Abstract
Background: Dendritic cells orchestrate pivotal immunological processes mediated by the production of cytokines and chemokines.
Objective: To assess whether neutralisation of tumour necrosis factor α (TNFα) during maturation of dendritic cells affects their phenotype and behaviour, which might explain the beneficial effects of TNFα neutralisation in rheumatoid arthritis.
Methods: Immature and fully matured dendritic cells were cultured from blood monocytes from patients with rheumatoid arthritis and healthy controls following standardised protocols. TNFα was neutralised by addition of the p55 soluble TNFα receptor, PEGsTNFRI. The effect of TNFα neutralisation on the phenotype (CD14, CD16, CD32, CD64, CD80, CD83, CD86, and MHC) of dendritic cells was investigated by flow cytometry. Expression of chemokines (CCL17, CCL18, CCL19, CCL22, CCL3, and CXCL8) and production of IL1ß and IL6 during dendritic cell differentiation and maturation were examined.
Results: Neutralisation of TNFα during the differentiation and maturation of dendritic cells did not result in an altered dendritic cell phenotype in the rheumatoid patients or the healthy controls. In contrast, the expression of CCL17, CCL18, CCL19, CCL22, CCL3, and CXCL8 by dendritic cells was significantly reduced when TNFα activity was inhibited during lipopolysaccharide triggered dendritic cell maturation. The production of IL1ß and IL6 by mature dendritic cells was inhibited by PEGsTNFRI.
Conclusions: Inhibition of TNFα activity during dendritic cell maturation leads to the development of semi-mature cells. These data suggest a novel pathway by which the neutralisation of TNFα might exert its therapeutic effects.
Full Text
The Full Text of this article is available as a PDF (114.0 KB).
Figure 1.
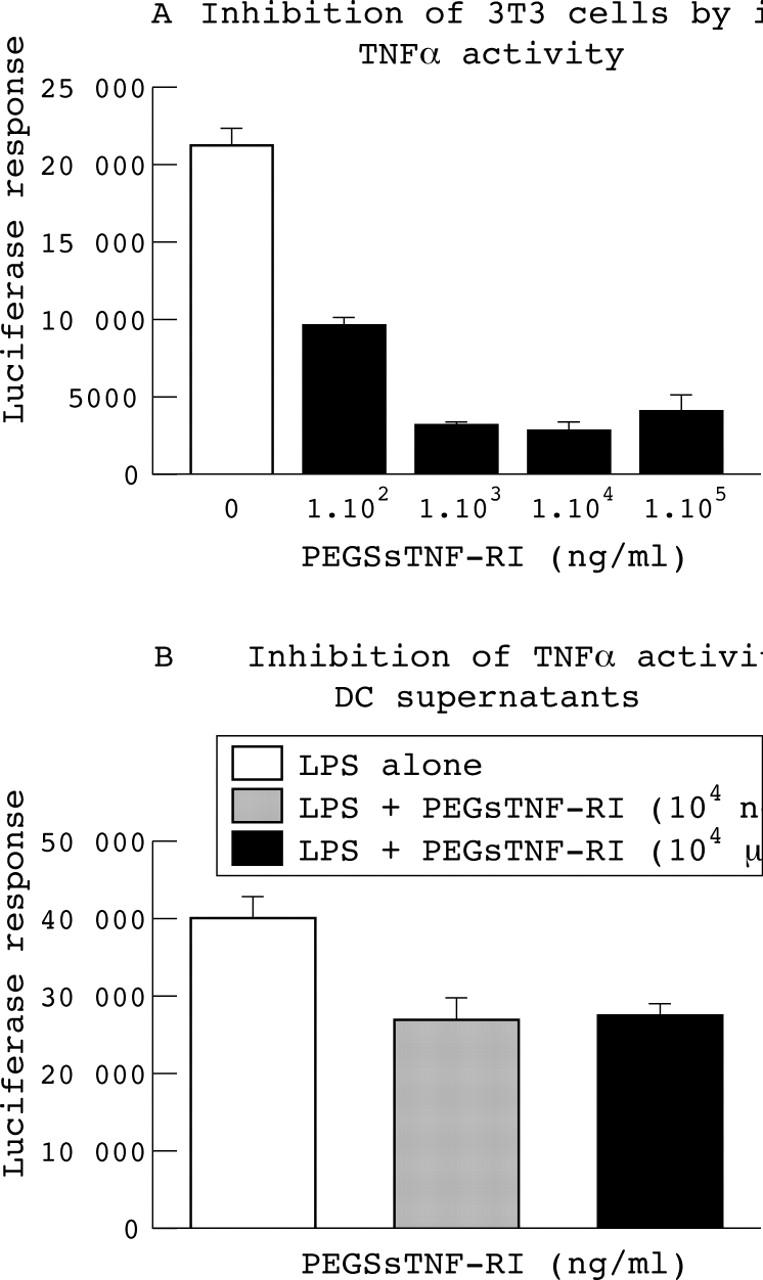
Inhibition of tumour necrosis α (TNFα) activity by the addition of the p55 soluble TNFα receptor PEGsTNFRI. (A) Inhibition of 3T3 cells (luciferase response) stimulated by TNFα (1 ng/ml) following the addition of various concentrations of PEGsTNFRI. (B) Neutralisation of TNFα activity in the supernatant of mature dendritic cells by addition of PEGsTNFRI. A 1000-fold excess of PEGsTNFRI was sufficient to provide maximum inhibition of TNFα activity, as a further 1000-fold excess did not cause any additional decrease in luciferase. DC, dendritic cell.
Figure 2.
Decrease in chemokine expression by mature dendritic cells when tumour necrosis α (TNFα) activity was inhibited. Expression of the chemokines CCL17, CCL18, CCL19, CCL22, CCL3, and CXCL8 by lipopolysaccharide matured dendritic cells was measured using real time polymerase chain reaction techniques. A clear decrease in chemokine expression was observed when TNFα activity was inhibited during the maturation process in both rheumatoid patients and healthy controls.
