Abstract
Objective: To assess the expression and localisation of the new metalloproteinase inhibitor RECK, an inhibitor of matrix metalloproteinase-14 (MMP-14) secretion and activity, in the synovial membrane of patients with rheumatoid arthritis (RA).
Methods: RECK expression in synovium samples from patients with RA, osteoarthritis (OA), and "trauma" were studied by quantitative real time reverse transcription-polymerase chain reaction (Q-PCR). RECK mRNA levels were compared with those of the enzyme MMP-14. RECK expression on cryostat sections of synovium was disclosed by goat-antihuman RECK monoclonal antibody. RECK protein was detected on synovial cryostat sections and measured by western blotting. RECK expression on macrophages was investigated by double staining of CD68 and RECK on cryostat sections and characterised by confocal microscopy. RECK expression on RA monocytes or normal monocytes was further investigated by FACS analysis.
Results: RECK expression in the synovial membrane of patients with RA was significantly lower than in OA and controls. MMP-14 mRNA levels were not significantly different between the three groups. In RA synovium, RECK protein was expressed mainly in the lining layer but also by macrophages around blood vessels. Fibroblasts and about 50% of the CD68 positive macrophages expressed RECK. In CD68 positive macrophages, RECK was only expressed in secretory granules and not on the membrane. The same pattern was found in M-CSF cultured macrophages of patients with RA and controls. In contrast, synovial fibroblasts showed a diffuse membrane expression within the synovium similar to cultured RA fibroblasts. RECK expression was low on the membrane of monocytes according to FACS analysis.
Conclusion: The new MMP inhibitor RECK is expressed in synovial membranes of RA, OA, and controls. RECK mRNA is lowest in RA synovial membranes. In contrast with fibroblasts, macrophages in the synovium express RECK only cytoplasmically and not on their membrane.
Full Text
The Full Text of this article is available as a PDF (322.4 KB).
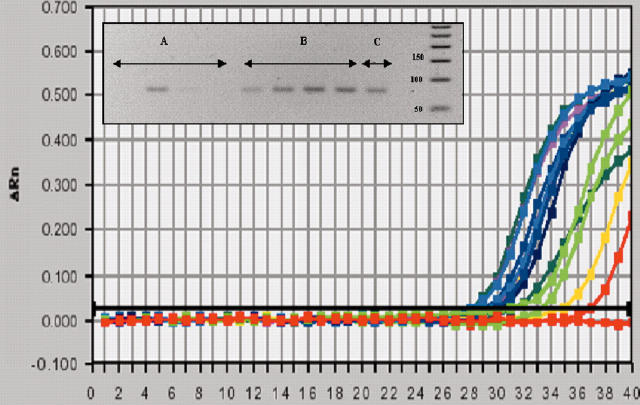
Figure 1.
Representative quantitative reverse transcriptase-polymerase chain reaction showing amplified RECK bands (99 bp) after 35 cycles from four different RA samples (A), four different trauma samples (B) and one breast cancer cell line sample (MDA-MB175) (C).
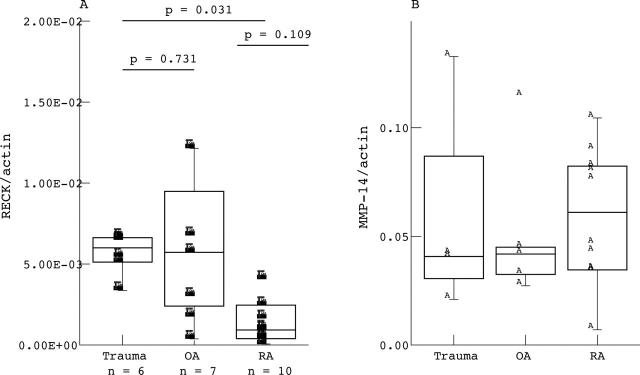
Figure 2.
Quantitative mRNA levels of RECK (A) and MMP-14 (B) in synovial membranes of patients with RA, OA, and trauma. RECK values were normalised to ß-actin signals. Note the significantly lower RECK mRNA levels in RA compared with trauma synovial specimen and the highly variable values within the OA membranes. Values represent the mean of 10 different patients with RA, 7 with OA, and 6 controls ("trauma" patients). Values were evaluated using the Mann-Whitney U test (significance p<0.05).
