Abstract
Background: CD4+ T cell responses to the G1 domain of aggrecan in patients with ankylosing spondylitis (AS) were recently reported. Whether such an immune response can be seen in the CD8+ subpopulation has not yet been determined.
Objective: To determine if HLA-B27 restricted G1-specific CD8+ T cells are present in AS and to analyse immunodominant CD8+ T cell epitopes.
Methods: Peripheral blood mononuclear cells of 45 patients with AS were stimulated with overlapping 18-mer peptides covering the whole G1 protein. Results were compared with those for patients with rheumatoid arthritis (RA) and healthy controls. For epitope analysis, G1-specific interferon gamma positive (IFNγ+) T cells were isolated by magnetic activated cell sorting. After in vitro expansion, CD8+ T cells were restimulated with 14 subpools of G1 peptides. T cells responding to G1 peptide subpools were quantified by flow cytometry according to IFNγ secretion. Predicted peptides were subsequently confirmed by stimulation with single peptides.
Results: G1-specific CD8+ T cell responses were found in 29/45 (64%) patients with AS, 18/35 (51%) patients with RA, but not in healthy controls. Five CD8+ T cell epitopes were identified as immunodominant in five patients. However, the T cell response was not HLA-B27 restricted. Nonamer peptides with an HLA-B27 binding motif did not induce a T cell response.
Conclusion: A G1 peptide-specific CD8+ T cell response is present in AS but also in patients with RA. It does not seem to be HLA-B27 restricted. Whether such a response has a role in the pathogenesis of AS needs clarification.
Full Text
The Full Text of this article is available as a PDF (309.8 KB).
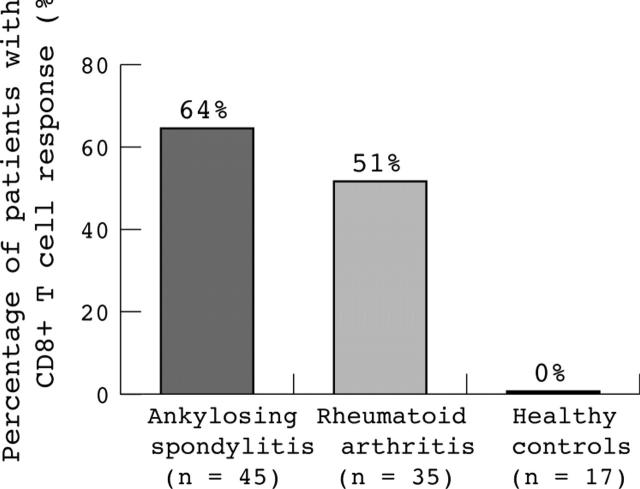
Figure 1.
Percentage of patients with AS (64%), with RA (51%), or healthy controls (0%) responding to in vitro stimulation with the whole peptide pool of overlapping G1 peptides of the proteoglycan aggrecan. Response was measured by IFNγ production of CD8+ T cells after antigen-specific stimulation in comparison with stimulation without antigen. For more details see "Patients and methods" section. Analysis was done with whole PB.
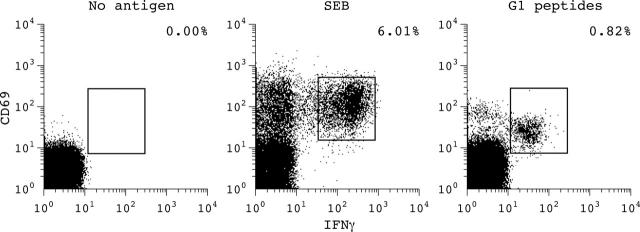
Figure 2.
Example of an antigen-specific response to the whole peptide pool of aggrecan derived G1 peptides compared with stimulation without antigen in a patient with AS. After staining for T cell surface markers and intracellular cytokines a gate for CD8+ T cells was set. The percentage of IFNγ/CD69 double positive cells of the CD8+ T cell subpopulation is indicated. Stimulation with SEB was used as a positive control.
