Abstract
Objectives: To analyse the association of interleukin 10 (IL10) promoter polymorphisms, which have been shown to be related to IL10 secretion capacity, with the response to long term treatment with etanercept in patients with rheumatoid arthritis (RA).
Methods: Fifty patients with active RA were treated for up to 4 years (median 39 months, range 3–52) with stable doses of etanercept as monotherapy. Treatment response was assessed as defined by the EULAR criteria in an intention to treat analysis, with the last observation carried forward. IL10 promoter microsatellite polymorphisms IL10.R and IL10.G were genotyped by fragment length analysis in patients and 189 healthy controls matched for ethnicity, age, and sex. Haplotypes were reconstructed using a method based on bayesian, coalescent theory with the PHASE software.
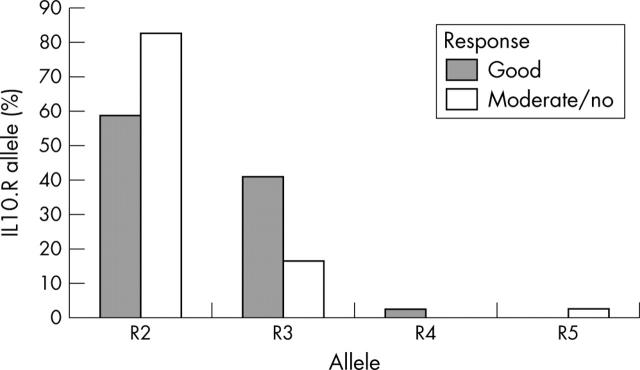
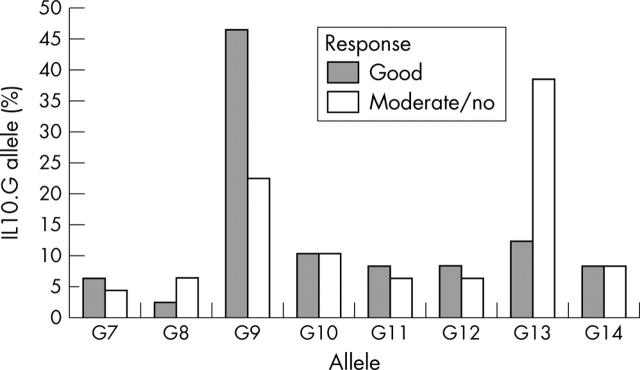
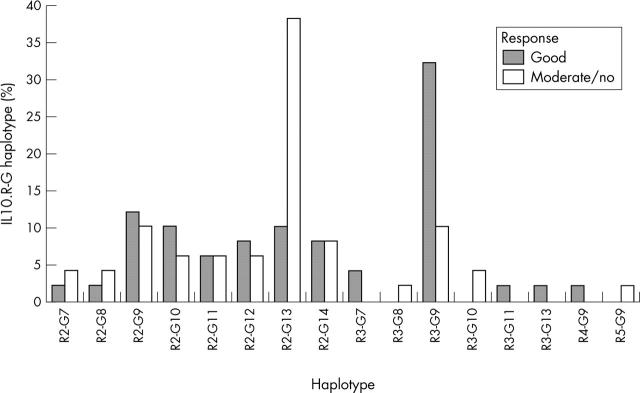
Results: IL10 microsatellite polymorphisms were not associated with susceptibility to RA. When patients with good treatment response (n = 25) were compared with patients with moderate (n = 17) or no response (n = 8), a significantly different distribution of the prevailing alleles R2, R3 and G9, G13, respectively, became evident. Good treatment response was associated with carriage of the R3 allele or R3-G9 haplotype, whereas the allele G13 and the haplotype R2-G13 predominated in patients with moderate or no response.
Conclusion: Genotyping of the IL10 promoter microsatellites may be useful in predicting the clinical response to etanercept in patients with RA. The high prevalence of the presumptive IL10 low producer allele R3 in patients with a favourable response suggests that IL10 promotes disease activity in RA under the specific condition of tumour necrosis factor antagonism.
Full Text
The Full Text of this article is available as a PDF (97.0 KB).
