Abstract
Objective: To investigate the effect of nitric oxide (NO) on mitochondrial activity and its relation with the apoptosis of human articular chondrocytes.
Materials and methods: Mitochondrial function was evaluated by analysing respiratory chain enzyme complexes, citrate synthase (CS) activities, and mitochondrial membrane potential (Δψm). The activities of the mitochondrial respiratory chain (MRC) complexes (complex I: NADH CoQ1 reductase, complex II: succinate dehydrogenase, complex III: ubiquinol cytochrome c reductase, complex IV: cytochrome c oxidase) and CS were measured in human articular chondrocytes isolated from normal cartilage. The Δψm was measured by 5,5',6,6'-tetracholoro-1,1',3,3'-tetraethylbenzimidazole carbocyanide iodide (JC-1) using flow cytometry. Apoptosis was analysed by flow cytometry. The mRNA expression of caspases was analysed by ribonuclease protection analysis and the detection of protein synthesis by western blotting. Sodium nitroprusside (SNP) was used as an NO compound donor.
Results: SNP at concentrations higher than 0.5 mmol/l for 24 hours induced cellular changes characteristic of apoptosis. SNP elicited mRNA expression of caspase-3 and caspase-7 and down regulated bcl-2 synthesis in a dose and time dependent manner. Furthermore, 0.5 mM SNP induced depolarisation of the mitochondrial membrane at 5, 12, and 24 hours. Analysis of the MRC showed that at 5 hours, 0.5 mM SNP reduced the activity of complex IV by 33%. The individual inhibition of mitochondrial complex IV with azide modified the Δψm and induced apoptosis.
Conclusions: This study suggests that the effect of NO on chondrocyte survival is mediated by its effect on complex IV of the MRC.
Full Text
The Full Text of this article is available as a PDF (207.2 KB).
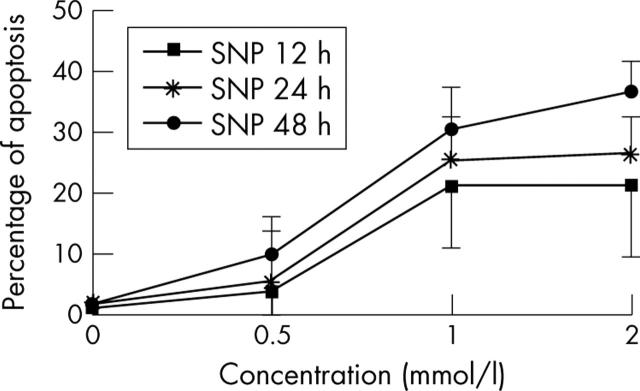
Figure 1.
Kinetics of the NO effect (SNP) on chondrocyte apoptosis. Cells (5x105 normal human chondrocytes), treated with SNP at different concentrations for 12, 24, and 48 hours were fixed in 70% ethanol at 4°C, then washed and incubated with RNase and PI for 15 minutes at room temperature in the dark. Data are expressed as a percentage of apoptotic (hypodiploid) nuclei. The graph shows that SNP dose dependently induces chondrocyte apoptosis.
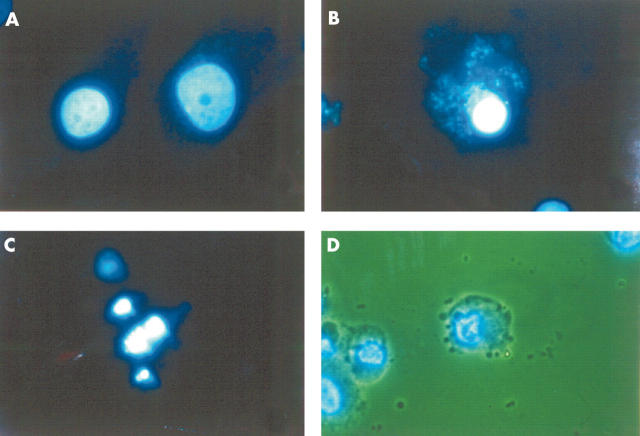
Figure 2.