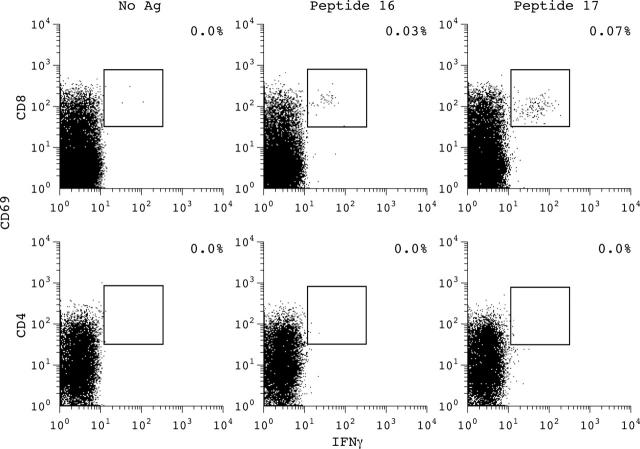
Figure 6.
Example of 18-mer peptides derived from the G1 domain of aggrecan inducing an antigen-specific CD8+ T cell response but not CD4+ T cell response compared with stimulation without antigen (Ag) in patient 1. After staining for T cell surface markers and intracellular cytokines a gate for CD8+ or CD4+ T cells was set. The percentage of IFNγ/CD69 double positive cells of the CD8+ or CD4+ T cell subpopulation is indicated.
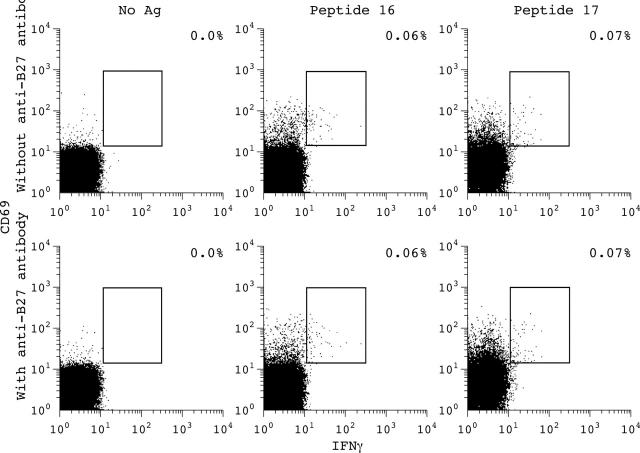
Figure 7.
Antigen-specific CD8+ T cell response to 18-mer single peptides (peptides 16,17) which cannot be blocked by an anti-HLA B27 antibody in patient 1. T cells were taken from PB.
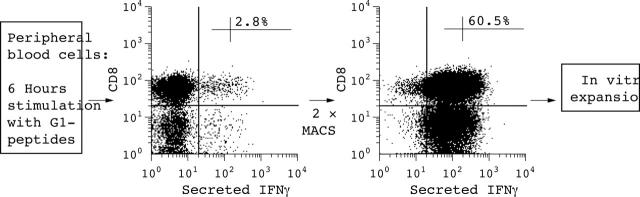
Figure 3.
Example of an antigen-specific IFNγ secretion assay after in vitro stimulation with aggrecan G1 peptides compared with stimulation without antigen in a patient with AS. After stimulation, IFNγ+ T cells were isolated twice by MACS. For more details see "Patients and methods" section.
Figure 4.