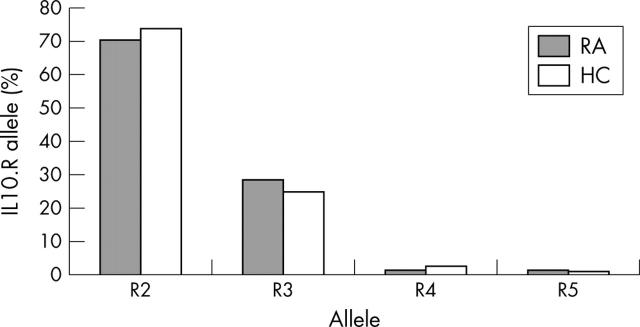
Figure 1.
IL10.R allele distribution in patients with RA and healthy controls (HC). Bars represent the allele frequencies in the respective population. Allele distribution was not significantly different between patients with RA (n = 100 alleles) and healthy controls (n = 378 alleles). χ2 (T4 statistics) = 0.62; p = 0.2232.
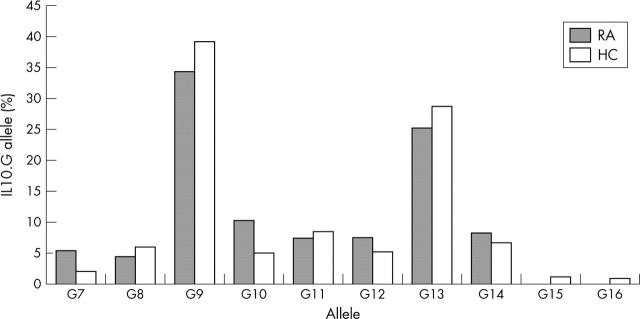
Figure 2.
IL10.G allele distribution in patients with RA and healthy controls (HC). Bars represent the allele frequencies in the respective population. Allele distribution was not significantly different between patients with RA (n = 100 alleles) and healthy controls (n = 378 alleles). χ2 (T4 statistics) = 8.09; p = 0.1684.
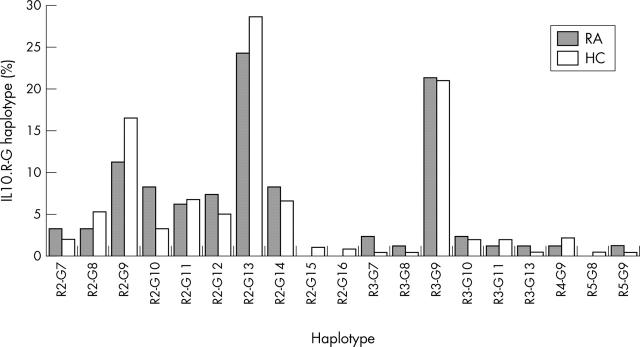
Figure 3.
IL10.R-G haplotype distribution in patients with RA and healthy controls (HC). Bars represent the haplotype frequencies in the respective population. Haplotype distribution was not significantly different between patients with RA (n = 100 alleles) and healthy controls (n = 378 alleles). χ2 (T4 statistics) = 12.08; p = 0.2140.
Figure 4.
IL10.R allele distribution in patients with RA as related to etanercept response. Bars represent the allele frequencies in the respective patient subgroup. Allele distribution was significantly different between patients responding well to etanercept treatment (n = 50 alleles) and patients responding moderately or not at all (n = 50 alleles). χ2 (T4 statistics) = 8.21; p = 0.0101.
Figure 5.
IL10.G allele distribution in patients with RA as related to etanercept response. Bars represent the allele frequencies in the respective patient subgroup. Allele distribution was significantly different between patients responding well to etanercept treatment (n = 50 alleles) and patients responding moderately or not at all (n = 50 alleles). χ2 (T4 statistics) = 10.93; p = 0.0346.
Figure 6.
