Abstract
Background: Dendritic cells (DC) have a role in the regulation of immunity and tolerance, attracting inflammatory cells by the production of various chemokines (CK). Fc gamma receptors (FcγR) may be involved in regulation of the DC function.
Objective: To assess the expression of CK by immature (iDC) and mature DC (mDC) and its regulation by FcγR in patients with RA and healthy donors (HC).
Methods: Expression of CK by DC from patients with RA and from HC was determined by real time quantitative PCR and ELISA. DC were derived from monocytes following standardised protocols. To study the potential regulation by FcγR, iDC were stimulated with immune complexes (IC) during lipopolysaccharide (LPS) induced maturation. The presence of CK was studied in synovial tissue from patients with RA, osteoarthritis, and healthy subjects by RT-PCR and immunohistochemistry.
Results: iDC from patients with RA had markedly increased mRNA levels of the CK CCL18 and CXCL8. Upon maturation with LPS, expression of CCL18, CCL19, CXCL8, CCL3, and CCL17 increased dramatically, reaching significantly higher levels in patients with RA. Monocytes failed to express these CK, except for CXCL8 and CCL3. IC-mediated triggering of the FcγR on DC from patients with highly active RA down regulated all CK, whereas the reverse was seen when DC from patients with low disease activity and healthy donors were stimulated. CCL18 was significantly increased in RA synovial tissue.
Conclusion: Increased CK expression by DC was found in patients with RA. This expression is partly regulated by FcγR triggering and results in an inhibitory DC subtype in RA upon FcγR-mediated triggering.
Full Text
The Full Text of this article is available as a PDF (230.5 KB).
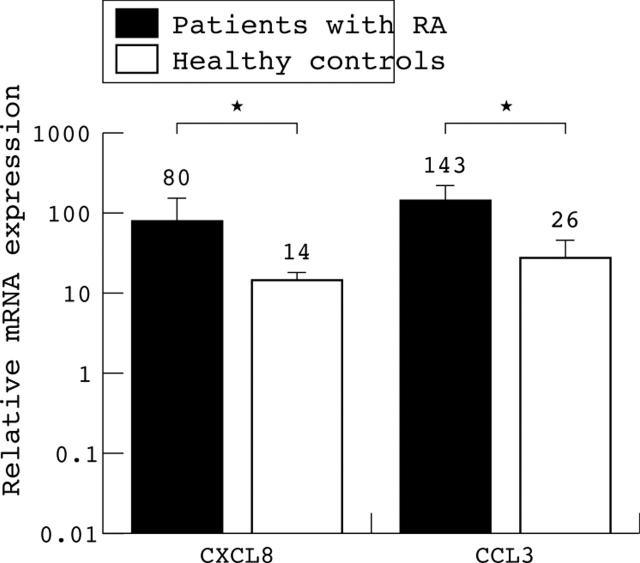
Figure 1.
CK mRNA expression by immature and mature monocyte derived dendritic cells from patients with RA and healthy controls. CK expression of iDC of patients with RA (black bars), healthy subjects (light grey bars), and after full maturation (patients with RA (dark grey bars), and healthy controls (white bars)). The mRNA expression is related to PBGD expression set to level 1. Note the log scale of the y axis. The mean level of mRNA expression is indicated by the number above the bar.
Figure 2.

CK expressed by iDC and mDC in relation to RA disease activity. (A) CK mRNA expression by iDC from 10 patients with active RA (black bars), six with inactive disease (grey bars), and 12 healthy controls (white bars). (B) Production of CK by mDC from the same group of patients with active (black bars) and inactive (grey bars) disease, and healthy donors (white bars). *p<0.05. The mRNA expression is related to PBGD expression set to level 1. Note the log scale of the y axis. The mean level of mRNA expression is indicated by the number above the bar.
Figure 3.
CK expression by monocytes from patients with RA and healthy controls. Relative expression of CK CXCL8 and CCL3 by monocytes of five patients with active RA (black bars) and five healthy donors (white bars), respectively. *p<0.05. The mRNA expression is related to PBGD expression set to level 1. Note the log scale of the y axis. The mean level of mRNA expression is indicated by the number above the bar.
Figure 4.
CK mRNA expression after triggering of FcγR on mature DC. Level of CCL18, CCL22, CCL17, CCL19, CXCL8, and CCL3 mRNA expression in the absence (black bars) or presence (white bars) of IC by mature DC from eight patients with RA and six healthy controls, respectively. *p<0.05. The mRNA expression is related to PBGD expression set to level 1. Note the log scale of the y axis. The mean level of mRNA expression is indicated by the number above the bar.
Figure 5.