Figure 3.
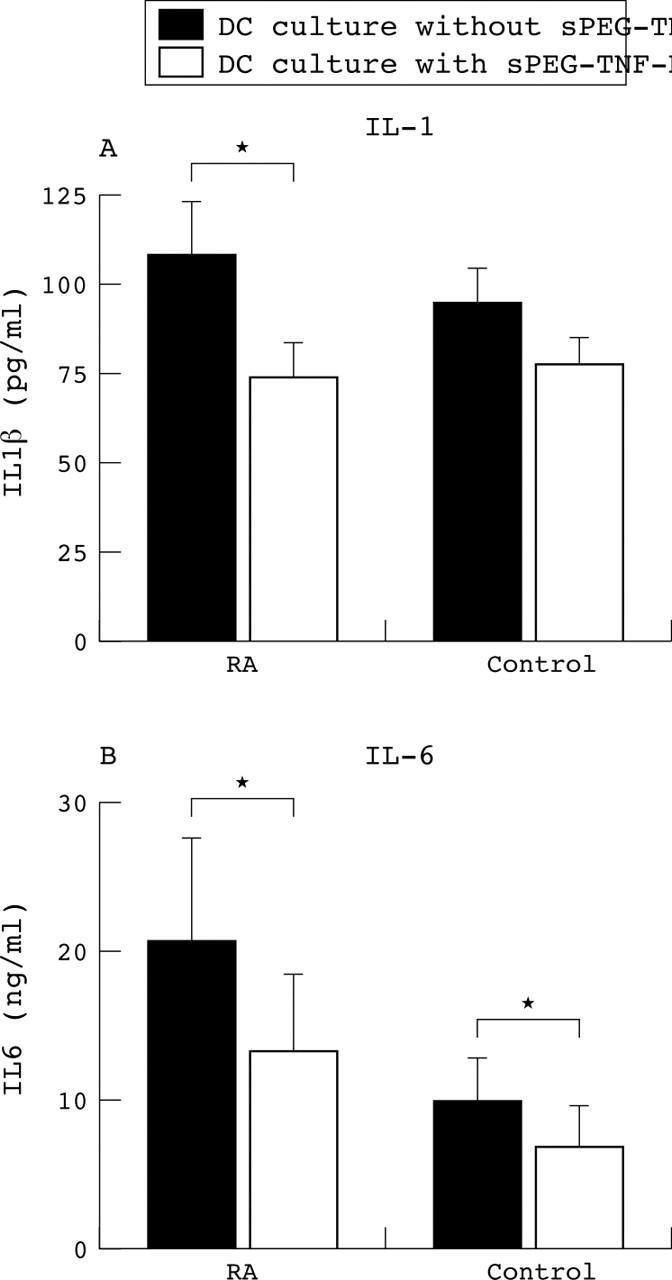
Inhibition of tumour necrosis factor α (TNFα) by PEGsTNFRI during dendritic cell maturation resulted in decreased production of interleukin 1ß (IL1ß) and interleukin 6 (IL-6). (A) Production of IL1ß. (B) IL6 secretion. Both proinflammatory mediators were significantly reduced when TNFα was neutralised during dendritic cell maturation triggered by the addition of lipopolysaccharide. Although the production of both IL1ß and IL6 by dendritic cells from rheumatoid patients was significantly greater than that from controls, the decreased production because by blockade of TNFα was similar in the two groups. DC, dendritic cells; RA, rheumatoid arthritis.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arend W. P. Cytokine imbalance in the pathogenesis of rheumatoid arthritis: the role of interleukin-1 receptor antagonist. Semin Arthritis Rheum. 2001 Apr;30(5 Suppl 2):1–6. doi: 10.1053/sarh.2001.23693. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y. J., Pulendran B., Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Steinman R. M. Dendritic cells and the control of immunity. Nature. 1998 Mar 19;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Bell D., Young J. W., Banchereau J. Dendritic cells. Adv Immunol. 1999;72:255–324. doi: 10.1016/s0065-2776(08)60023-1. [DOI] [PubMed] [Google Scholar]
- Blankenstein Thomas, Schüler Thomas. Cross-priming versus cross-tolerance: are two signals enough? Trends Immunol. 2002 Apr;23(4):171–173. doi: 10.1016/s1471-4906(02)02185-3. [DOI] [PubMed] [Google Scholar]
- Blaschke Sabine, Koziolek Michael, Schwarz Andreas, Benöhr Peter, Middel Peter, Schwarz Gerhard, Hummel Klaus-M, Müller Gerhard A. Proinflammatory role of fractalkine (CX3CL1) in rheumatoid arthritis. J Rheumatol. 2003 Sep;30(9):1918–1927. [PubMed] [Google Scholar]
- Brennan F. M., Maini R. N., Feldmann M. Role of pro-inflammatory cytokines in rheumatoid arthritis. Springer Semin Immunopathol. 1998;20(1-2):133–147. doi: 10.1007/BF00832003. [DOI] [PubMed] [Google Scholar]
- Burmester G. R., Stuhlmüller B., Keyszer G., Kinne R. W. Mononuclear phagocytes and rheumatoid synovitis. Mastermind or workhorse in arthritis? Arthritis Rheum. 1997 Jan;40(1):5–18. doi: 10.1002/art.1780400104. [DOI] [PubMed] [Google Scholar]
- Chomarat P., Banchereau J., Davoust J., Palucka A. K. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000 Dec;1(6):510–514. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- Choy E. H. S., Isenberg D. A., Garrood T., Farrow S., Ioannou Y., Bird H., Cheung N., Williams B., Hazleman B., Price R. Therapeutic benefit of blocking interleukin-6 activity with an anti-interleukin-6 receptor monoclonal antibody in rheumatoid arthritis: a randomized, double-blind, placebo-controlled, dose-escalation trial. Arthritis Rheum. 2002 Dec;46(12):3143–3150. doi: 10.1002/art.10623. [DOI] [PubMed] [Google Scholar]