IL10.R-G haplotype distribution in patients with RA as related to etanercept response. Bars represent the haplotype frequencies in the respective patient subgroup. Haplotype distribution was significantly different between patients responding well to etanercept treatment (n = 50 alleles) and patients responding moderately or not at all (n = 50 alleles). χ2 (T4 statistics) = 17.83; p = 0.0186.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Assmann G., Schulte H. Relation of high-density lipoprotein cholesterol and triglycerides to incidence of atherosclerotic coronary artery disease (the PROCAM experience). Prospective Cardiovascular Münster study. Am J Cardiol. 1992 Sep 15;70(7):733–737. doi: 10.1016/0002-9149(92)90550-i. [DOI] [PubMed] [Google Scholar]
- Baghai M., Osmon D. R., Wolk D. M., Wold L. E., Haidukewych G. J., Matteson E. L. Fatal sepsis in a patient with rheumatoid arthritis treated with etanercept. Mayo Clin Proc. 2001 Jun;76(6):653–656. doi: 10.4065/76.6.653. [DOI] [PubMed] [Google Scholar]
- Breedveld F. C., Emery P., Keystone E., Patel K., Furst D. E., Kalden J. R., St Clair E. W., Weisman M., Smolen J., Lipsky P. E. Infliximab in active early rheumatoid arthritis. Ann Rheum Dis. 2004 Feb;63(2):149–155. doi: 10.1136/ard.2003.013961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. Lori, Greene Mark H., Gershon Sharon K., Edwards Evelyne T., Braun M. Miles. Tumor necrosis factor antagonist therapy and lymphoma development: twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum. 2002 Dec;46(12):3151–3158. doi: 10.1002/art.10679. [DOI] [PubMed] [Google Scholar]
- Chernoff A. E., Granowitz E. V., Shapiro L., Vannier E., Lonnemann G., Angel J. B., Kennedy J. S., Rabson A. R., Wolff S. M., Dinarello C. A. A randomized, controlled trial of IL-10 in humans. Inhibition of inflammatory cytokine production and immune responses. J Immunol. 1995 May 15;154(10):5492–5499. [PubMed] [Google Scholar]
- Cush J. J., Splawski J. B., Thomas R., McFarlin J. E., Schulze-Koops H., Davis L. S., Fujita K., Lipsky P. E. Elevated interleukin-10 levels in patients with rheumatoid arthritis. Arthritis Rheum. 1995 Jan;38(1):96–104. doi: 10.1002/art.1780380115. [DOI] [PubMed] [Google Scholar]
- Eskdale J., Gallagher G. A polymorphic dinucleotide repeat in the human IL-10 promoter. Immunogenetics. 1995;42(5):444–445. doi: 10.1007/BF00179416. [DOI] [PubMed] [Google Scholar]
- Eskdale J., Gallagher G., Verweij C. L., Keijsers V., Westendorp R. G., Huizinga T. W. Interleukin 10 secretion in relation to human IL-10 locus haplotypes. Proc Natl Acad Sci U S A. 1998 Aug 4;95(16):9465–9470. doi: 10.1073/pnas.95.16.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskdale J., Keijsers V., Huizinga T., Gallagher G. Microsatellite alleles and single nucleotide polymorphisms (SNP) combine to form four major haplotype families at the human interleukin-10 (IL-10) locus. Genes Immun. 1999 Nov;1(2):151–155. doi: 10.1038/sj.gene.6363656. [DOI] [PubMed] [Google Scholar]
- Eskdale J., Kube D., Gallagher G. A second polymorphic dinucleotide repeat in the 5' flanking region of the human IL10 gene. Immunogenetics. 1996;45(1):82–83. doi: 10.1007/s002510050174. [DOI] [PubMed] [Google Scholar]
- Eskdale J., Kube D., Tesch H., Gallagher G. Mapping of the human IL10 gene and further characterization of the 5' flanking sequence. Immunogenetics. 1997;46(2):120–128. doi: 10.1007/s002510050250. [DOI] [PubMed] [Google Scholar]
- Eskdale J., McNicholl J., Wordsworth P., Jonas B., Huizinga T., Field M., Gallagher G. Interleukin-10 microsatellite polymorphisms and IL-10 locus alleles in rheumatoid arthritis susceptibility. Lancet. 1998 Oct 17;352(9136):1282–1283. doi: 10.1016/S0140-6736(05)70489-X. [DOI] [PubMed] [Google Scholar]