Expression of CK in synovial tissue from patients with RA compared with patients with OA and healthy controls. mRNA expression level of the CK CCL18, CCL19, CCL17, and CXCL8 in synovial tissue from five patients with RA (black bars), five patients with OA (grey bars), and five healthy donors (white bars). *p<0.05. The mRNA expression is related to PBGD expression set to level 1. Note the log scale of the y axis. The mean level of mRNA expression is indicated by the number above the bar.
Figure 6.
CCL18 (DC-CK1) production by iDC and mDC of patients with RA and healthy subjects before and after stimulation with anti-IgG complexes (HAGGs). (A) CCL18 protein production in supernatants of iDC and mDC from nine patients with active RA (black bars), five patients with inactive RA (grey bars), and six healthy donors (white bars). (B) CCL18 secretion by iDC after stimulation with LPS alone or LPS+HAGGs in nine patients with active RA (left panel) and six healthy donors (right panel).
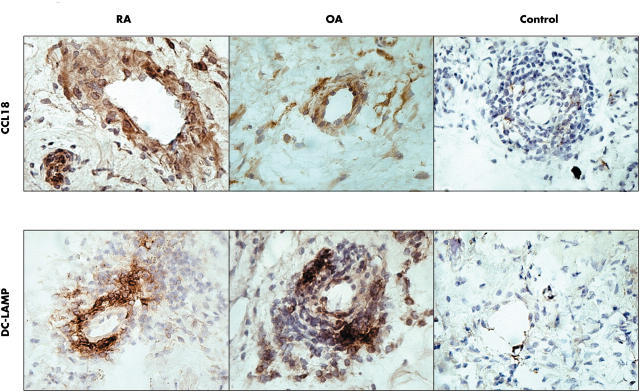
Figure 7.
Expression of CCL18 (DC-CK1) in the synovial tissue of patients with RA and OA and in healthy donors. The top row represents immunostaining for CCL18 of synovial tissue from a patient with RA, a patient with OA, and a healthy donor, respectively. The bottom row corresponds with immunostaining for DC-LAMP from the same subjects.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adema G. J., Hartgers F., Verstraten R., de Vries E., Marland G., Menon S., Foster J., Xu Y., Nooyen P., McClanahan T. A dendritic-cell-derived C-C chemokine that preferentially attracts naive T cells. Nature. 1997 Jun 12;387(6634):713–717. doi: 10.1038/42716. [DOI] [PubMed] [Google Scholar]
- Akahoshi T., Wada C., Endo H., Hirota K., Hosaka S., Takagishi K., Kondo H., Kashiwazaki S., Matsushima K. Expression of monocyte chemotactic and activating factor in rheumatoid arthritis. Regulation of its production in synovial cells by interleukin-1 and tumor necrosis factor. Arthritis Rheum. 1993 Jun;36(6):762–771. doi: 10.1002/art.1780360605. [DOI] [PubMed] [Google Scholar]
- Anderson Charles F., Mosser David M. Cutting edge: biasing immune responses by directing antigen to macrophage Fc gamma receptors. J Immunol. 2002 Apr 15;168(8):3697–3701. doi: 10.4049/jimmunol.168.8.3697. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Baekkevold E. S., Yamanaka T., Palframan R. T., Carlsen H. S., Reinholt F. P., von Andrian U. H., Brandtzaeg P., Haraldsen G. The CCR7 ligand elc (CCL19) is transcytosed in high endothelial venules and mediates T cell recruitment. J Exp Med. 2001 May 7;193(9):1105–1112. doi: 10.1084/jem.193.9.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y. J., Pulendran B., Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Steinman R. M. Dendritic cells and the control of immunity. Nature. 1998 Mar 19;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Bell D., Young J. W., Banchereau J. Dendritic cells. Adv Immunol. 1999;72:255–324. doi: 10.1016/s0065-2776(08)60023-1. [DOI] [PubMed] [Google Scholar]
- Bresnihan B., Tak P. P. Synovial tissue analysis in rheumatoid arthritis. Baillieres Best Pract Res Clin Rheumatol. 1999 Dec;13(4):645–659. doi: 10.1053/berh.1999.0051. [DOI] [PubMed] [Google Scholar]
- Clynes R., Maizes J. S., Guinamard R., Ono M., Takai T., Ravetch J. V. Modulation of immune complex-induced inflammation in vivo by the coordinate expression of activation and inhibitory Fc receptors. J Exp Med. 1999 Jan 4;189(1):179–185. doi: 10.1084/jem.189.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieu M. C., Vanbervliet B., Vicari A., Bridon J. M., Oldham E., Aït-Yahia S., Brière F., Zlotnik A., Lebecque S., Caux C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998 Jul 20;188(2):373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijstelbloem H. M., van de Winkel J. G., Kallenberg C. G. Inflammation in autoimmunity: receptors for IgG revisited. Trends Immunol. 2001 Sep;22(9):510–516. doi: 10.1016/s1471-4906(01)02014-2. [DOI] [PubMed] [Google Scholar]
