMed] [Google Scholar]
- Burgkart R., Glaser C., Hyhlik-Dürr A., Englmeier K. H., Reiser M., Eckstein F. Magnetic resonance imaging-based assessment of cartilage loss in severe osteoarthritis: accuracy, precision, and diagnostic value. Arthritis Rheum. 2001 Sep;44(9):2072–2077. doi: 10.1002/1529-0131(200109)44:9<2072::AID-ART357>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Burstein D., Bashir A., Gray M. L. MRI techniques in early stages of cartilage disease. Invest Radiol. 2000 Oct;35(10):622–638. doi: 10.1097/00004424-200010000-00008. [DOI] [PubMed] [Google Scholar]
- Eckstein F., Heudorfer L., Faber S. C., Burgkart R., Englmeier K-H, Reiser M. Long-term and resegmentation precision of quantitative cartilage MR imaging (qMRI). Osteoarthritis Cartilage. 2002 Dec;10(12):922–928. doi: 10.1053/joca.2002.0844. [DOI] [PubMed] [Google Scholar]
- Eckstein F., Lemberger B., Stammberger T., Englmeier K. H., Reiser M. Patellar cartilage deformation in vivo after static versus dynamic loading. J Biomech. 2000 Jul;33(7):819–825. doi: 10.1016/s0021-9290(00)00034-8. [DOI] [PubMed] [Google Scholar]
- Eckstein F., Reiser M., Englmeier K. H., Putz R. In vivo morphometry and functional analysis of human articular cartilage with quantitative magnetic resonance imaging--from image to data, from data to theory. Anat Embryol (Berl) 2001 Mar;203(3):147–173. doi: 10.1007/s004290000154. [DOI] [PubMed] [Google Scholar]
- Eckstein F., Tieschky M., Faber S. C., Haubner M., Kolem H., Englmeier K. H., Reiser M. Effect of physical exercise on cartilage volume and thickness in vivo: MR imaging study. Radiology. 1998 Apr;207(1):243–248. doi: 10.1148/radiology.207.1.9530322. [DOI] [PubMed] [Google Scholar]
- Eckstein F., Tieschky M., Faber S., Englmeier K. H., Reiser M. Functional analysis of articular cartilage deformation, recovery, and fluid flow following dynamic exercise in vivo. Anat Embryol (Berl) 1999 Oct;200(4):419–424. doi: 10.1007/s004290050291. [DOI] [PubMed] [Google Scholar]
- Froimson M. I., Ratcliffe A., Gardner T. R., Mow V. C. Differences in patellofemoral joint cartilage material properties and their significance to the etiology of cartilage surface fibrillation. Osteoarthritis Cartilage. 1997 Nov;5(6):377–386. doi: 10.1016/s1063-4584(97)80042-8. [DOI] [PubMed] [Google Scholar]
- Glaser Christian, Burgkart Rainer, Kutschera Andrea, Englmeier Karl-Hans, Reiser Maximilian, Eckstein Felix. Femoro-tibial cartilage metrics from coronal MR image data: Technique, test-retest reproducibility, and findings in osteoarthritis. Magn Reson Med. 2003 Dec;50(6):1229–1236. doi: 10.1002/mrm.10648. [DOI] [PubMed] [Google Scholar]
- Glüer C. C., Blake G., Lu Y., Blunt B. A., Jergas M., Genant H. K. Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporos Int. 1995;5(4):262–270. doi: 10.1007/BF01774016. [DOI] [PubMed] [Google Scholar]
- Graichen Heiko, von Eisenhart-Rothe Rüdiger, Vogl Thomas, Englmeier Karl-Hans, Eckstein Felix. Quantitative assessment of cartilage status in osteoarthritis by quantitative magnetic resonance imaging: technical validation for use in analysis of cartilage volume and further morphologic parameters. Arthritis Rheum. 2004 Mar;50(3):811–816. doi: 10.1002/art.20191. [DOI] [PubMed] [Google Scholar]
- Hehne H. J. Biomechanics of the patellofemoral joint and its clinical relevance. Clin Orthop Relat Res. 1990 Sep;(258):73–85. [PubMed] [Google Scholar]
- Hudelmaier M., Glaser C., Hohe J., Englmeier K. H., Reiser M., Putz R., Eckstein F. Age-related changes in the morphology and deformational behavior of knee joint cartilage. Arthritis Rheum. 2001 Nov;44(11):2556–2561. doi: 10.1002/1529-0131(200111)44:11<2556::aid-art436>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Hyhlik-Dürr A., Faber S., Burgkart R., Stammberger T., Maag K. P., Englmeier K. H., Reiser M., Eckstein F. Precision of tibial cartilage morphometry with a coronal water-excitation MR sequence. Eur Radiol. 2000;10(2):297–303. doi: 10.1007/s003300050047. [DOI] [PubMed] [Google Scholar]
