Abstract
Objective: To investigate the mode of action of methotrexate (MTX) in different types of models for rheumatoid arthritis (RA) and multiple sclerosis (MS).
Methods: Models for RA and MS were selected known to have different pathogenesis—that is, fibroblast induced arthritis in SCID mice, collagen induced arthritis (CIA), anticollagen II antibody induced arthritis (CAIA), and experimental autoimmune encephalomyelitis (EAE) in (Balb/cxB10.Q)F1 and B10.Q mice, and Pristane induced arthritis in DA rats (PIA). The MTX treatment was started 1 day after the onset of disease and continued for 14 days to compare effects on the different models.
Results: All models known to be critically dependent on T cell activation (CIA, PIA, and EAE) were effectively down regulated by titrated doses of MTX. In contrast, no effects were seen on fibroblast induced arthritis or CAIA. No effects were seen on the levels of anticollagen II antibodies in the CIA experiment.
Conclusion: The data show that MTX has strong ameliorative effect on both classical models of RA, like CIA and PIA, but also on a model for MS, EAE. It also suggests that MTX operates only in diseases which are preceded by, and dependent on, T cell activation. A comparison of CAIA and CIA suggested that MTX operates independently of arthritogenic antibodies. These results demonstrate that different animal models reflect the complexity of the corresponding human diseases and suggest that several models should be used for effective screening of new therapeutic agents.
Full Text
The Full Text of this article is available as a PDF (218.4 KB).
Figure 1.
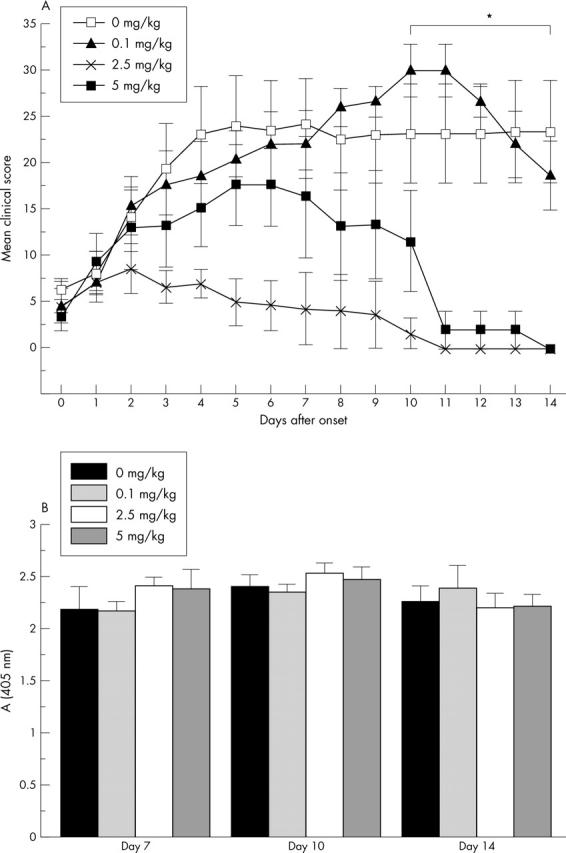
(A) MTX treatment of CIA. Day 0 indicates the onset of arthritis for each mouse and MTX is given at different doses starting 1 day after the onset. The mice were treated with 0.1 mg MTX/kg (n = 5), 2.5 mg MTX/kg (n = 3), 5 mg MTX/kg (n = 3) and a control group was treated with PBS (n = 5). The mean score of all mice for each day is given with the SEM. *Indicates significant differences in severity scores, p<0.05, for the groups treated with 2.5 mg/kg and 5 mg/kg in comparison with the control group. (B) Anti-CII antibody levels in the CIA experiment. The mice were bled at day 14 after the onset of arthritis. Mean values and SEM are indicated. No significant differences according to MTX treatment were seen.
Figure 2.