- Endo H., Akahoshi T., Takagishi K., Kashiwazaki S., Matsushima K. Elevation of interleukin-8 (IL-8) levels in joint fluids of patients with rheumatoid arthritis and the induction by IL-8 of leukocyte infiltration and synovitis in rabbit joints. Lymphokine Cytokine Res. 1991 Aug;10(4):245–252. [PubMed] [Google Scholar]
- Fernández Nieves, Renedo Marta, García-Rodríguez Carmen, Sánchez Crespo Mariano. Activation of monocytic cells through Fc gamma receptors induces the expression of macrophage-inflammatory protein (MIP)-1 alpha, MIP-1 beta, and RANTES. J Immunol. 2002 Sep 15;169(6):3321–3328. doi: 10.4049/jimmunol.169.6.3321. [DOI] [PubMed] [Google Scholar]
- Fillatreau Simon, Gray David. T cell accumulation in B cell follicles is regulated by dendritic cells and is independent of B cell activation. J Exp Med. 2003 Jan 20;197(2):195–206. doi: 10.1084/jem.20021750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster R., Schubel A., Breitfeld D., Kremmer E., Renner-Müller I., Wolf E., Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999 Oct 1;99(1):23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- Gerard C., Rollins B. J. Chemokines and disease. Nat Immunol. 2001 Feb;2(2):108–115. doi: 10.1038/84209. [DOI] [PubMed] [Google Scholar]
- Gibson U. E., Heid C. A., Williams P. M. A novel method for real time quantitative RT-PCR. Genome Res. 1996 Oct;6(10):995–1001. doi: 10.1101/gr.6.10.995. [DOI] [PubMed] [Google Scholar]
- Heid C. A., Stevens J., Livak K. J., Williams P. M. Real time quantitative PCR. Genome Res. 1996 Oct;6(10):986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- Kalergis Alexis M., Ravetch Jeffrey V. Inducing tumor immunity through the selective engagement of activating Fcgamma receptors on dendritic cells. J Exp Med. 2002 Jun 17;195(12):1653–1659. doi: 10.1084/jem.20020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Young Mo, Zhang Xiaoyu, Wagner Ulf G., Yang Hongyu, Beckenbaugh Robert D., Kurtin Paul J., Goronzy Jörg J., Weyand Cornelia M. CD8 T cells are required for the formation of ectopic germinal centers in rheumatoid synovitis. J Exp Med. 2002 May 20;195(10):1325–1336. doi: 10.1084/jem.20011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katschke K. J., Jr, Rottman J. B., Ruth J. H., Qin S., Wu L., LaRosa G., Ponath P., Park C. C., Pope R. M., Koch A. E. Differential expression of chemokine receptors on peripheral blood, synovial fluid, and synovial tissue monocytes/macrophages in rheumatoid arthritis. Arthritis Rheum. 2001 May;44(5):1022–1032. doi: 10.1002/1529-0131(200105)44:5<1022::AID-ANR181>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Kim S., Evans C. H., Ghivizzani S. C., Oligino T., Robbins P. D. Effective treatment of established murine collagen-induced arthritis by systemic administration of dendritic cells genetically modified to express IL-4. J Immunol. 2001 Mar 1;166(5):3499–3505. doi: 10.4049/jimmunol.166.5.3499. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Kunkel S. L., Burrows J. C., Evanoff H. L., Haines G. K., Pope R. M., Strieter R. M. Synovial tissue macrophage as a source of the chemotactic cytokine IL-8. J Immunol. 1991 Oct 1;147(7):2187–2195. [PubMed] [Google Scholar]
- Koch A. E., Kunkel S. L., Harlow L. A., Mazarakis D. D., Haines G. K., Burdick M. D., Pope R. M., Strieter R. M. Macrophage inflammatory protein-1 alpha. A novel chemotactic cytokine for macrophages in rheumatoid arthritis. J Clin Invest. 1994 Mar;93(3):921–928. doi: 10.1172/JCI117097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. E., Polverini P. J., Kunkel S. L., Harlow L. A., DiPietro L. A., Elner V. M., Elner S. G., Strieter R. M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992 Dec 11;258(5089):1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- Lindhout E., Vissers J. L., Hartgers F. C., Huijbens R. J., Scharenborg N. M., Figdor C. G., Adema G. J. The dendritic cell-specific CC-chemokine DC-CK1 is expressed by germinal center dendritic cells and attracts CD38-negative mantle zone B lymphocytes. J Immunol. 2001 Mar 1;166(5):3284–3289. doi: 10.4049/jimmunol.166.5.3284. [DOI] [PubMed] [Google Scholar]