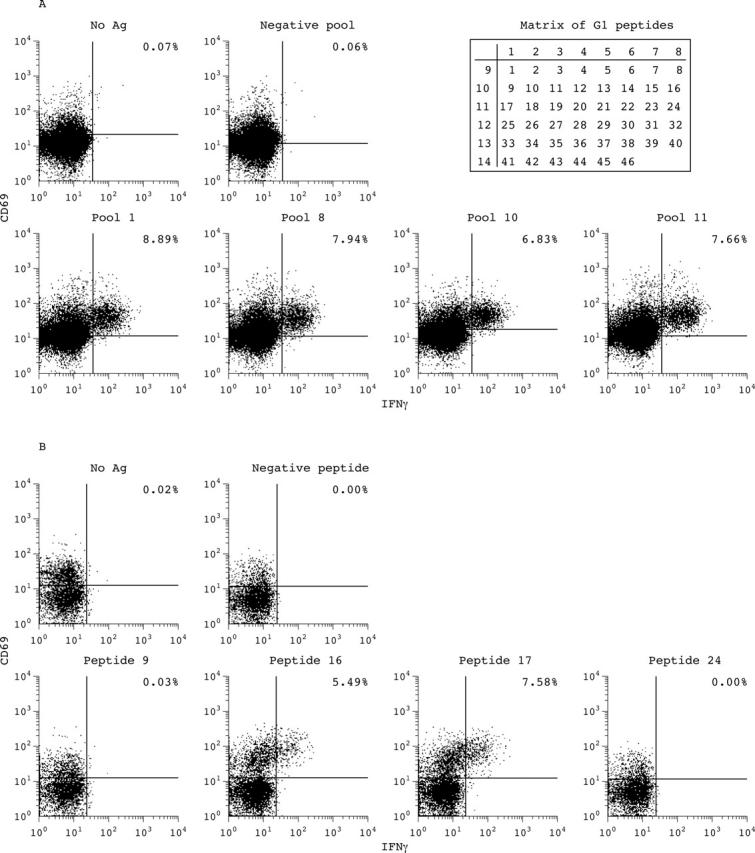
(A) Example of a patient with AS (patient 1) with an antigen-specific CD8+ T cell response to the subpools of peptides (pools 1, 8, 10, 11) derived from the G1 domain of aggrecan compared with stimulation without antigen. After staining for T cell surface markers and intracellular cytokines a gate for CD8+ T cells was set. The percentage of IFNγ/CD69 double positive cells of the CD8+ T cell subpopulation is indicated. Peptide subpools 1, 8, 10, and 11 were positive, indicating that the crossing peptides 9, 16, 17, and 24 might be stimulatory peptides. (B) Example of a patient with AS (patient 1) with an antigen (Ag)-specific CD8+ T cell response to single peptides 16 and 17, but not to peptides 9 and 24 and control peptide, derived from the G1 domain of aggrecan compared with stimulation without Ag. After staining for T cell surface markers and intracellular cytokines a gate for CD8+ T cells was set. The percentage of IFNγ/CD69 double positive cells of the CD8+ T cell subpopulation is indicated.
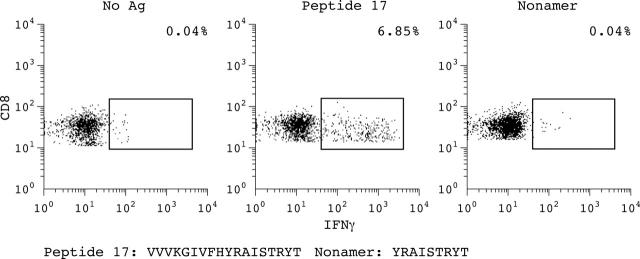
Figure 5.
