Abstract
Objective: To analyse patients with rheumatoid arthritis, treated with methotrexate in a weekly academic rheumatology clinic over 13 years, for continuation of courses and reasons for discontinuation.
Methods: All 248 patients with an analysable longitudinal course who took methotrexate in standard care between 1990 and 2003 were studied. Continuation of courses was analysed using life tables. All abnormal and severely abnormal values for aspartate aminotransferase (AST) >40 U/l, >80 U/l, albumin <35 g/l, <30 g/l, white blood cell (WBC) count <4.0x109/l, <3.0x109/l, and platelet count <150x109/l, <100x109/l, were identified. Responses of the clinician and subsequent laboratory values were reviewed.
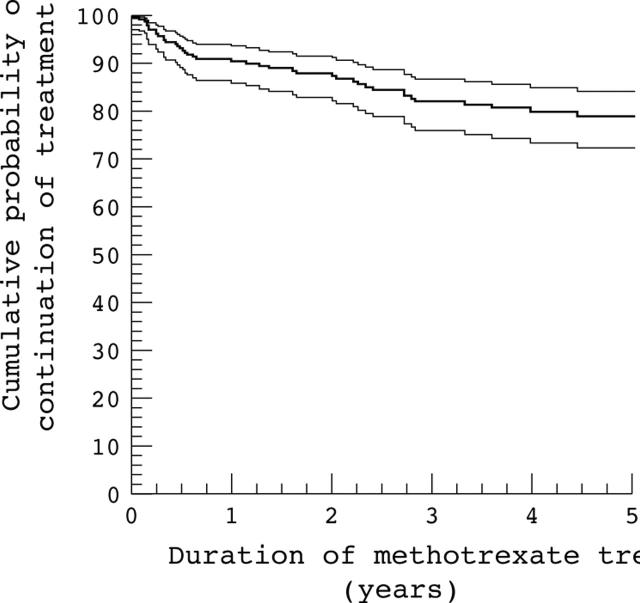
Results: Over 1007 person-years, the probability of continuing methotrexate over five years was 79% (95% confidence interval, 72% to 84%). Severe laboratory abnormalities occurred in 2.9 per 100 person-years, specifically 0.9 for AST >80 U/l, 1.1 for albumin <30 g/l, 0.7 for WBC <3.0x109/l, and 0.3 for platelets <100x109/l. No severe laboratory abnormality progressed to further severity or clinical disease. Permanent discontinuations of methotrexate occurred in 46 patients (19%), 26 (10% of all patients) for adverse effects, 15 (32.6%) for inefficacy; only two discontinuations resulted from laboratory abnormalities, both of WBC, possibly from other sources.
Conclusions: Methotrexate was associated with a high rate of continuation, and few clinically significant laboratory abnormalities. Discontinuation primarily reflected clinical rather than laboratory findings. Vigilance for methotrexate toxicity is required but methotrexate appears among the safest treatments for rheumatoid arthritis.
Full Text
The Full Text of this article is available as a PDF (214.0 KB).
Figure 1.
Cumulative probability (with 95% confidence interval) for continuation of methotrexate treatment in 248 patients with rheumatoid arthritis.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alarcón G. S., Tracy I. C., Strand G. M., Singh K., Macaluso M. Survival and drug discontinuation analyses in a large cohort of methotrexate treated rheumatoid arthritis patients. Ann Rheum Dis. 1995 Sep;54(9):708–712. doi: 10.1136/ard.54.9.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Bergquist S. R., Felson D. T., Prashker M. J., Freedberg K. A. The cost-effectiveness of liver biopsy in rheumatoid arthritis patients treated with methotrexate. Arthritis Rheum. 1995 Mar;38(3):326–333. doi: 10.1002/art.1780380306. [DOI] [PubMed] [Google Scholar]
- Bridges S. L., Jr, Alarcón G. S., Koopman W. J. Methotrexate-induced liver abnormalities in rheumatoid arthritis. J Rheumatol. 1989 Sep;16(9):1180–1183. [PubMed] [Google Scholar]
- Chan Edwin S. L., Cronstein Bruce N. Molecular action of methotrexate in inflammatory diseases. Arthritis Res. 2002 Mar 19;4(4):266–273. doi: 10.1186/ar419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Hyon K., Hernán Miguel A., Seeger John D., Robins James M., Wolfe Frederick. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002 Apr 6;359(9313):1173–1177. doi: 10.1016/S0140-6736(02)08213-2. [DOI] [PubMed] [Google Scholar]
- Hanrahan P. S., Scrivens G. A., Russell A. S. Prospective long term follow-up of methotrexate therapy in rheumatoid arthritis: toxicity, efficacy and radiological progression. Br J Rheumatol. 1989 Apr;28(2):147–153. doi: 10.1093/rheumatology/28.2.147. [DOI] [PubMed] [Google Scholar]
- Krause D., Schleusser B., Herborn G., Rau R. Response to methotrexate treatment is associated with reduced mortality in patients with severe rheumatoid arthritis. Arthritis Rheum. 2000 Jan;43(1):14–21. doi: 10.1002/1529-0131(200001)43:1<14::AID-ANR3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Kremer J. M., Alarcón G. S., Lightfoot R. W., Jr, Willkens R. F., Furst D. E., Williams H. J., Dent P. B., Weinblatt M. E. Methotrexate for rheumatoid arthritis. Suggested guidelines for monitoring liver toxicity. American College of Rheumatology. Arthritis Rheum. 1994 Mar;37(3):316–328. doi: 10.1002/art.1780370304. [DOI] [PubMed] [Google Scholar]
