Abstract
Objective: To clarify the effect of interleukin (IL) 18 on cartilage degeneration by studying the profile of IL18 receptor (IL18R) on chondrocytes and the direct effect of IL18 on production of matrix metalloproteinases (MMPs), aggrecanases, and tissue inhibitors of metalloproteinases (TIMPs) in articular chondrocytes.
Methods: Monolayer cultured human articular chondrocytes were isolated from non-arthritic subjects and patients with rheumatoid arthritis or osteoarthritis. Gene expression of IL18, IL18Rα, IL18Rß, MMPs, and aggrecanases was detected by RT-PCR. Protein levels of IL18Rα were analysed by flow cytometry. Protein levels of IL18, MMPs, and TIMPs were measured by ELISA. Aggrecanase-2 mRNA expression was quantitatively analysed by real time RT-PCR. Protein levels of signalling molecules were assayed by western blotting.
Results: IL18 mRNA was constitutively expressed in chondrocytes, and was enhanced by IL1ß stimulation. Flow cytometric analysis showed that IL1ß, tumour necrosis factor α, and IL18 up regulated IL18Rα expression levels. The level of IL18Rß mRNA was much lower than that of IL18Rα, and was slightly up regulated by IL1ß. In chondrocytes responding to IL18, IL18 (1–100 ng/ml) slightly increased the production of MMP-1, MMP-3, and MMP-13, which was blocked by NF-κB inhibitor and p38 mitogen activated protein kinase inhibitor. IL18 up regulated mRNA expression of aggrecanase-2, but not aggrecanase-1. IL18 also slightly stimulated TIMP-1 production?through extracellular signal regulated kinase activation.
Conclusion: IL18 induces production of MMPs from chondrocytes in inflammatory arthritis. Although the direct effect of IL18 on chondrocytes may not be pivotal for the induction of cartilage degeneration, IL18 seems to play some part in the degradation of articular cartilage in arthritis.
Full Text
The Full Text of this article is available as a PDF (208.4 KB).
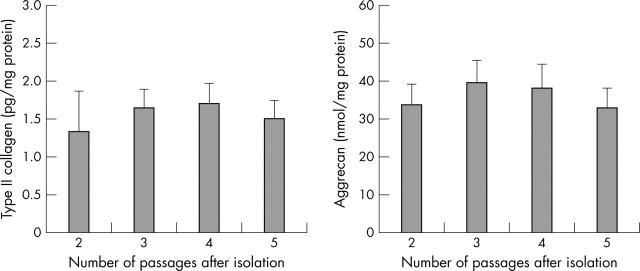
Figure 1.
Production of type II collagen and proteoglycan during the continuous culture of chondrocytes. The levels of production of type II collagen and proteoglycan released by cultured chondrocytes were analysed by an ELISA.
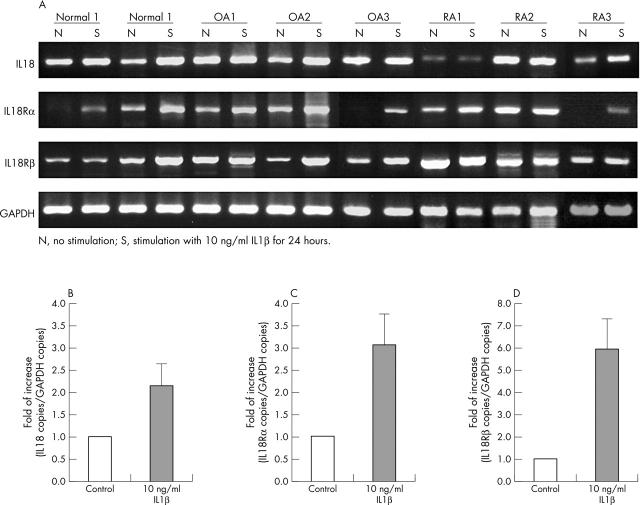
Figure 2.
Expression of mRNA for IL18, IL18Rα, and IL18Rß by cultured chondrocytes. The chondrocytes were isolated from normal human joint cartilage, OA and RA cartilage, and mRNA expression was detected by RT-PCR. PCR products were stained with ethidium bromide. Results are representative of individual experiments (A). Effect of IL1ß (10 ng/ml) on the expression of IL18 (B), IL18Rα (C), and IL18Rß (D) were analysed by real time PCR.