- Mamoune A., Durand V., Le Goff P., Pennec Y. L., Youinou P., Le Corre R. Abnormal distribution of CD45 isoforms expressed by CD4+ and CD8+ T cells in rheumatoid arthritis. Histol Histopathol. 2000 Apr;15(2):587–591. doi: 10.14670/HH-15.587. [DOI] [PubMed] [Google Scholar]
- Morita Y., Yang J., Gupta R., Shimizu K., Shelden E. A., Endres J., Mulé J. J., McDonagh K. T., Fox D. A. Dendritic cells genetically engineered to express IL-4 inhibit murine collagen-induced arthritis. J Clin Invest. 2001 May;107(10):1275–1284. doi: 10.1172/JCI11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser B., Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001 Feb;2(2):123–128. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- Ohl Lars, Henning Golo, Krautwald Stefan, Lipp Martin, Hardtke Svenja, Bernhardt Gunter, Pabst Oliver, Förster Reinhold. Cooperating mechanisms of CXCR5 and CCR7 in development and organization of secondary lymphoid organs. J Exp Med. 2003 May 5;197(9):1199–1204. doi: 10.1084/jem.20030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page Guillaume, Lebecque Serge, Miossec Pierre. Anatomic localization of immature and mature dendritic cells in an ectopic lymphoid organ: correlation with selective chemokine expression in rheumatoid synovium. J Immunol. 2002 May 15;168(10):5333–5341. doi: 10.4049/jimmunol.168.10.5333. [DOI] [PubMed] [Google Scholar]
- Pettit A. R., MacDonald K. P., O'Sullivan B., Thomas R. Differentiated dendritic cells expressing nuclear RelB are predominantly located in rheumatoid synovial tissue perivascular mononuclear cell aggregates. Arthritis Rheum. 2000 Apr;43(4):791–800. doi: 10.1002/1529-0131(200004)43:4<791::AID-ANR9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Pettit A. R., Thomas R. Dendritic cells: the driving force behind autoimmunity in rheumatoid arthritis? Immunol Cell Biol. 1999 Oct;77(5):420–427. doi: 10.1046/j.1440-1711.1999.00855.x. [DOI] [PubMed] [Google Scholar]
- Pricop L., Redecha P., Teillaud J. L., Frey J., Fridman W. H., Sautès-Fridman C., Salmon J. E. Differential modulation of stimulatory and inhibitory Fc gamma receptors on human monocytes by Th1 and Th2 cytokines. J Immunol. 2001 Jan 1;166(1):531–537. doi: 10.4049/jimmunol.166.1.531. [DOI] [PubMed] [Google Scholar]
- Radstake T. R. D. J., Blom A. B., Slöetjes A. W., van Gorselen E. O. F., Pesman G. J., Engelen L., Torensma R., van den Berg W. B., Figdor C. G., van Lent P. L. E. M. Increased FcgammaRII expression and aberrant tumour necrosis factor alpha production by mature dendritic cells from patients with active rheumatoid arthritis. Ann Rheum Dis. 2004 Dec;63(12):1556–1563. doi: 10.1136/ard.2003.016550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radstake T. R. D. J., van Lent P. L. E. M., Pesman G. J., Blom A. B., Sweep F. G. J., Rönnelid J., Adema G. J., Barrera P., van den Berg W. B. High production of proinflammatory and Th1 cytokines by dendritic cells from patients with rheumatoid arthritis, and down regulation upon FcgammaR triggering. Ann Rheum Dis. 2004 Jun;63(6):696–702. doi: 10.1136/ard.2003.010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathanaswami P., Hachicha M., Sadick M., Schall T. J., McColl S. R. Expression of the cytokine RANTES in human rheumatoid synovial fibroblasts. Differential regulation of RANTES and interleukin-8 genes by inflammatory cytokines. J Biol Chem. 1993 Mar 15;268(8):5834–5839. [PubMed] [Google Scholar]