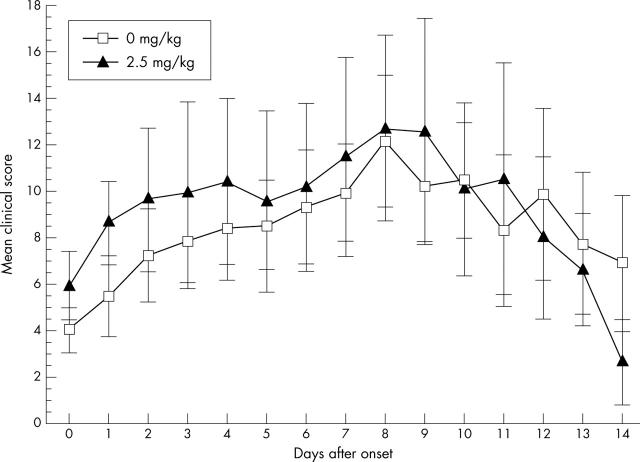
MTX treatment of CAIA. One group of mice were treated with MTX (2.5 mg/kg; n = 13) and a control group were treated with PBS (n = 13) starting 1 day after the onset of arthritis. Control mice were treated with PBS only. The mean score of all mice for each day is given with the SEM. No significant effect of MTX was seen.
Figure 3.
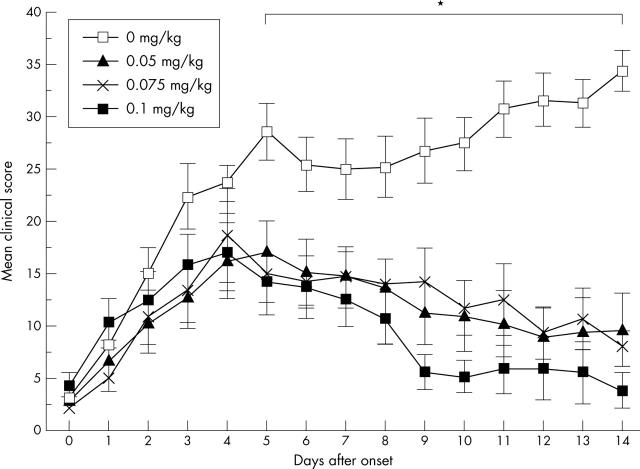
MTX treatment of PIA. Day 0 indicates onset of arthritis of each rat, and MTX was given at different doses starting 1 day after the onset. The rats were treated with 0.05 mg MTX/kg (n = 6), 0.075 mg MTX/kg (n = 6), 0.1 mg MTX/kg (n = 13), and a control group were treated with PBS (n = 6). The mean score of all rats for each day is given with the SEM. *Indicates significant differences in severity scores, p<0.05, for all MTX treated groups in comparison with the control group. In the group with highest MTX dose (0.1 mg MTX/kg) eight rats died at different times during the experiment.
Figure 4.
MTX treatment of fibroblast induced arthritis in SCID mice. MTX was given at different doses (5 mg/kg, 2.5 mg/kg, 0.1 mg/kg) and PBS was given in the control group (n = 9 in each group) starting 1 day after the injection of the fibroblasts, when all mice had already developed arthritis. Scoring started at day 1 when the arthritis began and treatment was given from this day and then daily until day 14. The mean diameter of the injected left knee of all mice in each group for each day is given with the SEM. No significant effect of MTX was seen.
Figure 5.
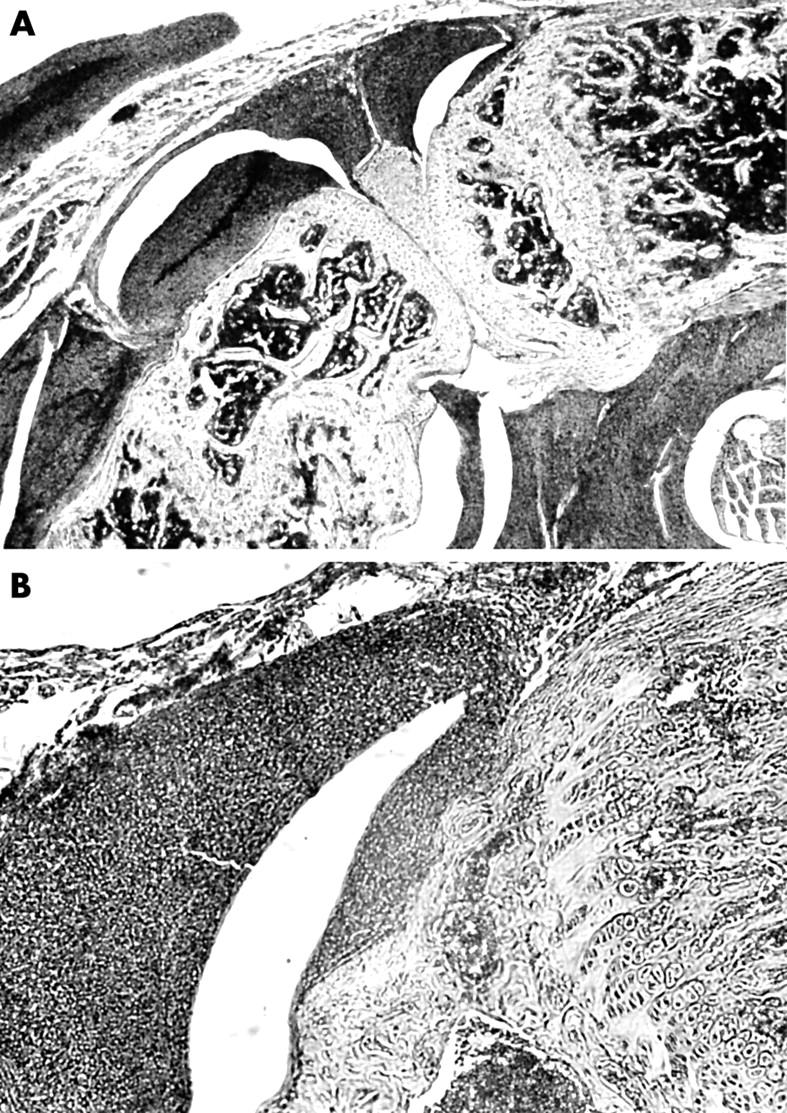
Histological aspect of a left SCID mouse knee joint 14 days after injection of 5x105 LS48 cells into the articular space. Original magnification x16 (A) and x40 (B). LS48 cells form a dense tissue attaching to and invading the articular structures. Stained with haematoxylin and eosin.
Figure 6.
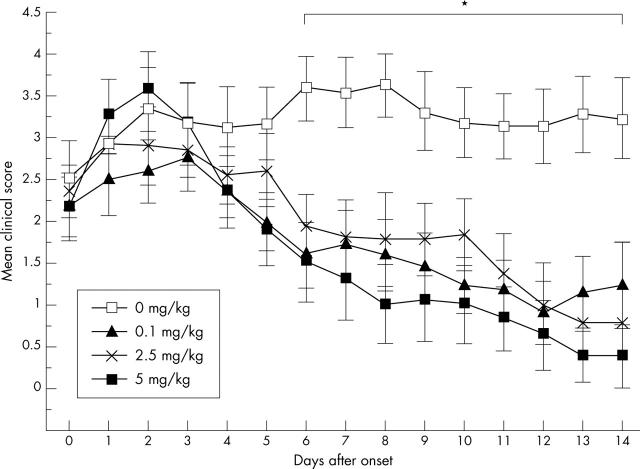
MTX treatment of EAE. Day 0 indicates onset of encephalomyelitis of each mouse and MTX was given at different doses starting 1 day after the onset. The mice were treated with 0.1 mg MTX/kg (n = 11), 2.5 mg MTX/kg (n = 10), 5 mg MTX/kg (n = 10) and a control group were treated with PBS (n = 12). The mean score of all mice each day is given with the SEM. *Indicates significant differences in encephalomyelitis severity scores, p<0.05 in comparison with the control group.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdul-Majid K. B., Jirholt J., Stadelmann C., Stefferl A., Kjellén P., Wallström E., Holmdahl R., Lassmann H., Olsson T., Harris R. A. Screening of several H-2 congenic mouse strains identified H-2(q) mice as highly susceptible to MOG-induced EAE with minimal adjuvant requirement. J Neuroimmunol. 2000 Nov 1;111(1-2):23–33. doi: 10.1016/s0165-5728(00)00360-x. [DOI] [PubMed] [Google Scholar]