Figure 3.
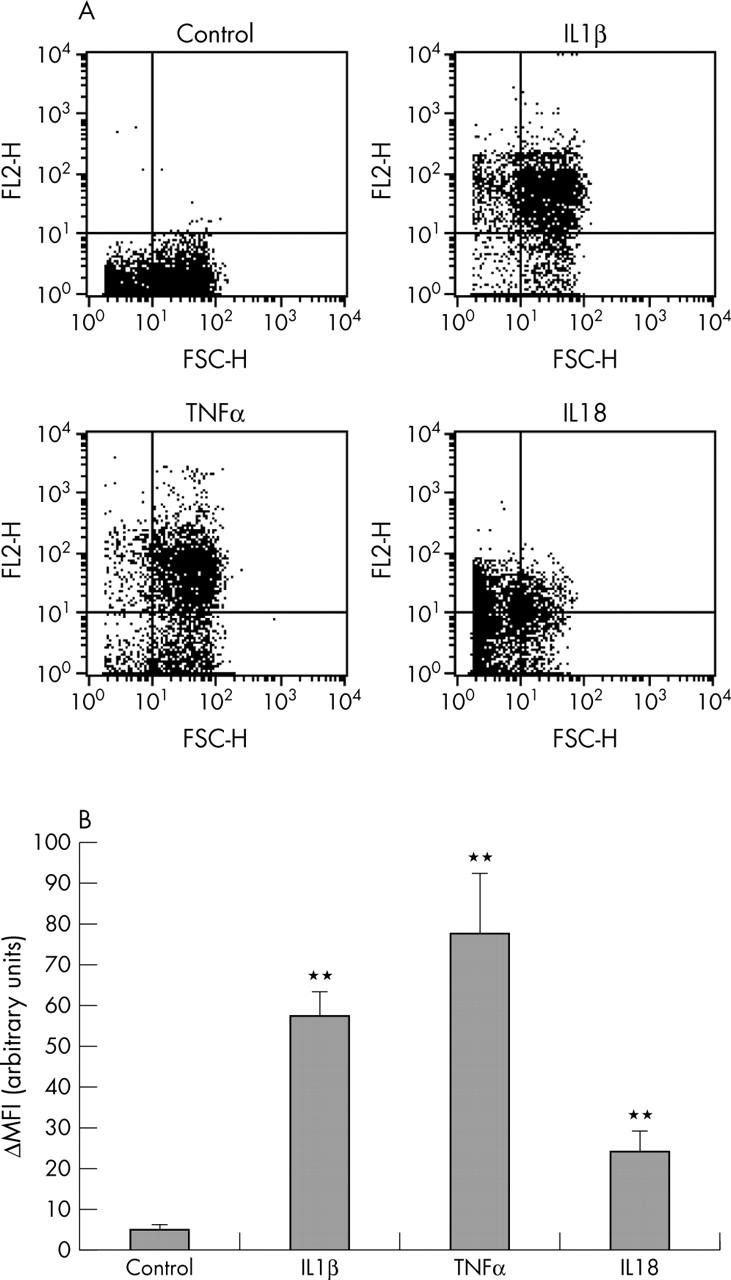
Flow cytometric analysis of IL18Rα expression on the surface of cultured OA chondrocytes. (A) Representative dot plots of cells incubated with or without IL1ß (5 ng/ml), TNFα (10 ng/ml), or IL18 (10 ng/ml) for 12 hours. (B) Changes in mean fluorescence intensity (ΔMFI). **p<0.01 v control.
Figure 4.