- Regnault A., Lankar D., Lacabanne V., Rodriguez A., Théry C., Rescigno M., Saito T., Verbeek S., Bonnerot C., Ricciardi-Castagnoli P. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med. 1999 Jan 18;189(2):371–380. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruth J. H., Rottman J. B., Katschke K. J., Jr, Qin S., Wu L., LaRosa G., Ponath P., Pope R. M., Koch A. E. Selective lymphocyte chemokine receptor expression in the rheumatoid joint. Arthritis Rheum. 2001 Dec;44(12):2750–2760. doi: 10.1002/1529-0131(200112)44:12<2750::aid-art462>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Ruth J. H., Volin M. V., Haines G. K., 3rd, Woodruff D. C., Katschke K. J., Jr, Woods J. M., Park C. C., Morel J. C., Koch A. E. Fractalkine, a novel chemokine in rheumatoid arthritis and in rat adjuvant-induced arthritis. Arthritis Rheum. 2001 Jul;44(7):1568–1581. doi: 10.1002/1529-0131(200107)44:7<1568::AID-ART280>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Salmon J. E., Pricop L. Human receptors for immunoglobulin G: key elements in the pathogenesis of rheumatic disease. Arthritis Rheum. 2001 Apr;44(4):739–750. doi: 10.1002/1529-0131(200104)44:4<739::AID-ANR129>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Santiago-Schwarz F., Anand P., Liu S., Carsons S. E. Dendritic cells (DCs) in rheumatoid arthritis (RA): progenitor cells and soluble factors contained in RA synovial fluid yield a subset of myeloid DCs that preferentially activate Th1 inflammatory-type responses. J Immunol. 2001 Aug 1;167(3):1758–1768. doi: 10.4049/jimmunol.167.3.1758. [DOI] [PubMed] [Google Scholar]
- Shi K., Hayashida K., Kaneko M., Hashimoto J., Tomita T., Lipsky P. E., Yoshikawa H., Ochi T. Lymphoid chemokine B cell-attracting chemokine-1 (CXCL13) is expressed in germinal center of ectopic lymphoid follicles within the synovium of chronic arthritis patients. J Immunol. 2001 Jan 1;166(1):650–655. doi: 10.4049/jimmunol.166.1.650. [DOI] [PubMed] [Google Scholar]
- Thomas R. Antigen-presenting cells in rheumatoid arthritis. Springer Semin Immunopathol. 1998;20(1-2):53–72. doi: 10.1007/BF00831999. [DOI] [PubMed] [Google Scholar]
- Thomas R., MacDonald K. P., Pettit A. R., Cavanagh L. L., Padmanabha J., Zehntner S. Dendritic cells and the pathogenesis of rheumatoid arthritis. J Leukoc Biol. 1999 Aug;66(2):286–292. doi: 10.1002/jlb.66.2.286. [DOI] [PubMed] [Google Scholar]
- Tokayer Amiel, Carsons Steven E., Chokshi Binny, Santiago-Schwarz Frances. High levels of interleukin 13 in rheumatoid arthritis sera are modulated by tumor necrosis factor antagonist therapy: association with dendritic cell growth activity. J Rheumatol. 2002 Mar;29(3):454–461. [PubMed] [Google Scholar]
- Vissers J. L., Hartgers F. C., Lindhout E., Teunissen M. B., Figdor C. G., Adema G. J. Quantitative analysis of chemokine expression by dendritic cell subsets in vitro and in vivo. J Leukoc Biol. 2001 May;69(5):785–793. [PubMed] [Google Scholar]
- Vulcano Marisa, Struyf Sofie, Scapini Patrizia, Cassatella Marco, Bernasconi Sergio, Bonecchi Raffaella, Calleri Angelica, Penna Giuseppe, Adorini Luciano, Luini Walter. Unique regulation of CCL18 production by maturing dendritic cells. J Immunol. 2003 Apr 1;170(7):3843–3849. doi: 10.4049/jimmunol.170.7.3843. [DOI] [PubMed] [Google Scholar]
- Youssef P. P., Smeets T. J., Bresnihan B., Cunnane G., Fitzgerald O., Breedveld F., Tak P. P. Microscopic measurement of cellular infiltration in the rheumatoid arthritis synovial membrane: a comparison of semiquantitative and quantitative analysis. Br J Rheumatol. 1998 Sep;37(9):1003–1007. doi: 10.1093/rheumatology/37.9.1003. [DOI] [PubMed] [Google Scholar]
- van Lent P. L., Nabbe K., Blom A. B., Holthuysen A. E., Sloetjes A., van de Putte L. B., Verbeek S., van den Berg W. B. Role of activatory Fc gamma RI and Fc gamma RIII and inhibitory Fc gamma RII in inflammation and cartilage destruction during experimental antigen-induced arthritis. Am J Pathol. 2001 Dec;159(6):2309–2320. doi: 10.1016/s0002-9440(10)63081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]