- Kim Y. J., Bonassar L. J., Grodzinsky A. J. The role of cartilage streaming potential, fluid flow and pressure in the stimulation of chondrocyte biosynthesis during dynamic compression. J Biomech. 1995 Sep;28(9):1055–1066. doi: 10.1016/0021-9290(94)00159-2. [DOI] [PubMed] [Google Scholar]
- Mow V. C., Ateshian G. A., Spilker R. L. Biomechanics of diarthrodial joints: a review of twenty years of progress. J Biomech Eng. 1993 Nov;115(4B):460–467. doi: 10.1115/1.2895525. [DOI] [PubMed] [Google Scholar]
- Mow V. C., Holmes M. H., Lai W. M. Fluid transport and mechanical properties of articular cartilage: a review. J Biomech. 1984;17(5):377–394. doi: 10.1016/0021-9290(84)90031-9. [DOI] [PubMed] [Google Scholar]
- Newton P. M., Mow V. C., Gardner T. R., Buckwalter J. A., Albright J. P. Winner of the 1996 Cabaud Award. The effect of lifelong exercise on canine articular cartilage. Am J Sports Med. 1997 May-Jun;25(3):282–287. doi: 10.1177/036354659702500302. [DOI] [PubMed] [Google Scholar]
- Peterfy C. G., Genant H. K. Emerging applications of magnetic resonance imaging in the evaluation of articular cartilage. Radiol Clin North Am. 1996 Mar;34(2):195-213, ix. [PubMed] [Google Scholar]
- Sah R. L., Kim Y. J., Doong J. Y., Grodzinsky A. J., Plaas A. H., Sandy J. D. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res. 1989;7(5):619–636. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- Stammberger T., Eckstein F., Englmeier K. H., Reiser M. Determination of 3D cartilage thickness data from MR imaging: computational method and reproducibility in the living. Magn Reson Med. 1999 Mar;41(3):529–536. doi: 10.1002/(sici)1522-2594(199903)41:3<529::aid-mrm15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Stammberger T., Eckstein F., Michaelis M., Englmeier K. H., Reiser M. Interobserver reproducibility of quantitative cartilage measurements: comparison of B-spline snakes and manual segmentation. Magn Reson Imaging. 1999 Sep;17(7):1033–1042. doi: 10.1016/s0730-725x(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Stammberger T., Hohe J., Englmeier K. H., Reiser M., Eckstein F. Elastic registration of 3D cartilage surfaces from MR image data for detecting local changes in cartilage thickness. Magn Reson Med. 2000 Oct;44(4):592–601. doi: 10.1002/1522-2594(200010)44:4<592::aid-mrm13>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Urban J. P. The chondrocyte: a cell under pressure. Br J Rheumatol. 1994 Oct;33(10):901–908. doi: 10.1093/rheumatology/33.10.901. [DOI] [PubMed] [Google Scholar]
- Vanwanseele B., Eckstein F., Knecht H., Spaepen A., Stüssi E. Longitudinal analysis of cartilage atrophy in the knees of patients with spinal cord injury. Arthritis Rheum. 2003 Dec;48(12):3377–3381. doi: 10.1002/art.11367. [DOI] [PubMed] [Google Scholar]
- Vanwanseele B., Eckstein F., Knecht H., Stüssi E., Spaepen A. Knee cartilage of spinal cord-injured patients displays progressive thinning in the absence of normal joint loading and movement. Arthritis Rheum. 2002 Aug;46(8):2073–2078. doi: 10.1002/art.10462. [DOI] [PubMed] [Google Scholar]
- Vanwanseele B., Lucchinetti E., Stüssi E. The effects of immobilization on the characteristics of articular cartilage: current concepts and future directions. Osteoarthritis Cartilage. 2002 May;10(5):408–419. doi: 10.1053/joca.2002.0529. [DOI] [PubMed] [Google Scholar]
- Waterton J. C., Solloway S., Foster J. E., Keen M. C., Gandy S., Middleton B. J., Maciewicz R. A., Watt I., Dieppe P. A., Taylor C. J. Diurnal variation in the femoral articular cartilage of the knee in young adult humans. Magn Reson Med. 2000 Jan;43(1):126–132. doi: 10.1002/(sici)1522-2594(200001)43:1<126::aid-mrm15>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]