Example of a patient with AS with an antigen-specific CD8+ T cell response to 18-mer single peptide 17, but not to a nonamer peptide that is part of this 18-mer peptide, derived from the G1 domain of aggrecan, compared with stimulation without antigen (Ag).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird Lucy A., Peh Chen Au, Kollnberger Simon, Elliott Tim, McMichael Andrew J., Bowness Paul. Lymphoblastoid cells express HLA-B27 homodimers both intracellularly and at the cell surface following endosomal recycling. Eur J Immunol. 2003 Mar;33(3):748–759. doi: 10.1002/eji.200323678. [DOI] [PubMed] [Google Scholar]
- Bollow M., Fischer T., Reisshauer H., Backhaus M., Sieper J., Hamm B., Braun J. Quantitative analyses of sacroiliac biopsies in spondyloarthropathies: T cells and macrophages predominate in early and active sacroiliitis- cellularity correlates with the degree of enhancement detected by magnetic resonance imaging. Ann Rheum Dis. 2000 Feb;59(2):135–140. doi: 10.1136/ard.59.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J., Bollow M., Eggens U., König H., Distler A., Sieper J. Use of dynamic magnetic resonance imaging with fast imaging in the detection of early and advanced sacroiliitis in spondylarthropathy patients. Arthritis Rheum. 1994 Jul;37(7):1039–1045. doi: 10.1002/art.1780370709. [DOI] [PubMed] [Google Scholar]
- Braun J., Bollow M., Neure L., Seipelt E., Seyrekbasan F., Herbst H., Eggens U., Distler A., Sieper J. Use of immunohistologic and in situ hybridization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arthritis Rheum. 1995 Apr;38(4):499–505. doi: 10.1002/art.1780380407. [DOI] [PubMed] [Google Scholar]
- Braun J., Bollow M., Remlinger G., Eggens U., Rudwaleit M., Distler A., Sieper J. Prevalence of spondylarthropathies in HLA-B27 positive and negative blood donors. Arthritis Rheum. 1998 Jan;41(1):58–67. doi: 10.1002/1529-0131(199801)41:1<58::AID-ART8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Braun J., Khan M. A., Sieper J. Enthesitis and ankylosis in spondyloarthropathy: what is the target of the immune response? Ann Rheum Dis. 2000 Dec;59(12):985–994. doi: 10.1136/ard.59.12.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewerton D. A., Hart F. D., Nicholls A., Caffrey M., James D. C., Sturrock R. D. Ankylosing spondylitis and HL-A 27. Lancet. 1973 Apr 28;1(7809):904–907. doi: 10.1016/s0140-6736(73)91360-3. [DOI] [PubMed] [Google Scholar]
- Brooks J. M., Murray R. J., Thomas W. A., Kurilla M. G., Rickinson A. B. Different HLA-B27 subtypes present the same immunodominant Epstein-Barr virus peptide. J Exp Med. 1993 Sep 1;178(3):879–887. doi: 10.1084/jem.178.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzás E. I., Brennan F. R., Mikecz K., Garzó M., Negroiu G., Holló K., Cs-Szabó G., Pintye E., Glant T. T. A proteoglycan (aggrecan)-specific T cell hybridoma induces arthritis in BALB/c mice. J Immunol. 1995 Sep 1;155(5):2679–2687. [PubMed] [Google Scholar]
- Buzás Edit I., Mikecz Katalin, Glant Tibor T. Aggrecan: A Target Molecule of Autoimmune Reactions. Pathol Oncol Res. 1996;2(4):219–228. doi: 10.1007/BF02904814. [DOI] [PubMed] [Google Scholar]
- Fiorillo M. T., Maragno M., Butler R., Dupuis M. L., Sorrentino R. CD8(+) T-cell autoreactivity to an HLA-B27-restricted self-epitope correlates with ankylosing spondylitis. J Clin Invest. 2000 Jul;106(1):47–53. doi: 10.1172/JCI9295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François R. J., Gardner D. L., Degrave E. J., Bywaters E. G. Histopathologic evidence that sacroiliitis in ankylosing spondylitis is not merely enthesitis. Arthritis Rheum. 2000 Sep;43(9):2011–2024. doi: 10.1002/1529-0131(200009)43:9<2011::AID-ANR12>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Glant T., Csongor J., Szücs T. Immunopathologic role of proteoglycan antigens in rheumatoid joint disease. Scand J Immunol. 1980;11(3):247–252. doi: 10.1111/j.1365-3083.1980.tb00232.x. [DOI] [PubMed] [Google Scholar]