Cellular changes induced by NO on normal human chondrocytes. 4',6-Dianidino-2-phenylindole dihydrochloride staining analysed by fluorescence microscopy (A–C) and by the combination of fluorescence and light microscopy (D). (A) Untreated cells (control). The normal morphology of a chondrocyte nucleus. (B, C) Cells treated with SNP (2 mmol/l) for 12 hours. Both panels show the typical morphology of an apoptotic nucleus, condensation (B) and fragmentation (C). (D) Cells treated with SNP (2 mmol/l) for 24 hours, showing simultaneous changes in the cytoplasmic membrane (bubbles) and in the nucleus margination of DNA.
Figure 3.
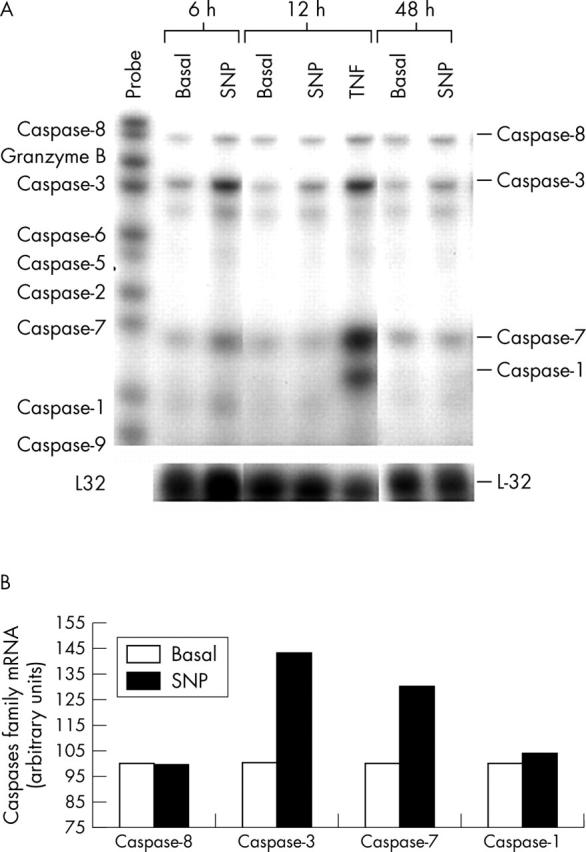
The time course of NO on mRNA expression of the caspases in normal human chondrocytes. (A) Confluent chondrocytic cells were incubated for the indicated time intervals (6, 12, and 48 hours), both in basal conditions or in the presence of SNP (0.5 mmol/l) or tumour necrosis factor α (TNFα, 10 ng/ml). After incubation, total RNA was isolated and the mRNA expression of the caspases was analysed by the RPA as reported in "Materials and methods". This autoradiograph represents a total of three experiments. (B) Densitometric analysis of the bands (at 6 hours of incubation) was conducted by computerised laser densitometry and normalised to the housekeeping L-32 mRNA level. Values are expressed as the percentage over control. TNFα was used as a positive control and as a comparator stimulus.
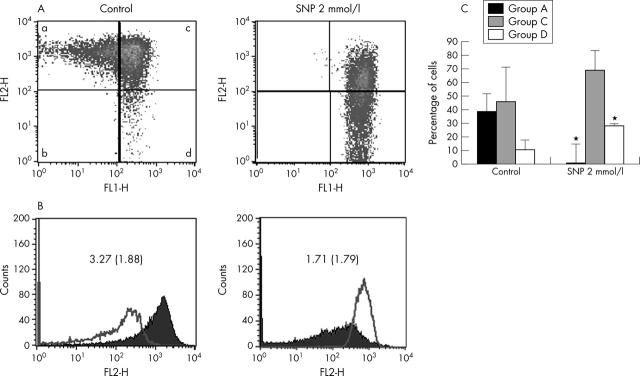
Figure 4.