- Feldmann M., Brennan F. M., Maini R. N. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- Klimiuk P. A., Goronzy J. J., Björ nsson J., Beckenbaugh R. D., Weyand C. M. Tissue cytokine patterns distinguish variants of rheumatoid synovitis. Am J Pathol. 1997 Nov;151(5):1311–1319. [PMC free article] [PubMed] [Google Scholar]
- Klimiuk P. A., Sierakowski S., Latosiewicz R., Cylwik J. P., Cylwik B., Skowronski J., Chwiecko J. Circulating tumour necrosis factor alpha and soluble tumour necrosis factor receptors in patients with different patterns of rheumatoid synovitis. Ann Rheum Dis. 2003 May;62(5):472–475. doi: 10.1136/ard.62.5.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroesen S., Widmer A. F., Tyndall A., Hasler P. Serious bacterial infections in patients with rheumatoid arthritis under anti-TNF-alpha therapy. Rheumatology (Oxford) 2003 May;42(5):617–621. doi: 10.1093/rheumatology/keg263. [DOI] [PubMed] [Google Scholar]
- Lard L. R., van Gaalen F. A., Schonkeren J. J. M., Pieterman E. J., Stoeken G., Vos K., Nelissen R. G. H. H., Westendorp R. G. J., Hoeben R. C., Breedveld F. C. Association of the -2849 interleukin-10 promoter polymorphism with autoantibody production and joint destruction in rheumatoid arthritis. Arthritis Rheum. 2003 Jul;48(7):1841–1848. doi: 10.1002/art.11160. [DOI] [PubMed] [Google Scholar]
- MacKay K., Milicic A., Lee D., Tikly M., Laval S., Shatford J., Wordsworth P. Rheumatoid arthritis susceptibility and interleukin 10: a study of two ethnically diverse populations. Rheumatology (Oxford) 2003 Jan;42(1):149–153. doi: 10.1093/rheumatology/keg054. [DOI] [PubMed] [Google Scholar]
- Martinez Alfonso, Salido Marina, Bonilla Gema, Pascual-Salcedo Dora, Fernandez-Arquero Miguel, de Miguel Sonia, Balsa Alejandro, de la Concha Emilio G., Fernandez-Gutierrez Benjamin. Association of the major histocompatibility complex with response to infliximab therapy in rheumatoid arthritis patients. Arthritis Rheum. 2004 Apr;50(4):1077–1082. doi: 10.1002/art.20154. [DOI] [PubMed] [Google Scholar]
- Miterski Bianca, Drynda Susanne, Böschow Gundula, Klein Wolfram, Oppermann Joachim, Kekow Jörn, Epplen Jörg Thomas. Complex genetic predisposition in adult and juvenile rheumatoid arthritis. BMC Genet. 2004 Feb 4;5:2–2. doi: 10.1186/1471-2156-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan N., Edwards E. T., Cupps T. R., Oliverio P. J., Sandberg G., Crayton H., Richert J. R., Siegel J. N. Demyelination occurring during anti-tumor necrosis factor alpha therapy for inflammatory arthritides. Arthritis Rheum. 2001 Dec;44(12):2862–2869. doi: 10.1002/1529-0131(200112)44:12<2862::aid-art474>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Moore K. W., de Waal Malefyt R., Coffman R. L., O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Moreland L. W., Cohen S. B., Baumgartner S. W., Tindall E. A., Bulpitt K., Martin R., Weinblatt M., Taborn J., Weaver A., Burge D. J. Long-term safety and efficacy of etanercept in patients with rheumatoid arthritis. J Rheumatol. 2001 Jun;28(6):1238–1244. [PubMed] [Google Scholar]
- Mugnier Benedicte, Balandraud Nathalie, Darque Albert, Roudier Chantal, Roudier Jean, Reviron Denis. Polymorphism at position -308 of the tumor necrosis factor alpha gene influences outcome of infliximab therapy in rheumatoid arthritis. Arthritis Rheum. 2003 Jul;48(7):1849–1852. doi: 10.1002/art.11168. [DOI] [PubMed] [Google Scholar]
- Padyukov L., Lampa J., Heimbürger M., Ernestam S., Cederholm T., Lundkvist I., Andersson P., Hermansson Y., Harju A., Klareskog L. Genetic markers for the efficacy of tumour necrosis factor blocking therapy in rheumatoid arthritis. Ann Rheum Dis. 2003 Jun;62(6):526–529. doi: 10.1136/ard.62.6.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez L., Orte J., Brieva J. A. Terminal differentiation of spontaneous rheumatoid factor-secreting B cells from rheumatoid arthritis patients depends on endogenous interleukin-10. Arthritis Rheum. 1995 Dec;38(12):1771–1776. doi: 10.1002/art.1780381210. [DOI] [PubMed] [Google Scholar]