- Guerassimov A., Duffy C., Zhang Y., Banerjee S., Leroux J. Y., Reimann A., Webber C., Delaunay N., Vipparti V., Ronbeck L. Immunity to cartilage link protein in patients with juvenile rheumatoid arthritis. J Rheumatol. 1997 May;24(5):959–964. [PubMed] [Google Scholar]
- Guerassimov A., Zhang Y., Banerjee S., Cartman A., Webber C., Esdaile J., Fitzcharles M. A., Poole A. R. Autoimmunity to cartilage link protein in patients with rheumatoid arthritis and ankylosing spondylitis. J Rheumatol. 1998 Aug;25(8):1480–1484. [PubMed] [Google Scholar]
- Guerassimov A., Zhang Y., Cartman A., Rosenberg L. C., Esdaile J., Fitzcharles M. A., Poole A. R. Immune responses to cartilage link protein and the G1 domain of proteoglycan aggrecan in patients with osteoarthritis. Arthritis Rheum. 1999 Mar;42(3):527–533. doi: 10.1002/1529-0131(199904)42:3<527::AID-ANR18>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Hermann E., Fleischer B., Meyer zum Büschenfelde K. H. Bacteria-specific cytotoxic CD8+ T cells: a missing link in the pathogenesis of the HLA-B27-associated spondylarthropathies. Ann Med. 1994 Oct;26(5):365–369. doi: 10.3109/07853899409148352. [DOI] [PubMed] [Google Scholar]
- Hermann E., Yu D. T., Meyer zum Büschenfelde K. H., Fleischer B. HLA-B27-restricted CD8 T cells derived from synovial fluids of patients with reactive arthritis and ankylosing spondylitis. Lancet. 1993 Sep 11;342(8872):646–650. doi: 10.1016/0140-6736(93)91760-j. [DOI] [PubMed] [Google Scholar]
- Kern F., Faulhaber N., Frömmel C., Khatamzas E., Prösch S., Schönemann C., Kretzschmar I., Volkmer-Engert R., Volk H. D., Reinke P. Analysis of CD8 T cell reactivity to cytomegalovirus using protein-spanning pools of overlapping pentadecapeptides. Eur J Immunol. 2000 Jun;30(6):1676–1682. doi: 10.1002/1521-4141(200006)30:6<1676::AID-IMMU1676>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Kern F., Surel I. P., Brock C., Freistedt B., Radtke H., Scheffold A., Blasczyk R., Reinke P., Schneider-Mergener J., Radbruch A. T-cell epitope mapping by flow cytometry. Nat Med. 1998 Aug;4(8):975–978. doi: 10.1038/nm0898-975. [DOI] [PubMed] [Google Scholar]
- Kollnberger Simon, Bird Lucy, Sun Mei-Yi, Retiere Christelle, Braud Veronique M., McMichael Andrew, Bowness Paul. Cell-surface expression and immune receptor recognition of HLA-B27 homodimers. Arthritis Rheum. 2002 Nov;46(11):2972–2982. doi: 10.1002/art.10605. [DOI] [PubMed] [Google Scholar]
- Kuon W., Holzhütter H. G., Appel H., Grolms M., Kollnberger S., Traeder A., Henklein P., Weiss E., Thiel A., Lauster R. Identification of HLA-B27-restricted peptides from the Chlamydia trachomatis proteome with possible relevance to HLA-B27-associated diseases. J Immunol. 2001 Oct 15;167(8):4738–4746. doi: 10.4049/jimmunol.167.8.4738. [DOI] [PubMed] [Google Scholar]
- Leroux J. Y., Guerassimov A., Cartman A., Delaunay N., Webber C., Rosenberg L. C., Banerjee S., Poole A. R. Immunity to the G1 globular domain of the cartilage proteoglycan aggrecan can induce inflammatory erosive polyarthritis and spondylitis in BALB/c mice but immunity to G1 is inhibited by covalently bound keratan sulfate in vitro and in vivo. J Clin Invest. 1996 Feb 1;97(3):621–632. doi: 10.1172/JCI118458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksymowych W. P. Ankylosing spondylitis--at the interface of bone and cartilage. J Rheumatol. 2000 Oct;27(10):2295–2301. [PubMed] [Google Scholar]