- Cronstein B. N., Naime D., Ostad E. The antiinflammatory mechanism of methotrexate. Increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J Clin Invest. 1993 Dec;92(6):2675–2682. doi: 10.1172/JCI116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei K., Eugster H. P., Bopst M., Constantinescu C. S., Lavi E., Fontana A. Tumor necrosis factor alpha and lymphotoxin alpha are not required for induction of acute experimental autoimmune encephalomyelitis. J Exp Med. 1997 Jun 16;185(12):2177–2182. doi: 10.1084/jem.185.12.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata S., Matsubara T., Saura R., Tateishi H., Hirohata K. Inhibition of in vitro vascular endothelial cell proliferation and in vivo neovascularization by low-dose methotrexate. Arthritis Rheum. 1989 Sep;32(9):1065–1073. doi: 10.1002/anr.1780320903. [DOI] [PubMed] [Google Scholar]
- Hjelmström P., Juedes A. E., Fjell J., Ruddle N. H. B-cell-deficient mice develop experimental allergic encephalomyelitis with demyelination after myelin oligodendrocyte glycoprotein sensitization. J Immunol. 1998 Nov 1;161(9):4480–4483. [PubMed] [Google Scholar]
- Holmdahl R., Andersson M., Goldschmidt T. J., Gustafsson K., Jansson L., Mo J. A. Type II collagen autoimmunity in animals and provocations leading to arthritis. Immunol Rev. 1990 Dec;118:193–232. doi: 10.1111/j.1600-065x.1990.tb00817.x. [DOI] [PubMed] [Google Scholar]
- Holmdahl R., Rubin K., Klareskog L., Larsson E., Wigzell H. Characterization of the antibody response in mice with type II collagen-induced arthritis, using monoclonal anti-type II collagen antibodies. Arthritis Rheum. 1986 Mar;29(3):400–410. doi: 10.1002/art.1780290314. [DOI] [PubMed] [Google Scholar]
- Johansson A. C., Sundler M., Kjellén P., Johannesson M., Cook A., Lindqvist A. K., Nakken B., Bolstad A. I., Jonsson R., Alarcón-Riquelme M. Genetic control of collagen-induced arthritis in a cross with NOD and C57BL/10 mice is dependent on gene regions encoding complement factor 5 and FcgammaRIIb and is not associated with loci controlling diabetes. Eur J Immunol. 2001 Jun;31(6):1847–1856. doi: 10.1002/1521-4141(200106)31:6<1847::aid-immu1847>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Kleinau S., Martinsson P., Heyman B. Induction and suppression of collagen-induced arthritis is dependent on distinct fcgamma receptors. J Exp Med. 2000 May 1;191(9):1611–1616. doi: 10.1084/jem.191.9.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y. Y., Feige U., Sarosi I., Bolon B., Tafuri A., Morony S., Capparelli C., Li J., Elliott R., McCabe S. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999 Nov 18;402(6759):304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- Lehmann J., Jüngel A., Lehmann I., Busse F., Biskop M., Saalbach A., Emmrich F., Sack U. Grafting of fibroblasts isolated from the synovial membrane of rheumatoid arthritis (RA) patients induces chronic arthritis in SCID mice-A novel model for studying the arthritogenic role of RA fibroblasts in vivo. J Autoimmun. 2000 Nov;15(3):301–313. doi: 10.1006/jaut.2000.0435. [DOI] [PubMed] [Google Scholar]
- Malfait A. M., Williams R. O., Malik A. S., Maini R. N., Feldmann M. Chronic relapsing homologous collagen-induced arthritis in DBA/1 mice as a model for testing disease-modifying and remission-inducing therapies. Arthritis Rheum. 2001 May;44(5):1215–1224. doi: 10.1002/1529-0131(200105)44:5<1215::AID-ANR206>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Marinova-Mutafchieva L., Williams R. O., Mason L. J., Mauri C., Feldmann M., Maini R. N. Dynamics of proinflammatory cytokine expression in the joints of mice with collagen-induced arthritis (CIA). Clin Exp Immunol. 1997 Mar;107(3):507–512. doi: 10.1046/j.1365-2249.1997.2901181.x. [DOI] [PubMed] [Google Scholar]
- Müssener A., Litton M. J., Lindroos E., Klareskog L. Cytokine production in synovial tissue of mice with collagen-induced arthritis (CIA). Clin Exp Immunol. 1997 Mar;107(3):485–493. doi: 10.1046/j.1365-2249.1997.3181214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima A., Hakoda M., Yamanaka H., Kamatani N., Kashiwazaki S. Divergent effects of methotrexate on the clonal growth of T and B lymphocytes and synovial adherent cells from patients with rheumatoid arthritis. Ann Rheum Dis. 1996 Apr;55(4):237–242. doi: 10.1136/ard.55.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa F., Matsuno H., Yudoh K., Katayama R., Sawai T., Uzuki M., Kimura T. Methotrexate inhibits rheumatoid synovitis by inducing apoptosis. J Rheumatol. 2001 Aug;28(8):1800–1808. [PubMed] [Google Scholar]
- Nandakumar Kutty Selva, Svensson Lars, Holmdahl Rikard. Collagen type II-specific monoclonal antibody-induced arthritis in mice: description of the disease and the influence of age, sex, and genes. Am J Pathol. 2003 Nov;163(5):1827–1837. doi: 10.1016/S0002-9440(10)63542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath M. F., Hildner K., Becker C., Schlaak J. F., Barbulescu K., Germann T., Schmitt E., Schirmacher P., Haralambous S., Pasparakis M. Methotrexate specifically modulates cytokine production by T cells and macrophages in murine collagen-induced arthritis (CIA): a mechanism for methotrexate-mediated immunosuppression. Clin Exp Immunol. 1999 Jan;115(1):42–55. doi: 10.1046/j.1365-2249.1999.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson Peter, Holmberg Jens, Tordsson Jesper, Lu Shemin, Akerström Bo, Holmdahl Rikard. Positional identification of Ncf1 as a gene that regulates arthritis severity in rats. Nat Genet. 2002 Dec 2;33(1):25–32. doi: 10.1038/ng1058. [DOI] [PubMed] [Google Scholar]