- Cravens Petra D., Lipsky Peter E. Dendritic cells, chemokine receptors and autoimmune inflammatory diseases. Immunol Cell Biol. 2002 Oct;80(5):497–505. doi: 10.1046/j.1440-1711.2002.01118.x. [DOI] [PubMed] [Google Scholar]
- Debandt M., Vittecoq O., Descamps V., Le Loët X., Meyer O. Anti-TNF-alpha-induced systemic lupus syndrome. Clin Rheumatol. 2003 Feb;22(1):56–61. doi: 10.1007/s10067-002-0654-5. [DOI] [PubMed] [Google Scholar]
- Endo H., Akahoshi T., Takagishi K., Kashiwazaki S., Matsushima K. Elevation of interleukin-8 (IL-8) levels in joint fluids of patients with rheumatoid arthritis and the induction by IL-8 of leukocyte infiltration and synovitis in rabbit joints. Lymphokine Cytokine Res. 1991 Aug;10(4):245–252. [PubMed] [Google Scholar]
- Feldmann M., Brennan F. M., Maini R. Cytokines in autoimmune disorders. Int Rev Immunol. 1998;17(1-4):217–228. doi: 10.3109/08830189809084493. [DOI] [PubMed] [Google Scholar]
- Fleischmann Roy M., Schechtman Joy, Bennett Ralph, Handel Malcolm L., Burmester Gerd-Rudiger, Tesser John, Modafferi Dennis, Poulakos Jennifer, Sun Gordon. Anakinra, a recombinant human interleukin-1 receptor antagonist (r-metHuIL-1ra), in patients with rheumatoid arthritis: A large, international, multicenter, placebo-controlled trial. Arthritis Rheum. 2003 Apr;48(4):927–934. doi: 10.1002/art.10870. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek T. B., Torensma R., van Vliet S. J., van Duijnhoven G. C., Adema G. J., van Kooyk Y., Figdor C. G. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000 Mar 3;100(5):575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- Gerard C., Rollins B. J. Chemokines and disease. Nat Immunol. 2001 Feb;2(2):108–115. doi: 10.1038/84209. [DOI] [PubMed] [Google Scholar]
- Halla J. T., Koopman W. J., Fallahi S., Oh S. J., Gay R. E., Schrohenloher R. E. Rheumatoid myositis. Clinical and histologic features and possible pathogenesis. Arthritis Rheum. 1984 Jul;27(7):737–743. doi: 10.1002/art.1780270703. [DOI] [PubMed] [Google Scholar]
- Haringman J. J., Kraan M. C., Smeets T. J. M., Zwinderman K. H., Tak P. P. Chemokine blockade and chronic inflammatory disease: proof of concept in patients with rheumatoid arthritis. Ann Rheum Dis. 2003 Aug;62(8):715–721. doi: 10.1136/ard.62.8.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helle M., Boeije L., de Groot E., de Vos A., Aarden L. Sensitive ELISA for interleukin-6. Detection of IL-6 in biological fluids: synovial fluids and sera. J Immunol Methods. 1991 Apr 8;138(1):47–56. doi: 10.1016/0022-1759(91)90063-l. [DOI] [PubMed] [Google Scholar]