- Manz R., Assenmacher M., Pflüger E., Miltenyi S., Radbruch A. Analysis and sorting of live cells according to secreted molecules, relocated to a cell-surface affinity matrix. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):1921–1925. doi: 10.1073/pnas.92.6.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis R. U., Margolis R. K. Aggrecan-versican-neurocan family proteoglycans. Methods Enzymol. 1994;245:105–126. doi: 10.1016/0076-6879(94)45008-0. [DOI] [PubMed] [Google Scholar]
- McGonagle D., Conaghan P. G., O'Connor P., Gibbon W., Green M., Wakefield R., Ridgway J., Emery P. The relationship between synovitis and bone changes in early untreated rheumatoid arthritis: a controlled magnetic resonance imaging study. Arthritis Rheum. 1999 Aug;42(8):1706–1711. doi: 10.1002/1529-0131(199908)42:8<1706::AID-ANR20>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- McGonagle D., Emery P. Classification of inflammatory arthritis. Lancet. 1999 Feb 20;353(9153):671–671. doi: 10.1016/S0140-6736(05)75464-7. [DOI] [PubMed] [Google Scholar]
- Mear J. P., Schreiber K. L., Münz C., Zhu X., Stevanović S., Rammensee H. G., Rowland-Jones S. L., Colbert R. A. Misfolding of HLA-B27 as a result of its B pocket suggests a novel mechanism for its role in susceptibility to spondyloarthropathies. J Immunol. 1999 Dec 15;163(12):6665–6670. [PubMed] [Google Scholar]
- Mikecz K., Glant T. T., Baron M., Poole A. R. Isolation of proteoglycan-specific T lymphocytes from patients with ankylosing spondylitis. Cell Immunol. 1988 Mar;112(1):55–63. doi: 10.1016/0008-8749(88)90275-4. [DOI] [PubMed] [Google Scholar]
- Poole A. R. The histopathology of ankylosing spondylitis: are there unifying hypotheses? Am J Med Sci. 1998 Oct;316(4):228–233. doi: 10.1097/00000441-199810000-00002. [DOI] [PubMed] [Google Scholar]
- Sieper J., Kingsley G. Recent advances in the pathogenesis of reactive arthritis. Immunol Today. 1996 Apr;17(4):160–163. doi: 10.1016/0167-5699(96)80612-8. [DOI] [PubMed] [Google Scholar]
- Thiel A., Wu P., Lauster R., Braun J., Radbruch A., Sieper J. Analysis of the antigen-specific T cell response in reactive arthritis by flow cytometry. Arthritis Rheum. 2000 Dec;43(12):2834–2842. doi: 10.1002/1529-0131(200012)43:12<2834::AID-ANR25>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Ugrinovic S., Mertz A., Wu P., Braun J., Sieper J. A single nonamer from the Yersinia 60-kDa heat shock protein is the target of HLA-B27-restricted CTL response in Yersinia-induced reactive arthritis. J Immunol. 1997 Dec 1;159(11):5715–5723. [PubMed] [Google Scholar]
- Waldrop S. L., Pitcher C. J., Peterson D. M., Maino V. C., Picker L. J. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J Clin Invest. 1997 Apr 1;99(7):1739–1750. doi: 10.1172/JCI119338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Guerassimov A., Leroux J. Y., Cartman A., Webber C., Lalic R., de Miguel E., Rosenberg L. C., Poole A. R. Arthritis induced by proteoglycan aggrecan G1 domain in BALB/c mice. Evidence for t cell involvement and the immunosuppressive influence of keratan sulfate on recognition of t and b cell epitopes. J Clin Invest. 1998 Apr 15;101(8):1678–1686. doi: 10.1172/JCI1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J., Zhang Y., Thiel A., Rudwaleit M., Shi S-L, Radbruch A., Poole R., Braun J., Sieper J. Predominant cellular immune response to the cartilage autoantigenic G1 aggrecan in ankylosing spondylitis and rheumatoid arthritis. Rheumatology (Oxford) 2003 Feb 28;42(7):846–855. doi: 10.1093/rheumatology/keg230. [DOI] [PubMed] [Google Scholar]
- van der Linden S., Valkenburg H. A., Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984 Apr;27(4):361–368. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]