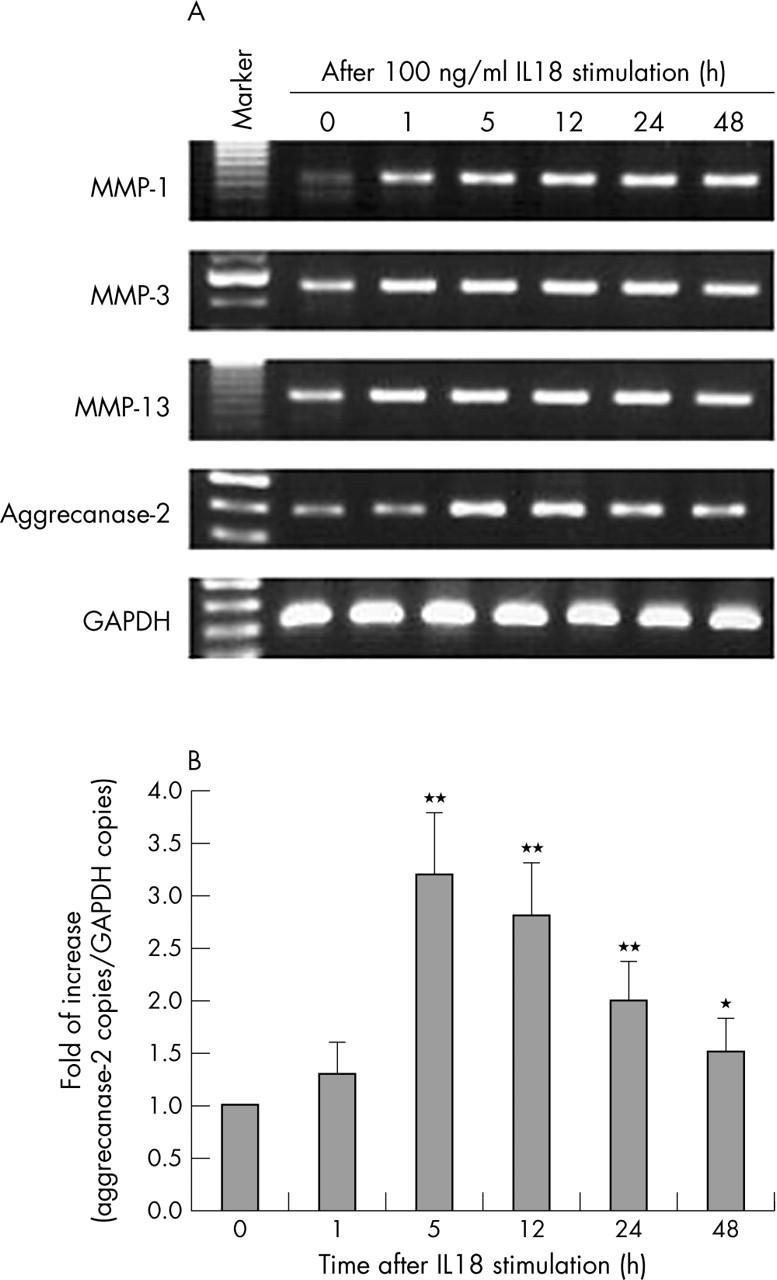
Time course of IL18 effects on gene expression of MMP-1, MMP-3, MMP-13, and aggrecanase-2 in OA chondrocytes. (A) Representative results from chondrocytes that responded to IL18 by RT-PCR. (B) Expression level of aggrecanase-2 analysed by quantitative real time RT-PCR. The details are described in "Patients and methods". The copy number of aggrecanase-2 mRNA was standardised by the copy number of GAPDH mRNA. Compared with the control (without IL18): *p<0.05, **p<0.01.
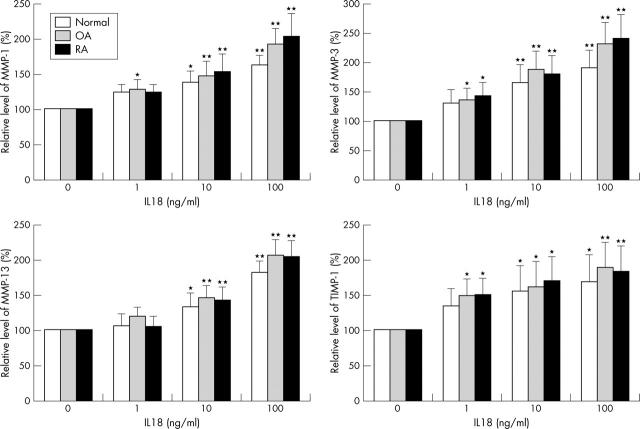
Figure 5.
Dose-response of IL18 effects on MMP-1, MMP-3, MMP-13, and TIMP-1 production in chondrocytes. After a 48 hour incubation with indicated concentrations of IL18, the supernatants were harvested. The levels of MMPs and TIMP-1 in the supernatants were assayed by ELISA. Here only the results from the chondrocytes responsive to IL18 were shown. Compared with the control (0 ng/ml IL18): *p<0.05; **p<0.01.