- Phillips Kristine, Husni M. Elaine, Karlson Elizabeth W., Coblyn Jonathan S. Experience with etanercept in an academic medical center: are infection rates increased? Arthritis Rheum. 2002 Feb;47(1):17–21. doi: 10.1002/art1.10243. [DOI] [PubMed] [Google Scholar]
- Reparon-Schuijt C. C., van Esch W. J., van Kooten C., Levarht E. W., Breedveld F. C., Verweij C. L. Functional analysis of rheumatoid factor-producing B cells from the synovial fluid of rheumatoid arthritis patients. Arthritis Rheum. 1998 Dec;41(12):2211–2220. doi: 10.1002/1529-0131(199812)41:12<2211::AID-ART17>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Reparon-Schuijt C. C., van Esch W. J., van Kooten C., Schellekens G. A., de Jong B. A., van Venrooij W. J., Breedveld F. C., Verweij C. L. Secretion of anti-citrulline-containing peptide antibody by B lymphocytes in rheumatoid arthritis. Arthritis Rheum. 2001 Jan;44(1):41–47. doi: 10.1002/1529-0131(200101)44:1<41::AID-ANR6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Robinson W. H., Genovese M. C., Moreland L. W. Demyelinating and neurologic events reported in association with tumor necrosis factor alpha antagonism: by what mechanisms could tumor necrosis factor alpha antagonists improve rheumatoid arthritis but exacerbate multiple sclerosis? Arthritis Rheum. 2001 Sep;44(9):1977–1983. doi: 10.1002/1529-0131(200109)44:9<1977::AID-ART345>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Shakoor Najia, Michalska Margaret, Harris Charlotte A., Block Joel A. Drug-induced systemic lupus erythematosus associated with etanercept therapy. Lancet. 2002 Feb 16;359(9306):579–580. doi: 10.1016/S0140-6736(02)07714-0. [DOI] [PubMed] [Google Scholar]
- Sham P. C., Curtis D. Monte Carlo tests for associations between disease and alleles at highly polymorphic loci. Ann Hum Genet. 1995 Jan;59(Pt 1):97–105. doi: 10.1111/j.1469-1809.1995.tb01608.x. [DOI] [PubMed] [Google Scholar]
- Stephens M., Smith N. J., Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001 Mar 9;68(4):978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens Matthew, Donnelly Peter. A comparison of bayesian methods for haplotype reconstruction from population genotype data. Am J Hum Genet. 2003 Oct 20;73(5):1162–1169. doi: 10.1086/379378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanidworanun C., Strober W. Predominant role of tumor necrosis factor-alpha in human monocyte IL-10 synthesis. J Immunol. 1993 Dec 15;151(12):6853–6861. [PubMed] [Google Scholar]
- Westendorp R. G., Langermans J. A., Huizinga T. W., Elouali A. H., Verweij C. L., Boomsma D. I., Vandenbroucke J. P., Vandenbrouke J. P. Genetic influence on cytokine production and fatal meningococcal disease. Lancet. 1997 Jan 18;349(9046):170–173. doi: 10.1016/s0140-6736(96)06413-6. [DOI] [PubMed] [Google Scholar]
- Weyand C. M., Klimiuk P. A., Goronzy J. J. Heterogeneity of rheumatoid arthritis: from phenotypes to genotypes. Springer Semin Immunopathol. 1998;20(1-2):5–22. doi: 10.1007/BF00831996. [DOI] [PubMed] [Google Scholar]
- den Broeder A. A., Joosten L. A. B., Saxne T., Heinegård D., Fenner H., Miltenburg A. M. M., Frasa W. L. H., van Tits L. J., Buurman W. A., van Riel P. L. C. M. Long term anti-tumour necrosis factor alpha monotherapy in rheumatoid arthritis: effect on radiological course and prognostic value of markers of cartilage turnover and endothelial activation. Ann Rheum Dis. 2002 Apr;61(4):311–318. doi: 10.1136/ard.61.4.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gestel A. M., Prevoo M. L., van 't Hof M. A., van Rijswijk M. H., van de Putte L. B., van Riel P. L. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum. 1996 Jan;39(1):34–40. doi: 10.1002/art.1780390105. [DOI] [PubMed] [Google Scholar]