- Jonuleit H., Schmitt E., Steinbrink K., Enk A. H. Dendritic cells as a tool to induce anergic and regulatory T cells. Trends Immunol. 2001 Jul;22(7):394–400. doi: 10.1016/s1471-4906(01)01952-4. [DOI] [PubMed] [Google Scholar]
- Kaliński P., Hilkens C. M., Wierenga E. A., Kapsenberg M. L. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999 Dec;20(12):561–567. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- Keane J., Gershon S., Wise R. P., Mirabile-Levens E., Kasznica J., Schwieterman W. D., Siegel J. N., Braun M. M. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001 Oct 11;345(15):1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Kunkel S. L., Harlow L. A., Johnson B., Evanoff H. L., Haines G. K., Burdick M. D., Pope R. M., Strieter R. M. Enhanced production of monocyte chemoattractant protein-1 in rheumatoid arthritis. J Clin Invest. 1992 Sep;90(3):772–779. doi: 10.1172/JCI115950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A., Sallusto F. The instructive role of dendritic cells on T cell responses: lineages, plasticity and kinetics. Curr Opin Immunol. 2001 Jun;13(3):291–298. doi: 10.1016/s0952-7915(00)00218-1. [DOI] [PubMed] [Google Scholar]
- Leung Bernard P., Conacher Margaret, Hunter David, McInnes Iain B., Liew Foo Y., Brewer James M. A novel dendritic cell-induced model of erosive inflammatory arthritis: distinct roles for dendritic cells in T cell activation and induction of local inflammation. J Immunol. 2002 Dec 15;169(12):7071–7077. doi: 10.4049/jimmunol.169.12.7071. [DOI] [PubMed] [Google Scholar]
- Lu Lina, Thomson Angus W. Manipulation of dendritic cells for tolerance induction in transplantation and autoimmune disease. Transplantation. 2002 Jan 15;73(1 Suppl):S19–S22. doi: 10.1097/00007890-200201151-00008. [DOI] [PubMed] [Google Scholar]
- Ludewig B., Odermatt B., Ochsenbein A. F., Zinkernagel R. M., Hengartner H. Role of dendritic cells in the induction and maintenance of autoimmune diseases. Immunol Rev. 1999 Jun;169:45–54. doi: 10.1111/j.1600-065x.1999.tb01305.x. [DOI] [PubMed] [Google Scholar]
- Lutz Manfred B., Schuler Gerold. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002 Sep;23(9):445–449. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- Maini R., St Clair E. W., Breedveld F., Furst D., Kalden J., Weisman M., Smolen J., Emery P., Harriman G., Feldmann M. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999 Dec 4;354(9194):1932–1939. doi: 10.1016/s0140-6736(99)05246-0. [DOI] [PubMed] [Google Scholar]