Effect of NO on mitochondrial activity. (A) Fluorescence activated cell sorter analysis of mitochondrial membrane potential in human chondrocytes. Untreated and treated normal chondrocytes (5x105) with NO donor (SNP 0.5, 1, and 2 mmol/l for 5, 12, and 24 hours) were stained with 5,5,6,6-tetrachloro-1,1,3,3-tetraethylbenzimidazole carbocyanide iodide (JC-1) and analysed by flow cytometry. Photomultiplier settings were adjusted to detect JC-1 monomer fluorescence signals on the filter 1 (FL1) detector (green fluorescence) and JC-1 aggregate fluorescence signals on the FL2 detector (red fluorescence). The study showed that chondrocytes could be classified into four subgroups A-D as described in the text. Shown is an example of chondrocytes treated with 2 mM SNP for 5 hours, which shows that SNP increases the population of cells with depolarised mitochondria and decreases that of cells with normal polarisation. (B) Quantification of red and green fluorescence. Histograms represent the JC-1 fluorescence of normal cells (left) and those treated with 2 mM SNP for 5 hours (right). Green fluorescence (open graph) increases while red fluorescence (solid graph) decreases in the SNP treated chondrocytes, suggesting a reduction of the mitochondrial membrane potential and, therefore, a decrease in the red/green ratio. Shown is an example at 5 hours. Results are the mean (SD) from six different experiments. (C) Quantification of depolarised chondrocytes. Untreated cells and cells treated (5x105 normal chondrocytes) with SNP at different concentrations for 5, 12, and 24 hours were analysed by flow cytometry on a FACScan (Becton and Dickinson, Mountain View, CA). The proportion of cells with mitochondrial depolarisation (group D) is greater in SNP treated chondrocytes than in untreated cells. Furthermore, SNP reduced the proportion of cells with normal polarisation (group A). Shown are the results of six experiments at 5 hours. Bars show the mean (SD). *p⩽0.01 versus untreated chondrocytes.
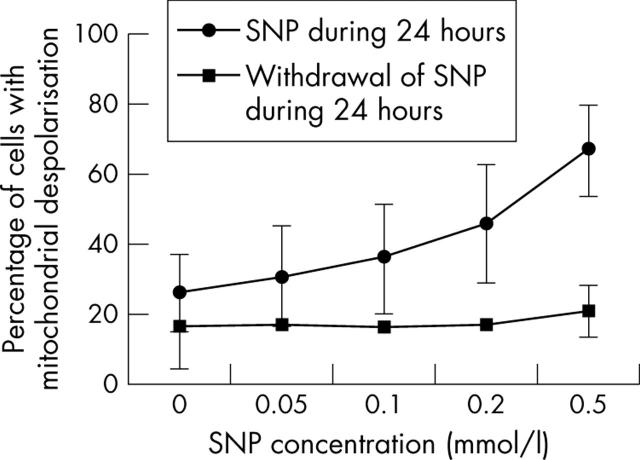
Figure 5.
The effect of NO on mitochondrial depolarisation is reversible. Cells (5x105 normal human chondrocytes) were treated with 2 mM SNP for 24 hours. The medium was then removed and cells were washed, and new medium without SNP was added. After 24 hours, cells were analysed by flow cytometry on a FACScan (Becton and Dickinson, Mountain View, CA). The proportion of cells with mitochondrial depolarisation (group D) was quantified. The effect of SNP on Δψm was reversible. Shown are the results of six experiments at 5 hours. Bars show the mean (SD).
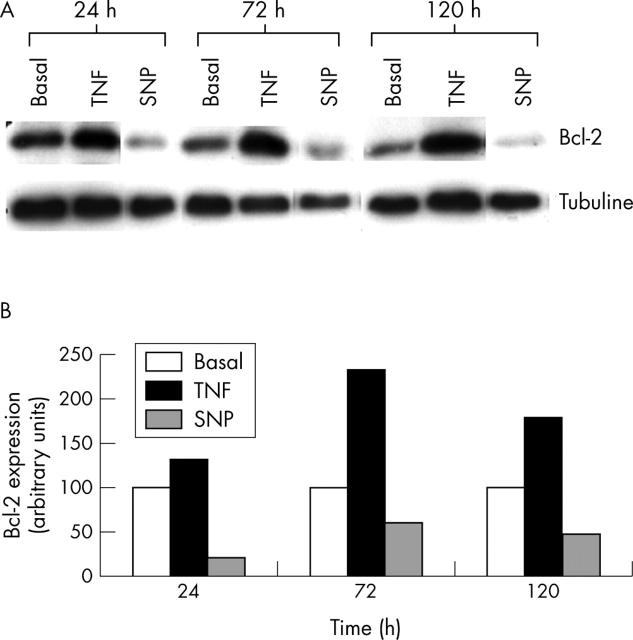
Figure 6.