Figure 3.
RECK and ß-actin expression in synovial membranes of four different patients with RA using western blotting. Synovial membranes were chosen after they appeared positive for RECK in Q-PCR. Note that the content of cells (ß-actin) varied extensively between the four patients and correlated with the amount of RECK expression.
Figure 4.
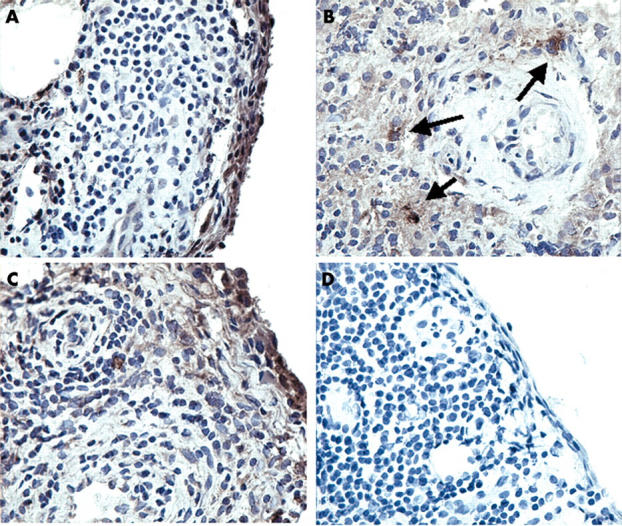
RECK and MMP-14 protein expression in synovial specimens from patients with RA as determined by immunolocalisation. Immunolocalisation using a specific mouse-antihuman RECK was performed on cryostat sections of patients with RA. RECK was mainly expressed within the intimal lining layer (A) and by large cells, probably macrophages, lying around blood vessels (B). MMP-14 was found in corresponding regions (C). As a control, a non-relevant IgG2 antibody was used, which showed no staining (D). Original magnification x250.
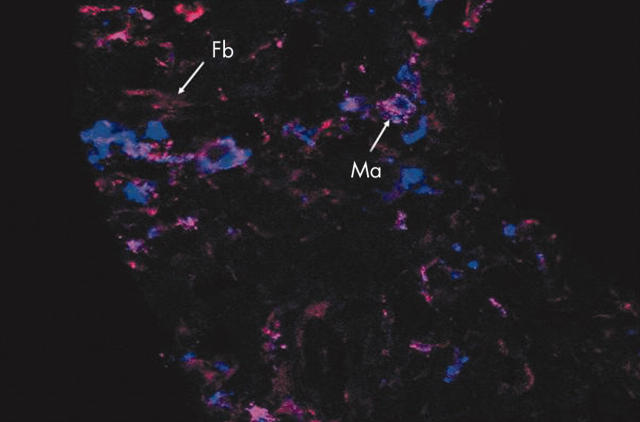
Figure 5.
Comparison of RECK and the macrophage marker CD68 in serial cryostat sections of synovium from five patients with RA. Double staining for CD68 and RECK using Alexa 647 labelled anti-CD68 (blue fluorescent) and Alexa 568 labelled anti-RECK (red fluorescent), respectively. Colocalisation of RECK and CD68 was clearly present in around 50% of the macrophage-like type A cells. Original magnification x400. Ma, macrophage; Fb, fibroblast.
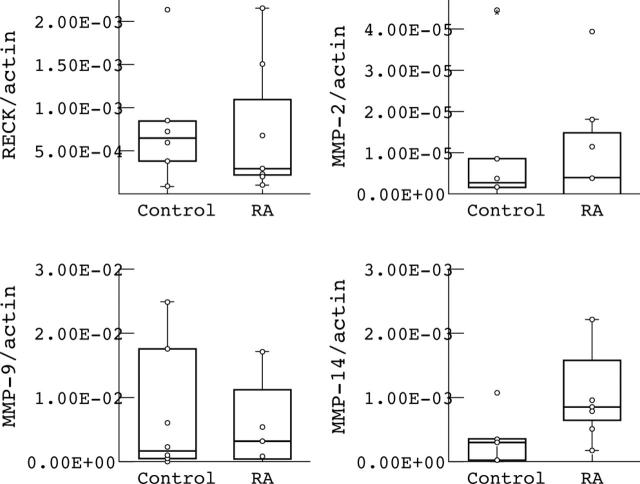
Figure 6.
