Abstract
Objective: To determine the safety/tolerability and efficacy of anakinra in patients with SLE with leading joint involvement.
Methods: In patients with SLE with active polyarthritis and no other uncontrolled systemic/organ manifestations, 100 mg/day anakinra was self administered subcutaneously for 3 months. Disease activity was assessed by VAS, number of swollen/tender joints, ECLAM score, and serological and immunological measures.
Results: Four patients with SLE were studied; anakinra was safe in all four patients and no drug related serious adverse events occurred. A subjective benefit was seen in all patients and a trend towards better activity measures after 4 weeks. After an initial response, one patient left the study because of an arthritic flare after 6 weeks.
Conclusion: In this study anakinra was apparently safe and well tolerated and led to clinical and serological improvement. Anakinra might be an interesting alternative in individual patients with lupus arthritis not responding to conventional treatments.
Full Text
The Full Text of this article is available as a PDF (65.6 KB).
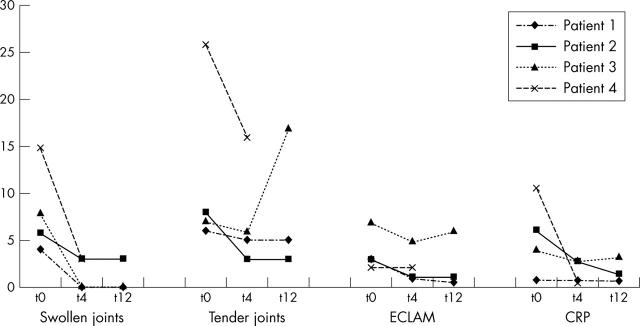
Figure 1.
Follow up data (t0, t4, and t12 weeks) for the number of swollen joints, number of tender joints, ECLAM and CRP (mg/dl) for all four patients. Note: no t12 (12 weeks) data were available for patient 4 (drop out because of uncontrolled disease activity).