- Kremer J. M., Lee R. G., Tolman K. G. Liver histology in rheumatoid arthritis patients receiving long-term methotrexate therapy. A prospective study with baseline and sequential biopsy samples. Arthritis Rheum. 1989 Feb;32(2):121–127. doi: 10.1002/anr.1780320202. [DOI] [PubMed] [Google Scholar]
- Mielants H., Veys E. M., Van der Straeten C., Ackerman C., Goemaere S. The efficacy and toxicity of a constant low dose of methotrexate as a treatment for intractable rheumatoid arthritis: an open prospective study. J Rheumatol. 1991 Jul;18(7):978–983. [PubMed] [Google Scholar]
- Morgan S. L., Baggott J. E., Vaughn W. H., Austin J. S., Veitch T. A., Lee J. Y., Koopman W. J., Krumdieck C. L., Alarcón G. S. Supplementation with folic acid during methotrexate therapy for rheumatoid arthritis. A double-blind, placebo-controlled trial. Ann Intern Med. 1994 Dec 1;121(11):833–841. doi: 10.7326/0003-4819-121-11-199412010-00002. [DOI] [PubMed] [Google Scholar]
- Pincus T., Brooks R. H., Callahan L. F. Prediction of long-term mortality in patients with rheumatoid arthritis according to simple questionnaire and joint count measures. Ann Intern Med. 1994 Jan 1;120(1):26–34. doi: 10.7326/0003-4819-120-1-199401010-00005. [DOI] [PubMed] [Google Scholar]
- Pincus T., Callahan L. F. The 'side effects' of rheumatoid arthritis: joint destruction, disability and early mortality. Br J Rheumatol. 1993 Mar;32 (Suppl 1):28–37. [PubMed] [Google Scholar]
- Pincus T., Marcum S. B., Callahan L. F., Adams R. F., Barber J., Barth W. F., Gordon G. V., Huston J. W., Polk J. R., Whelton J. C. Longterm drug therapy for rheumatoid arthritis in seven rheumatology private practices: I. Nonsteroidal antiinflammatory drugs. J Rheumatol. 1992 Dec;19(12):1874–1884. [PubMed] [Google Scholar]
- Pincus T., Marcum S. B., Callahan L. F. Longterm drug therapy for rheumatoid arthritis in seven rheumatology private practices: II. Second line drugs and prednisone. J Rheumatol. 1992 Dec;19(12):1885–1894. [PubMed] [Google Scholar]
- Rau R., Karger T., Herborn G., Frenzel H. Liver biopsy findings in patients with rheumatoid arthritis undergoing longterm treatment with methotrexate. J Rheumatol. 1989 Apr;16(4):489–493. [PubMed] [Google Scholar]
- Rau R., Schleusser B., Herborn G., Karger T. Long-term treatment of destructive rheumatoid arthritis with methotrexate. J Rheumatol. 1997 Oct;24(10):1881–1889. [PubMed] [Google Scholar]
- Sany J., Anaya J. M., Lussiez V., Couret M., Combe B., Daures J. P. Treatment of rheumatoid arthritis with methotrexate: a prospective open longterm study of 191 cases. J Rheumatol. 1991 Sep;18(9):1323–1327. [PubMed] [Google Scholar]
- Shergy W. J., Polisson R. P., Caldwell D. S., Rice J. R., Pisetsky D. S., Allen N. B. Methotrexate-associated hepatotoxicity: retrospective analysis of 210 patients with rheumatoid arthritis. Am J Med. 1988 Dec;85(6):771–774. doi: 10.1016/s0002-9343(88)80019-6. [DOI] [PubMed] [Google Scholar]
- Sokka Tuulikki, Pincus Theodore. Contemporary disease modifying antirheumatic drugs (DMARD) in patients with recent onset rheumatoid arthritis in a US private practice: methotrexate as the anchor drug in 90% and new DMARD in 30% of patients. J Rheumatol. 2002 Dec;29(12):2521–2524. [PubMed] [Google Scholar]
- Wolfe F., Hawley D. J., Cathey M. A. Termination of slow acting antirheumatic therapy in rheumatoid arthritis: a 14-year prospective evaluation of 1017 consecutive starts. J Rheumatol. 1990 Aug;17(8):994–1002. [PubMed] [Google Scholar]
- Yazici Yusuf, Erkan Doruk, Paget Stephen A. Monitoring by rheumatologists for methotrexate-, etanercept-, infliximab-, and anakinra-associated adverse events. Arthritis Rheum. 2003 Oct;48(10):2769–2772. doi: 10.1002/art.11277. [DOI] [PubMed] [Google Scholar]
- Yazici Yusuf, Erkan Doruk, Paget Stephen A. Monitoring methotrexate hepatic toxicity in rheumatoid arthritis: is it time to update the guidelines? J Rheumatol. 2002 Aug;29(8):1586–1589. [PubMed] [Google Scholar]
- Zachariae H., Søgaard H. Methotrexate-induced liver cirrhosis. A follow-up. Dermatologica. 1987;175(4):178–182. doi: 10.1159/000248822. [DOI] [PubMed] [Google Scholar]