- Mellman I., Steinman R. M. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001 Aug 10;106(3):255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- Menges Mauritius, Rössner Susanne, Voigtländer Constanze, Schindler Heike, Kukutsch Nicole A., Bogdan Christian, Erb Klaus, Schuler Gerold, Lutz Manfred B. Repetitive injections of dendritic cells matured with tumor necrosis factor alpha induce antigen-specific protection of mice from autoimmunity. J Exp Med. 2002 Jan 7;195(1):15–21. doi: 10.1084/jem.20011341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan N., Edwards E. T., Cupps T. R., Oliverio P. J., Sandberg G., Crayton H., Richert J. R., Siegel J. N. Demyelination occurring during anti-tumor necrosis factor alpha therapy for inflammatory arthritides. Arthritis Rheum. 2001 Dec;44(12):2862–2869. doi: 10.1002/1529-0131(200112)44:12<2862::aid-art474>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Morita Y., Yang J., Gupta R., Shimizu K., Shelden E. A., Endres J., Mulé J. J., McDonagh K. T., Fox D. A. Dendritic cells genetically engineered to express IL-4 inhibit murine collagen-induced arthritis. J Clin Invest. 2001 May;107(10):1275–1284. doi: 10.1172/JCI11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page Guillaume, Lebecque Serge, Miossec Pierre. Anatomic localization of immature and mature dendritic cells in an ectopic lymphoid organ: correlation with selective chemokine expression in rheumatoid synovium. J Immunol. 2002 May 15;168(10):5333–5341. doi: 10.4049/jimmunol.168.10.5333. [DOI] [PubMed] [Google Scholar]
- Panayi G. S., Lanchbury J. S., Kingsley G. H. The importance of the T cell in initiating and maintaining the chronic synovitis of rheumatoid arthritis. Arthritis Rheum. 1992 Jul;35(7):729–735. doi: 10.1002/art.1780350702. [DOI] [PubMed] [Google Scholar]
- Pasare Chandrashekhar, Medzhitov Ruslan. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003 Jan 16;299(5609):1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- Pettit A. R., MacDonald K. P., O'Sullivan B., Thomas R. Differentiated dendritic cells expressing nuclear RelB are predominantly located in rheumatoid synovial tissue perivascular mononuclear cell aggregates. Arthritis Rheum. 2000 Apr;43(4):791–800. doi: 10.1002/1529-0131(200004)43:4<791::AID-ANR9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Radstake T. R. D. J., Blom A. B., Slöetjes A. W., van Gorselen E. O. F., Pesman G. J., Engelen L., Torensma R., van den Berg W. B., Figdor C. G., van Lent P. L. E. M. Increased FcgammaRII expression and aberrant tumour necrosis factor alpha production by mature dendritic cells from patients with active rheumatoid arthritis. Ann Rheum Dis. 2004 Dec;63(12):1556–1563. doi: 10.1136/ard.2003.016550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radstake T. R. D. J., van Lent P. L. E. M., Pesman G. J., Blom A. B., Sweep F. G. J., Rönnelid J., Adema G. J., Barrera P., van den Berg W. B. High production of proinflammatory and Th1 cytokines by dendritic cells from patients