Treatment with NO reduced the activation of the bcl-2 protein on human chondrocytic cells. (A) Confluent OA chondrocytes were incubated for the indicated times (24, 72, and 120 hours) in basal conditions or in the presence of SNP (0.5 mmol/l) or TNFα (10 ng/ml). Aliquots of total cell lysates were subjected to SDS-polyacrylamide gel electrophoresis, and immunoblotting was performed using the anti-bcl-2 antibody as described in "Materials and methods". This autoradiograph is representative of two experiments. (B) Densitometric analyses of the bands were conducted by computerised laser densitometry and normalised to tubuline. Values are expressed as the percentage over control. TNFα was used as a positive control and a comparator stimulus.
Figure 7.
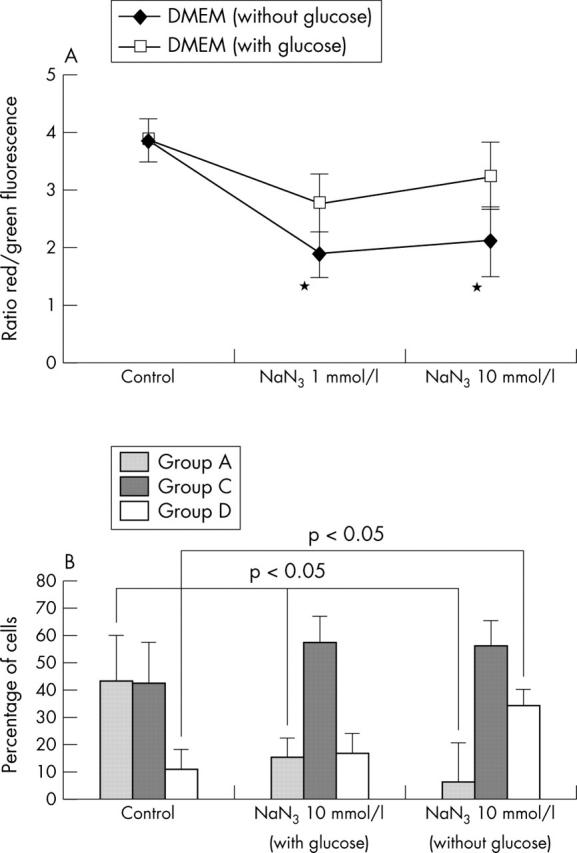
Effect of MRC inhibitor on Δψm. (A) Normal human chondrocytes (5x105) were incubated with NaN3 for 5, 12, and 24 hours. Cells were then analysed by flow cytometry to quantify the Δψm. Results are shown as the ratio of red/green fluorescence. Shown are the results of six experiments at 5 hours. Bars show the mean (SD). p = 0.05 treated v untreated chondrocytes. See "Materials and methods" for a description of the complexes. (B) Quantification of depolarised chondrocytes. Untreated and treated cells (5 x 105 normal human chondrocytes) with NaN3 at different concentrations for 5, 12, and 24 hours were analysed by flow cytometry on a FACScan. NaN3 reduced the proportion of cells with normal polarisation in chondrocytes (group A) cultured in medium with and without glucose. However, the proportion of cells with mitochondrial depolarisation (group D) was higher in chondrocytes stimulated with NaN3 and cultured in medium without glucose. Shown are the results of six experiments at 5 hours. Bars show the mean (SD). *p⩽0.05 treated v untreated chondrocytes.