Figure 6.
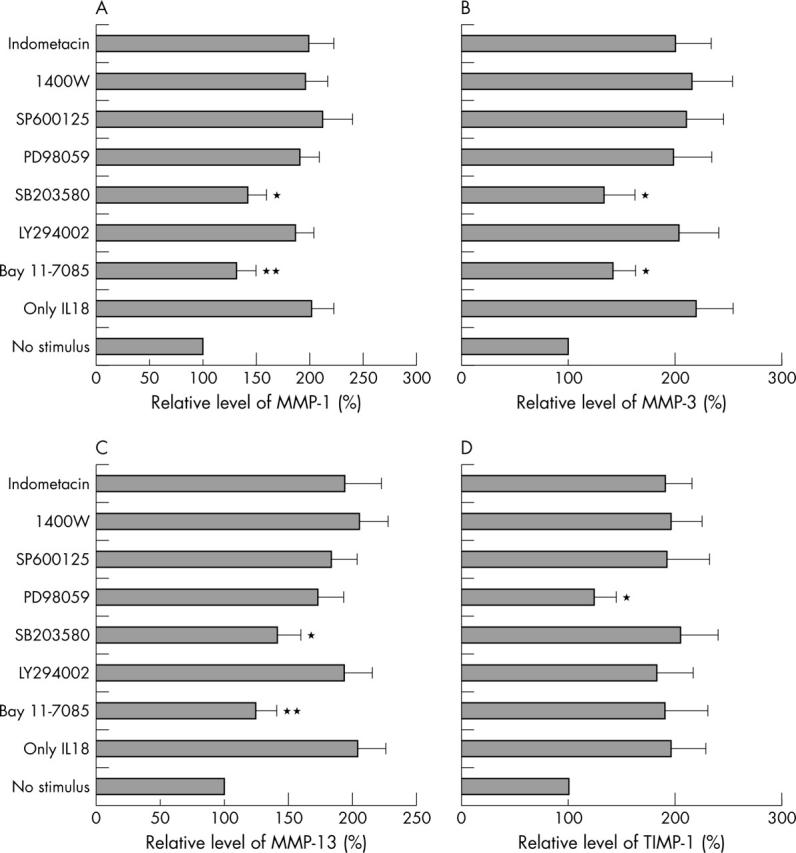
Effect of specific inhibitors on IL18 enhanced production of MMP-1, MMP-3, MMP-13, and TIMP-1 in OA chondrocytes. In IL18 responsive chondrocytes, specific inhibitor of NF-κB (20 µM Bay11-7085), PI3K (10 µM LY294002), p38 MAPK (10 µM SB203580), MEK (30 µM PD98059), JNK (20 µM SP600125), inducible nitric oxide synthase (100 µM 1400W), or cyclo-oxygenase (1 µM indometacin) was added to the culture medium 30 minutes before the addition of 100 ng/ml IL18. Compared with the control group (only IL18): *p<0.05; **p<0.01.
Figure 7.
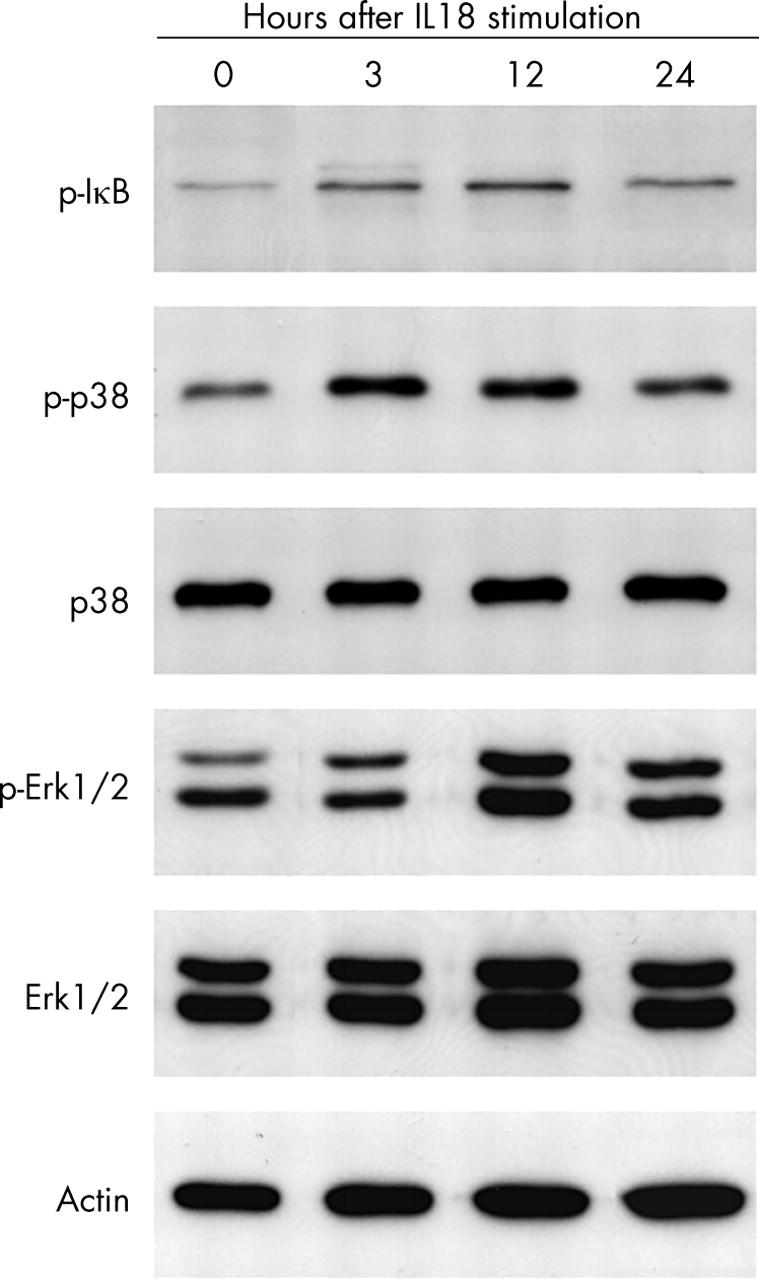
Phosphorylation of IκB, p38 MAPK, and Erk1/2 induced by IL18 in responsive OA chondrocytes. Chondrocyte lysates were prepared at the indicated times after stimulation with 100 ng/ml IL18. Whole cell lysates were subjected to western blotting with specific antibodies.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. Preston, Acott Ted S. Involvement of the Erk-MAP kinase pathway in TNFalpha regulation of trabecular matrix metalloproteinases and TIMPs. Invest Ophthalmol Vis Sci. 2003 Jan;44(1):164–169. doi: 10.1167/iovs.01-1201. [DOI] [PubMed] [Google Scholar]
- Altman R., Asch E., Bloch D., Bole G., Borenstein D., Brandt K., Christy W., Cooke T. D., Greenwald R., Hochberg M. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986 Aug;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- Arend W. P., Dayer J. M. Inhibition of the production and effects of interleukin-1 and tumor necrosis factor alpha in rheumatoid arthritis. Arthritis Rheum. 1995 Feb;38(2):151–160. doi: 10.1002/art.1780380202. [DOI] [PubMed] [Google Scholar]
- Ariel Amiram, Novick Daniela, Rubinstein Menachem, Dinarello Charles A., Lider Ofer, Hershkoviz Rami. IL-12 and IL-18 induce MAP kinase-dependent adhesion of T cells to extracellular matrix components. J Leukoc Biol. 2002 Jul;72(1):192–198. [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Bau Brigitte, Gebhard Pia M., Haag Jochen, Knorr Thomas, Bartnik Eckart, Aigner Thomas. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum. 2002 Oct;46(10):2648–2657. doi: 10.1002/art.10531. [DOI] [PubMed] [Google Scholar]
- Bigg H. F., McLeod R., Waters J., Cawston T. E., Nolan J. F., Clark I. M. Induction of human tissue inhibitor of metalloproteinase-1 gene expression by all-trans retinoic acid in combination with basic fibroblast growth factor involves both p42/44 and p38 MAP kinases. Ann N Y Acad Sci. 1999 Jun 30;878:506–509. doi: 10.1111/j.1749-6632.1999.tb07710.x. [DOI] [PubMed] [Google Scholar]