Quantitative mRNA levels of RECK and various MMPs (MMP-2, MMP-9, MMP-14) in monocytes of patients with RA and controls. RECK and MMP values were normalised to ß-actin signals. Note that no significant differences were found in RECK and MMPs mRNA levels between RA and controls. Values represent the mean of six different patients with RA and controls. Values were evaluated by the Mann-Whitney U test (significance p<0.05).
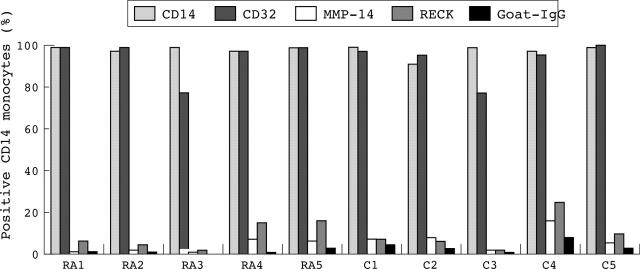
Figure 7.
Expression of RECK, MMP-14, CD14, and CD32 (FcγRII) on monocytes from five patients with RA and five healthy controls as determined by flow cytometric analysis. Monocytes were detected in sodium citrate treated blood. The percentage of positive cells was determined. Data were evaluated by the Mann-Whitney U test (p<0.05). Note that only low levels of cells express RECK and MMP-14. No significant differences in RECK expression on monocytes was found between patients with RA and healthy controls. RA, rheumatoid arthritis; C, control
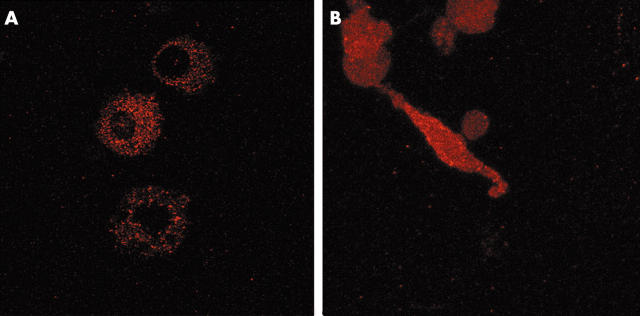
Figure 8.
Immunolocalisation of RECK in RA monocytes treated for 7 days with M-CSF and fibroblasts isolated from RA synovial membranes, and subsequently cultured for 3 days. Confocal microscopy shows that in macrophages, RECK is only expressed intracellularly and not on its membrane (A). In contrast, synovial fibroblasts showed a dispersed pattern and RECK was clearly present on the membrane (B). Original magnification x400.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrera P., Blom A., van Lent P. L., van Bloois L., Beijnen J. H., van Rooijen N., de Waal Malefijt M. C., van de Putte L. B., Storm G., van den Berg W. B. Synovial macrophage depletion with clodronate-containing liposomes in rheumatoid arthritis. Arthritis Rheum. 2000 Sep;43(9):1951–1959. doi: 10.1002/1529-0131(200009)43:9<1951::AID-ANR5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Blom Arjen B., Radstake Timothy R. D. J., Holthuysen Astrid E. M., Slöetjes Annet W., Pesman Gerard J., Sweep Fred G. J., van de Loo Fons A. J., Joosten L. A. B., Barrera Pilar, van Lent Peter L. E. M. Increased expression of Fcgamma receptors II and III on macrophages of rheumatoid arthritis patients results in higher production of tumor necrosis factor alpha and matrix metalloproteinase. Arthritis Rheum. 2003 Apr;48(4):1002–1014. doi: 10.1002/art.10871. [DOI] [PubMed] [Google Scholar]
- Firestein G. S. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum. 1996 Nov;39(11):1781–1790. doi: 10.1002/art.1780391103. [DOI] [PubMed] [Google Scholar]
- Gravallese E. M., Pettit A. R., Lee R., Madore R., Manning C., Tsay A., Gaspar J., Goldring M. B., Goldring S. R., Oettgen P. Angiopoietin-1 is expressed in the synovium of patients with rheumatoid arthritis and is induced by tumour necrosis factor alpha. Ann Rheum Dis. 2003 Feb;62(2):100–107. doi: 10.1136/ard.62.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida Joji, Wilhelmson Krista L., Price Matthew A., Wilson Christopher M., Pei Duanqing, Furcht Leo T., McCarthy James B. Membrane type-1 matrix metalloproteinase promotes human melanoma invasion and growth. J Invest Dermatol. 2004 Jan;122(1):167–176. doi: 10.1046/j.0022-202X.2003.22114.x. [DOI] [PubMed] [Google Scholar]