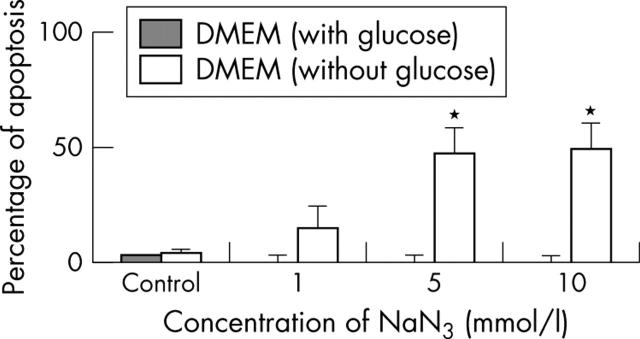
Figure 8.
Effect of an MRC inhibitor on chondrocyte apoptosis. Normal human chondrocytes (5x105) were cultured in DMEM and glucose-free DMEM, and incubated with NaN3 (a mitochondrial inhibitor of complex IV) for 12, 24, and 48 hours. Cells were then analysed by flow cytometry to quantify the percentage of apoptosis. Shown are the results of six experiments at 24 hours. Bars show the mean (SD). *p = 0.05 treated v untreated chondrocytes. See "Materials and methods" for a description of the technique.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida A., Almeida J., Bolaños J. P., Moncada S. Different responses of astrocytes and neurons to nitric oxide: the role of glycolytically generated ATP in astrocyte protection. Proc Natl Acad Sci U S A. 2001 Dec 11;98(26):15294–15299. doi: 10.1073/pnas.261560998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin A. R., Dave M., Attur M., Abramson S. B. COX-2, NO, and cartilage damage and repair. Curr Rheumatol Rep. 2000 Dec;2(6):447–453. doi: 10.1007/s11926-000-0019-5. [DOI] [PubMed] [Google Scholar]
- Bates J. N., Baker M. T., Guerra R., Jr, Harrison D. G. Nitric oxide generation from nitroprusside by vascular tissue. Evidence that reduction of the nitroprusside anion and cyanide loss are required. Biochem Pharmacol. 1991 Dec 11;42 (Suppl):S157–S165. doi: 10.1016/0006-2952(91)90406-u. [DOI] [PubMed] [Google Scholar]
- Blanco F. J., Guitian R., Vázquez-Martul E., de Toro F. J., Galdo F. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998 Feb;41(2):284–289. doi: 10.1002/1529-0131(199802)41:2<284::AID-ART12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Blanco F. J., Ochs R. L., Schwarz H., Lotz M. Chondrocyte apoptosis induced by nitric oxide. Am J Pathol. 1995 Jan;146(1):75–85. [PMC free article] [PubMed] [Google Scholar]
- Borderie D., Le Marechal H., Ekindjian O. G., Hernvann A. Nitric oxide modifies glycolytic pathways in cultured human synoviocytes. Cell Biol Int. 2000;24(5):285–289. doi: 10.1006/cbir.2000.0498. [DOI] [PubMed] [Google Scholar]
- Brown G. C. Nitric oxide regulates mitochondrial respiration and cell functions by inhibiting cytochrome oxidase. FEBS Lett. 1995 Aug 7;369(2-3):136–139. doi: 10.1016/0014-5793(95)00763-y. [DOI] [PubMed] [Google Scholar]
- Cleeter M. W., Cooper J. M., Darley-Usmar V. M., Moncada S., Schapira A. H. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 1994 May 23;345(1):50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999 Jul 15;341(Pt 2):233–249. [PMC free article] [PubMed] [Google Scholar]
- Del Carlo Marcello, Jr, Loeser Richard F. Nitric oxide-mediated chondrocyte cell death requires the generation of additional reactive oxygen species. Arthritis Rheum. 2002 Feb;46(2):394–403. doi: 10.1002/art.10056. [DOI] [PubMed] [Google Scholar]
- Gottlieb Roberta A., Granville David J. Analyzing mitochondrial changes during apoptosis. Methods. 2002 Apr;26(4):341–347. doi: 10.1016/S1046-2023(02)00040-3. [DOI] [PubMed] [Google Scholar]
- Green D. R., Reed J. C. Mitochondria and apoptosis. Science. 1998 Aug 28;281(5381):1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Ochs R. L., Komiya S., Lotz M. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum. 1998 Sep;41(9):1632–1638. doi: 10.1002/1529-0131(199809)41:9<1632::AID-ART14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Horton W. E., Jr, Feng L., Adams C. Chondrocyte apoptosis in development, aging and disease. Matrix Biol. 1998 Jun;17(2):107–115. doi: 10.1016/s0945-053x(98)90024-5. [DOI] [PubMed] [Google Scholar]