- Boileau Christelle, Martel-Pelletier Johanne, Moldovan Florina, Jouzeau Jean-Yves, Netter Patrick, Manning Pamela T., Pelletier Jean-Pierre. The in situ up-regulation of chondrocyte interleukin-1-converting enzyme and interleukin-18 levels in experimental osteoarthritis is mediated by nitric oxide. Arthritis Rheum. 2002 Oct;46(10):2637–2647. doi: 10.1002/art.10518. [DOI] [PubMed] [Google Scholar]
- Caterson B., Flannery C. R., Hughes C. E., Little C. B. Mechanisms involved in cartilage proteoglycan catabolism. Matrix Biol. 2000 Aug;19(4):333–344. doi: 10.1016/s0945-053x(00)00078-0. [DOI] [PubMed] [Google Scholar]
- Cawston T. E. Metalloproteinase inhibitors and the prevention of connective tissue breakdown. Pharmacol Ther. 1996;70(3):163–182. doi: 10.1016/0163-7258(96)00015-0. [DOI] [PubMed] [Google Scholar]
- Chua C. C., Chua B. H. Tumor necrosis factor-alpha induces mRNA for collagenase and TIMP in human skin fibroblasts. Connect Tissue Res. 1990;25(2):161–170. doi: 10.3109/03008209009006990. [DOI] [PubMed] [Google Scholar]
- Dodge G. R., Poole A. R. Immunohistochemical detection and immunochemical analysis of type II collagen degradation in human normal, rheumatoid, and osteoarthritic articular cartilages and in explants of bovine articular cartilage cultured with interleukin 1. J Clin Invest. 1989 Feb;83(2):647–661. doi: 10.1172/JCI113929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y., Valbracht J., Lotz M. Selective activation of the mitogen-activated protein kinase subgroups c-Jun NH2 terminal kinase and p38 by IL-1 and TNF in human articular chondrocytes. J Clin Invest. 1996 Nov 15;98(10):2425–2430. doi: 10.1172/JCI119056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracie J. A., Forsey R. J., Chan W. L., Gilmour A., Leung B. P., Greer M. R., Kennedy K., Carter R., Wei X. Q., Xu D. A proinflammatory role for IL-18 in rheumatoid arthritis. J Clin Invest. 1999 Nov;104(10):1393–1401. doi: 10.1172/JCI7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F. K., Wu S. G. Phosphatidylinositol 3-kinase modulates IL-18-induced nuclear factor-kappa B activation. Acta Pharmacol Sin. 2000 Apr;21(4):321–324. [PubMed] [Google Scholar]