with rheumatoid arthritis, and down regulation upon FcgammaR triggering. Ann Rheum Dis. 2004 Jun;63(6):696–702. doi: 10.1136/ard.2003.010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radstake T. R. D. J., van der Voort R., ten Brummelhuis M., de Waal Malefijt M., Looman M., Figdor C. G., van den Berg W. B., Barrera P., Adema G. J. Increased expression of CCL18, CCL19, and CCL17 by dendritic cells from patients with rheumatoid arthritis, and regulation by Fc gamma receptors. Ann Rheum Dis. 2004 Aug 26;64(3):359–367. doi: 10.1136/ard.2003.017566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Koops H., Kalden J. R. The balance of Th1/Th2 cytokines in rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2001 Dec;15(5):677–691. doi: 10.1053/berh.2001.0187. [DOI] [PubMed] [Google Scholar]
- Smeets R. L., van de Loo F. A. J., Joosten L. A. B., Arntz O. J., Bennink M. B., Loesberg W. A., Dmitriev I. P., Curiel D. T., Martin M. U., van den Berg W. B. Effectiveness of the soluble form of the interleukin-1 receptor accessory protein as an inhibitor of interleukin-1 in collagen-induced arthritis. Arthritis Rheum. 2003 Oct;48(10):2949–2958. doi: 10.1002/art.11234. [DOI] [PubMed] [Google Scholar]
- Smeets Tom J. M., Kraan Maarten C., van Loon Marieke E., Tak Paul-Peter. Tumor necrosis factor alpha blockade reduces the synovial cell infiltrate early after initiation of treatment, but apparently not by induction of apoptosis in synovial tissue. Arthritis Rheum. 2003 Aug;48(8):2155–2162. doi: 10.1002/art.11098. [DOI] [PubMed] [Google Scholar]
- Steinman R. M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Steinman Ralph Marvin, Nussenzweig Michel C. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A. 2002 Jan 2;99(1):351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervoordeldonk Margriet J. B. M., Tak Paul P. Cytokines in rheumatoid arthritis. Curr Rheumatol Rep. 2002 Jun;4(3):208–217. doi: 10.1007/s11926-002-0067-0. [DOI] [PubMed] [Google Scholar]
- Vissers J. L., Hartgers F. C., Lindhout E., Teunissen M. B., Figdor C. G., Adema G. J. Quantitative analysis of chemokine expression by dendritic cell subsets in vitro and in vivo. J Leukoc Biol. 2001 May;69(5):785–793. [PubMed] [Google Scholar]
- Weyand Cornelia M., Goronzy Jorg J. Ectopic germinal center formation in rheumatoid synovitis. Ann N Y Acad Sci. 2003 Apr;987:140–149. doi: 10.1111/j.1749-6632.2003.tb06042.x. [DOI] [PubMed] [Google Scholar]
- van der Meer J. W., Endres S., Lonnemann G., Cannon J. G., Ikejima T., Okusawa S., Gelfand J. A., Dinarello C. A. Concentrations of immunoreactive human tumor necrosis factor alpha produced by human mononuclear cells in vitro. J Leukoc Biol. 1988 Mar;43(3):216–223. doi: 10.1002/jlb.43.3.216. [DOI] [PubMed] [Google Scholar]