- Johnson K., Jung A., Murphy A., Andreyev A., Dykens J., Terkeltaub R. Mitochondrial oxidative phosphorylation is a downstream regulator of nitric oxide effects on chondrocyte matrix synthesis and mineralization. Arthritis Rheum. 2000 Jul;43(7):1560–1570. doi: 10.1002/1529-0131(200007)43:7<1560::AID-ANR21>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Kim H. A., Lee Y. J., Seong S. C., Choe K. W., Song Y. W. Apoptotic chondrocyte death in human osteoarthritis. J Rheumatol. 2000 Feb;27(2):455–462. [PubMed] [Google Scholar]
- Kim Song-Ja, Ju Jung-Won, Oh Chun-Do, Yoon Young-Mee, Song Woo Keun, Kim Jae-Hong, Yoo Yung Joon, Bang Ok-Sun, Kang Shin-Sung, Chun Jang-Soo. ERK-1/2 and p38 kinase oppositely regulate nitric oxide-induced apoptosis of chondrocytes in association with p53, caspase-3, and differentiation status. J Biol Chem. 2001 Oct 31;277(2):1332–1339. doi: 10.1074/jbc.M107231200. [DOI] [PubMed] [Google Scholar]
- Kluck R. M., Bossy-Wetzel E., Green D. R., Newmeyer D. D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997 Feb 21;275(5303):1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Kroemer G., Zamzami N., Susin S. A. Mitochondrial control of apoptosis. Immunol Today. 1997 Jan;18(1):44–51. doi: 10.1016/s0167-5699(97)80014-x. [DOI] [PubMed] [Google Scholar]
- Kösel S., Hofhaus G., Maassen A., Vieregge P., Graeber M. B. Role of mitochondria in Parkinson disease. Biol Chem. 1999 Jul-Aug;380(7-8):865–870. doi: 10.1515/BC.1999.106. [DOI] [PubMed] [Google Scholar]
- Leary Scot C., Hill Bruce C., Lyons Carrie N., Carlson Christopher G., Michaud Denise, Kraft Claudia S., Ko Kenton, Glerum D. Moira, Moyes Christopher D. Chronic treatment with azide in situ leads to an irreversible loss of cytochrome c oxidase activity via holoenzyme dissociation. J Biol Chem. 2002 Jan 16;277(13):11321–11328. doi: 10.1074/jbc.M112303200. [DOI] [PubMed] [Google Scholar]
- Lee R. B., Urban J. P. Evidence for a negative Pasteur effect in articular cartilage. Biochem J. 1997 Jan 1;321(Pt 1):95–102. doi: 10.1042/bj3210095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard J. V., Schapira A. H. Mitochondrial respiratory chain disorders I: mitochondrial DNA defects. Lancet. 2000 Jan 22;355(9200):299–304. doi: 10.1016/s0140-6736(99)05225-3. [DOI] [PubMed] [Google Scholar]
- Lizasoain I., Moro M. A., Knowles R. G., Darley-Usmar V., Moncada S. Nitric oxide and peroxynitrite exert distinct effects on mitochondrial respiration which are differentially blocked by glutathione or glucose. Biochem J. 1996 Mar 15;314(Pt 3):877–880. doi: 10.1042/bj3140877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler M., Kroemer G. The mitochondrion in cell death control: certainties and incognita. Exp Cell Res. 2000 Apr 10;256(1):19–26. doi: 10.1006/excr.2000.4833. [DOI] [PubMed] [Google Scholar]
- Lotz M. The role of nitric oxide in articular cartilage damage. Rheum Dis Clin North Am. 1999 May;25(2):269–282. doi: 10.1016/s0889-857x(05)70067-3. [DOI] [PubMed] [Google Scholar]
- Maneiro Emilia, Martín Miguel A., de Andres María C., López-Armada Maria J., Fernández-Sueiro José L., del Hoyo Pilar, Galdo Fausto, Arenas Joaquin, Blanco Francisco J. Mitochondrial respiratory activity is altered in osteoarthritic human articular chondrocytes. Arthritis Rheum. 2003 Mar;48(3):700–708. doi: 10.1002/art.10837. [DOI] [PubMed] [Google Scholar]