- Langlois Stéphanie, Gingras Denis, Béliveau Richard. Membrane type 1-matrix metalloproteinase (MT1-MMP) cooperates with sphingosine 1-phosphate to induce endothelial cell migration and morphogenic differentiation. Blood. 2003 Dec 11;103(8):3020–3028. doi: 10.1182/blood-2003-08-2968. [DOI] [PubMed] [Google Scholar]
- Lioté F., Champy R., Moenner M., Boval-Boizard B., Badet J. Elevated angiogenin levels in synovial fluid from patients with inflammatory arthritis and secretion of angiogenin by cultured synovial fibroblasts. Clin Exp Immunol. 2003 Apr;132(1):163–168. doi: 10.1046/j.1365-2249.2003.02117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migita K., Tanaka F., Yamasaki S., Shibatomi K., Ida H., Kawakami A., Aoyagi T., Kawabe Y., Eguchi K. Regulation of rheumatoid synoviocyte proliferation by endogenous p53 induction. Clin Exp Immunol. 2001 Nov;126(2):334–338. doi: 10.1046/j.1365-2249.2001.01677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulherin D., Fitzgerald O., Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996 Jan;39(1):115–124. doi: 10.1002/art.1780390116. [DOI] [PubMed] [Google Scholar]
- Murphy G., Stanton H., Cowell S., Butler G., Knäuper V., Atkinson S., Gavrilovic J. Mechanisms for pro matrix metalloproteinase activation. APMIS. 1999 Jan;107(1):38–44. doi: 10.1111/j.1699-0463.1999.tb01524.x. [DOI] [PubMed] [Google Scholar]
- Nagase H., Suzuki K., Morodomi T., Enghild J. J., Salvesen G. Activation mechanisms of the precursors of matrix metalloproteinases 1, 2 and 3. Matrix Suppl. 1992;1:237–244. [PubMed] [Google Scholar]
- Oh J., Takahashi R., Kondo S., Mizoguchi A., Adachi E., Sasahara R. M., Nishimura S., Imamura Y., Kitayama H., Alexander D. B. The membrane-anchored MMP inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell. 2001 Dec 14;107(6):789–800. doi: 10.1016/s0092-8674(01)00597-9. [DOI] [PubMed] [Google Scholar]
- Petrow P. K., Wernicke D., Schulze Westhoff C., Hummel K. M., Bräuer R., Kriegsmann J., Gromnica-Ihle E., Gay R. E., Gay S. Characterisation of the cell type-specificity of collagenase 3 mRNA expression in comparison with membrane type 1 matrix metalloproteinase and gelatinase A in the synovial membrane in rheumatoid arthritis. Ann Rheum Dis. 2002 May;61(5):391–397. doi: 10.1136/ard.61.5.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee Jin-Sae, Coussens Lisa M. RECKing MMP function: implications for cancer development. Trends Cell Biol. 2002 May;12(5):209–211. doi: 10.1016/s0962-8924(02)02280-8. [DOI] [PubMed] [Google Scholar]
- Sasahara Regina Maki, Brochado Sheila Maria, Takahashi Chiaki, Oh Junseo, Maria-Engler Silvya Stuchi, Granjeiro José Mauro, Noda Makoto, Sogayar Mari Cleide. Transcriptional control of the RECK metastasis/angiogenesis suppressor gene. Cancer Detect Prev. 2002;26(6):435–443. doi: 10.1016/s0361-090x(02)00123-x. [DOI] [PubMed] [Google Scholar]
- Seiki M. Membrane-type matrix metalloproteinases. APMIS. 1999 Jan;107(1):137–143. doi: 10.1111/j.1699-0463.1999.tb01536.x. [DOI] [PubMed] [Google Scholar]