- Kalina U., Kauschat D., Koyama N., Nuernberger H., Ballas K., Koschmieder S., Bug G., Hofmann W. K., Hoelzer D., Ottmann O. G. IL-18 activates STAT3 in the natural killer cell line 92, augments cytotoxic activity, and mediates IFN-gamma production by the stress kinase p38 and by the extracellular regulated kinases p44erk-1 and p42erk-21. J Immunol. 2000 Aug 1;165(3):1307–1313. doi: 10.4049/jimmunol.165.3.1307. [DOI] [PubMed] [Google Scholar]
- Kawashima Masanori, Miossec Pierre. Heterogeneity of response of rheumatoid synovium cell subsets to interleukin-18 in relation to differential interleukin-18 receptor expression. Arthritis Rheum. 2003 Mar;48(3):631–637. doi: 10.1002/art.10825. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Reznikov L. L., Stuyt R. J., Selzman C. H., Fantuzzi G., Hoshino T., Young H. A., Dinarello C. A. Functional reconstitution and regulation of IL-18 activity by the IL-18R beta chain. J Immunol. 2001 Jan 1;166(1):148–154. doi: 10.4049/jimmunol.166.1.148. [DOI] [PubMed] [Google Scholar]
- Li Kelvin W., Wang Aaron S., Sah Robert L. Microenvironment regulation of extracellular signal-regulated kinase activity in chondrocytes: effects of culture configuration, interleukin-1, and compressive stress. Arthritis Rheum. 2003 Mar;48(3):689–699. doi: 10.1002/art.10849. [DOI] [PubMed] [Google Scholar]
- Liacini Abdelhamid, Sylvester Judith, Li Wen Qing, Zafarullah Muhammad. Inhibition of interleukin-1-stimulated MAP kinases, activating protein-1 (AP-1) and nuclear factor kappa B (NF-kappa B) transcription factors down-regulates matrix metalloproteinase gene expression in articular chondrocytes. Matrix Biol. 2002 Apr;21(3):251–262. doi: 10.1016/s0945-053x(02)00007-0. [DOI] [PubMed] [Google Scholar]
- Martel-Pelletier J., McCollum R., Fujimoto N., Obata K., Cloutier J. M., Pelletier J. P. Excess of metalloproteases over tissue inhibitor of metalloprotease may contribute to cartilage degradation in osteoarthritis and rheumatoid arthritis. Lab Invest. 1994 Jun;70(6):807–815. [PubMed] [Google Scholar]
- Martel-Pelletier J., Zafarullah M., Kodama S., Pelletier J. P. In vitro effects of interleukin 1 on the synthesis of metalloproteases, TIMP, plasminogen activators and inhibitors in human articular cartilage. J Rheumatol Suppl. 1991 Feb;27:80–84. [PubMed] [Google Scholar]
- Morel J. C., Park C. C., Kumar P., Koch A. E. Interleukin-18 induces rheumatoid arthritis synovial fibroblast CXC chemokine production through NFkappaB activation. Lab Invest. 2001 Oct;81(10):1371–1383. doi: 10.1038/labinvest.3780351. [DOI] [PubMed] [Google Scholar]