- Moriya R., Uehara T., Nomura Y. Mechanism of nitric oxide-induced apoptosis in human neuroblastoma SH-SY5Y cells. FEBS Lett. 2000 Nov 10;484(3):253–260. doi: 10.1016/s0014-5793(00)02167-0. [DOI] [PubMed] [Google Scholar]
- Notoya K., Jovanovic D. V., Reboul P., Martel-Pelletier J., Mineau F., Pelletier J. P. The induction of cell death in human osteoarthritis chondrocytes by nitric oxide is related to the production of prostaglandin E2 via the induction of cyclooxygenase-2. J Immunol. 2000 Sep 15;165(6):3402–3410. doi: 10.4049/jimmunol.165.6.3402. [DOI] [PubMed] [Google Scholar]
- Otte P. Basic cell metabolism of articular cartilage. Manometric studies. Z Rheumatol. 1991 Sep-Oct;50(5):304–312. [PubMed] [Google Scholar]
- Petit P. X., Lecoeur H., Zorn E., Dauguet C., Mignotte B., Gougeon M. L. Alterations in mitochondrial structure and function are early events of dexamethasone-induced thymocyte apoptosis. J Cell Biol. 1995 Jul;130(1):157–167. doi: 10.1083/jcb.130.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit P. X., Zamzami N., Vayssière J. L., Mignotte B., Kroemer G., Castedo M. Implication of mitochondria in apoptosis. Mol Cell Biochem. 1997 Sep;174(1-2):185–188. [PubMed] [Google Scholar]
- Studer R., Jaffurs D., Stefanovic-Racic M., Robbins P. D., Evans C. H. Nitric oxide in osteoarthritis. Osteoarthritis Cartilage. 1999 Jul;7(4):377–379. doi: 10.1053/joca.1998.0216. [DOI] [PubMed] [Google Scholar]
- Takabayashi A., Kawai Y., Iwata S., Kanai M., Denno R., Kawada K., Obama K., Taki Y. Nitric oxide induces a decrease in the mitochondrial membrane potential of peripheral blood lymphocytes, especially in natural killer cells. Antioxid Redox Signal. 2000 Winter;2(4):673–680. doi: 10.1089/ars.2000.2.4-673. [DOI] [PubMed] [Google Scholar]
- Tomita M., Sato E. F., Nishikawa M., Yamano Y., Inoue M. Nitric oxide regulates mitochondrial respiration and functions of articular chondrocytes. Arthritis Rheum. 2001 Jan;44(1):96–104. doi: 10.1002/1529-0131(200101)44:1<96::AID-ANR13>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Ushmorov A., Ratter F., Lehmann V., Dröge W., Schirrmacher V., Umansky V. Nitric-oxide-induced apoptosis in human leukemic lines requires mitochondrial lipid degradation and cytochrome C release. Blood. 1999 Apr 1;93(7):2342–2352. [PubMed] [Google Scholar]
- Vander Heiden M. G., Chandel N. S., Li X. X., Schumacker P. T., Colombini M., Thompson C. B. Outer mitochondrial membrane permeability can regulate coupled respiration and cell survival. Proc Natl Acad Sci U S A. 2000 Apr 25;97(9):4666–4671. doi: 10.1073/pnas.090082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vayssiere J. L., Petit P. X., Risler Y., Mignotte B. Commitment to apoptosis is associated with changes in mitochondrial biogenesis and activity in cell lines conditionally immortalized with simian virus 40. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11752–11756. doi: 10.1073/pnas.91.24.11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamzami N., Marchetti P., Castedo M., Zanin C., Vayssière J. L., Petit P. X., Kroemer G. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J Exp Med. 1995 May 1;181(5):1661–1672. doi: 10.1084/jem.181.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]