- Span Paul N., Sweep C. G. J., Manders Peggy, Beex Louk V. A. M., Leppert David, Lindberg Raija L. P. Matrix metalloproteinase inhibitor reversion-inducing cysteine-rich protein with Kazal motifs: a prognostic marker for good clinical outcome in human breast carcinoma. Cancer. 2003 Jun 1;97(11):2710–2715. doi: 10.1002/cncr.11395. [DOI] [PubMed] [Google Scholar]
- Sweeney Susan E., Firestein Gary S. Rheumatoid arthritis: regulation of synovial inflammation. Int J Biochem Cell Biol. 2004 Mar;36(3):372–378. doi: 10.1016/s1357-2725(03)00259-0. [DOI] [PubMed] [Google Scholar]
- Takahashi C., Sheng Z., Horan T. P., Kitayama H., Maki M., Hitomi K., Kitaura Y., Takai S., Sasahara R. M., Horimoto A. Regulation of matrix metalloproteinase-9 and inhibition of tumor invasion by the membrane-anchored glycoprotein RECK. Proc Natl Acad Sci U S A. 1998 Oct 27;95(22):13221–13226. doi: 10.1073/pnas.95.22.13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Junko, Kajita Masahiro, Suenaga Naoko, Fujii Katsuyuki, Seiki Motoharu. Sequence-specific silencing of MT1-MMP expression suppresses tumor cell migration and invasion: importance of MT1-MMP as a therapeutic target for invasive tumors. Oncogene. 2003 Nov 27;22(54):8716–8722. doi: 10.1038/sj.onc.1206962. [DOI] [PubMed] [Google Scholar]
- Vu T. H., Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000 Sep 1;14(17):2123–2133. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- Wang He, Olszewski Bronia, Rosebury Wendy, Wang Dongkai, Robertson Andrew, Keiser Joan A. Impaired angiogenesis in SHR is associated with decreased KDR and MT1-MMP expression. Biochem Biophys Res Commun. 2004 Mar 5;315(2):363–368. doi: 10.1016/j.bbrc.2004.01.059. [DOI] [PubMed] [Google Scholar]
- Weaver Valerie M. Membrane-associated MMP regulators: novel cell adhesion tumor suppressor proteins? Dev Cell. 2002 Jan;2(1):6–7. doi: 10.1016/s1534-5807(01)00117-4. [DOI] [PubMed] [Google Scholar]
- Yamanaka H., Makino K., Takizawa M., Nakamura H., Fujimoto N., Moriya H., Nemori R., Sato H., Seiki M., Okada Y. Expression and tissue localization of membrane-types 1, 2, and 3 matrix metalloproteinases in rheumatoid synovium. Lab Invest. 2000 May;80(5):677–687. doi: 10.1038/labinvest.3780071. [DOI] [PubMed] [Google Scholar]
- Yanni G., Whelan A., Feighery C., Bresnihan B. Synovial tissue macrophages and joint erosion in rheumatoid arthritis. Ann Rheum Dis. 1994 Jan;53(1):39–44. doi: 10.1136/ard.53.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvaifler N. J., Tsai V., Alsalameh S., von Kempis J., Firestein G. S., Lotz M. Pannocytes: distinctive cells found in rheumatoid arthritis articular cartilage erosions. Am J Pathol. 1997 Mar;150(3):1125–1138. [PMC free article] [PubMed] [Google Scholar]
- van Lent Peter L. E. M., Figdor Carl G., Barrera Pilar, van Ginkel Karin, Slöetjes Annet, van den Berg Wim B., Torensma Ruurd. Expression of the dendritic cell-associated C-type lectin DC-SIGN by inflammatory matrix metalloproteinase-producing macrophages in rheumatoid arthritis synovium and interaction with intercellular adhesion molecule 3-positive T cells. Arthritis Rheum. 2003 Feb;48(2):360–369. doi: 10.1002/art.10786. [DOI] [PubMed] [Google Scholar]