- Morel J. C., Park C. C., Woods J. M., Koch A. E. A novel role for interleukin-18 in adhesion molecule induction through NF kappa B and phosphatidylinositol (PI) 3-kinase-dependent signal transduction pathways. J Biol Chem. 2001 Jul 27;276(40):37069–37075. doi: 10.1074/jbc.M103574200. [DOI] [PubMed] [Google Scholar]
- Möller Burkhard, Kessler Uta, Rehart Stefan, Kalina Uwe, Ottmann Oliver G., Kaltwasser Joachim Peter, Hoelzer Dieter, Kukoc-Zivojnov Natasa. Expression of interleukin-18 receptor in fibroblast-like synoviocytes. Arthritis Res. 2001 Nov 14;4(2):139–144. doi: 10.1186/ar390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura H., Tsutsi H., Komatsu T., Yutsudo M., Hakura A., Tanimoto T., Torigoe K., Okura T., Nukada Y., Hattori K. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995 Nov 2;378(6552):88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- Olee T., Hashimoto S., Quach J., Lotz M. IL-18 is produced by articular chondrocytes and induces proinflammatory and catabolic responses. J Immunol. 1999 Jan 15;162(2):1096–1100. [PubMed] [Google Scholar]
- Onodera S., Kaneda K., Mizue Y., Koyama Y., Fujinaga M., Nishihira J. Macrophage migration inhibitory factor up-regulates expression of matrix metalloproteinases in synovial fibroblasts of rheumatoid arthritis. J Biol Chem. 2000 Jan 7;275(1):444–450. doi: 10.1074/jbc.275.1.444. [DOI] [PubMed] [Google Scholar]
- Plater-Zyberk C., Joosten L. A., Helsen M. M., Sattonnet-Roche P., Siegfried C., Alouani S., van De Loo F. A., Graber P., Aloni S., Cirillo R. Therapeutic effect of neutralizing endogenous IL-18 activity in the collagen-induced model of arthritis. J Clin Invest. 2001 Dec;108(12):1825–1832. doi: 10.1172/JCI12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torigoe K., Ushio S., Okura T., Kobayashi S., Taniai M., Kunikata T., Murakami T., Sanou O., Kojima H., Fujii M. Purification and characterization of the human interleukin-18 receptor. J Biol Chem. 1997 Oct 10;272(41):25737–25742. doi: 10.1074/jbc.272.41.25737. [DOI] [PubMed] [Google Scholar]
- Wei X. Q., Leung B. P., Arthur H. M., McInnes I. B., Liew F. Y. Reduced incidence and severity of collagen-induced arthritis in mice lacking IL-18. J Immunol. 2001 Jan 1;166(1):517–521. doi: 10.4049/jimmunol.166.1.517. [DOI] [PubMed] [Google Scholar]
- van den Berg W. B., van de Loo F. A., Otterness I., Arntz O., Joosten L. A. In vivo evidence for a key role of IL-1 in cartilage destruction in experimental arthritis. Agents Actions Suppl. 1991;32:159–163. doi: 10.1007/978-3-0348-7405-2_21. [DOI] [PubMed] [Google Scholar]