- Segal R., Mozes E., Yaron M., Tartakovsky B. The effects of methotrexate on the production and activity of interleukin-1. Arthritis Rheum. 1989 Apr;32(4):370–377. doi: 10.1002/anr.1780320403. [DOI] [PubMed] [Google Scholar]
- Smolen Josef S., Steiner Günter. Therapeutic strategies for rheumatoid arthritis. Nat Rev Drug Discov. 2003 Jun;2(6):473–488. doi: 10.1038/nrd1109. [DOI] [PubMed] [Google Scholar]
- Stuart J. M., Dixon F. J. Serum transfer of collagen-induced arthritis in mice. J Exp Med. 1983 Aug 1;158(2):378–392. doi: 10.1084/jem.158.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson L., Jirholt J., Holmdahl R., Jansson L. B cell-deficient mice do not develop type II collagen-induced arthritis (CIA). Clin Exp Immunol. 1998 Mar;111(3):521–526. doi: 10.1046/j.1365-2249.1998.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson Lars, Abdul-Majid Khairul-Bariah, Bauer Jan, Lassmann Hans, Harris Robert A., Holmdahl Rikard. A comparative analysis of B cell-mediated myelin oligodendrocyte glycoprotein-experimental autoimmune encephalomyelitis pathogenesis in B cell-deficient mice reveals an effect on demyelination. Eur J Immunol. 2002 Jul;32(7):1939–1946. doi: 10.1002/1521-4141(200207)32:7<1939::AID-IMMU1939>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Terato K., Hasty K. A., Reife R. A., Cremer M. A., Kang A. H., Stuart J. M. Induction of arthritis with monoclonal antibodies to collagen. J Immunol. 1992 Apr 1;148(7):2103–2108. [PubMed] [Google Scholar]
- Vingsbo C., Sahlstrand P., Brun J. G., Jonsson R., Saxne T., Holmdahl R. Pristane-induced arthritis in rats: a new model for rheumatoid arthritis with a chronic disease course influenced by both major histocompatibility complex and non-major histocompatibility complex genes. Am J Pathol. 1996 Nov;149(5):1675–1683. [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Rollins S. A., Madri J. A., Matis L. A. Anti-C5 monoclonal antibody therapy prevents collagen-induced arthritis and ameliorates established disease. Proc Natl Acad Sci U S A. 1995 Sep 12;92(19):8955–8959. doi: 10.1073/pnas.92.19.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson W. C., Townes A. S. Genetic susceptibility to murine collagen II autoimmune arthritis. Proposed relationship to the IgG2 autoantibody subclass response, complement C5, major histocompatibility complex (MHC) and non-MHC loci. J Exp Med. 1985 Dec 1;162(6):1878–1891. doi: 10.1084/jem.162.6.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]