Abstract
Background: Rheumatoid arthritis (RA) synovium is characterised by enhanced NF-κB activity and proinflammatory cytokines. Cryopyrin (CIAS-1, NALP-3, PYPAF-1) has been shown to regulate NF-κB and caspase-1 activation.
Objective: To study the expression of cryopyrin, its effector molecule ASC, and its putative antagonist pyrin in RA and osteoarthritis (OA) synovium, and the main two cellular constituents of synovial lining, cultured fibroblast-like synoviocytes (FLS) and macrophages.
Methods: FLS and macrophages were cultured in the presence of inflammatory mediators. Real time polymerase chain reaction was used to quantify message levels in synovial biopsy specimens and cells. In situ hybridisation was employed to localise expression of cryopyrin mRNA.
Results: Cryopyrin mRNA was raised in RA synovium and detected in both lining and sublining regions. FLS from RA and OA tissue expressed low baseline levels of cryopyrin transcripts that were induced by tumour necrosis factor α (TNFα). In contrast, macrophages differentiated in vitro expressed relatively high cryopyrin levels, which were further induced by TNFα, but not by interleukin 1ß. ASC mRNA levels were comparable in RA and OA tissue, FLS, and macrophages, and were depressed by TNFα in macrophages. Pyrin expression was higher in RA synovium than in OA tissue, and virtually undetectable in FLS but high in macrophages where it was unchanged by TNFα treatment.
Conclusion: These results suggest that enhanced cryopyrin levels in RA synovium are due to a greater numbers of tissue macrophages, and demonstrate transcriptional regulation of cryopyrin in a chronic inflammatory disease.
Full Text
The Full Text of this article is available as a PDF (147.0 KB).
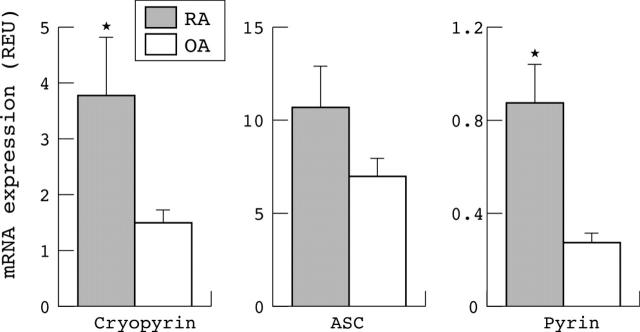
Figure 1.
Expression of cryopyrin, ASC, and pyrin mRNA in synovial tissue from patients with RA and OA undergoing joint replacement surgery, as determined by real time qPCR. REU (relative expression units) data are standardised to known dilutions of PBMC cDNA and normalised by GAPDH to control for cellularity. Results are from 14 patients for each group. *p<0.05 by Student's t test on log transformed data. Both the cryopyrin and pyrin message was significantly raised in RA.
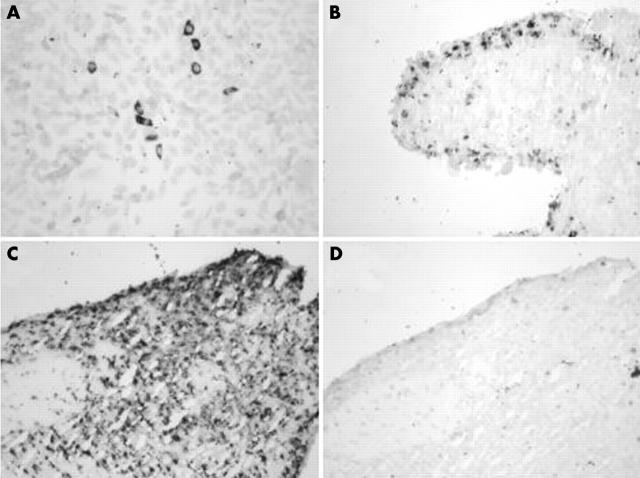
Figure 2.
Detection of cryopyrin mRNA in synovial tissue by in situ hybridisation. (A) CHO cells transfected with a cryopyrin-GFP fusion construct and hybridised with cryopyrin antisense probe to demonstrate its specificity. No signal was obtained with cryopyrin sense probe (not shown). (B) RA synovium hybridised with MMP-3 antisense probe shows typical lining distribution. MMP-3 sense probe did not hybridise (not shown). (C) Cryopyrin mRNA is expressed in both lining and sublining in RA synovium as shown by the antisense probe. (D) The cryopyrin sense probe did not hybridise to RA synovium (serial section, same area as in C).
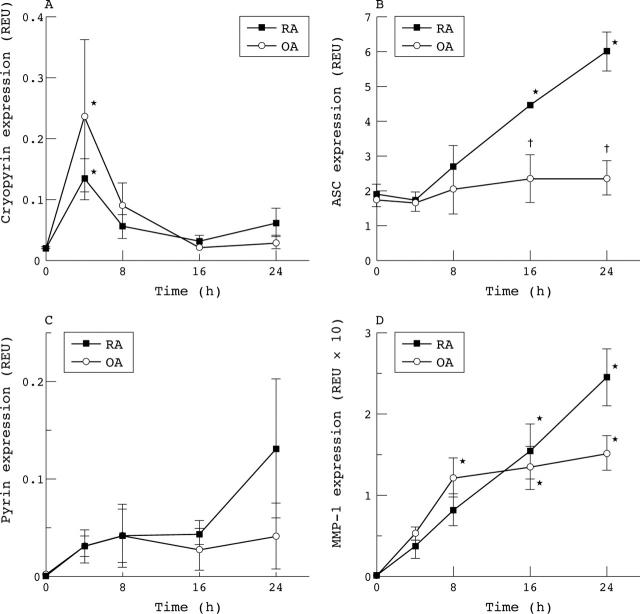
Figure 3.
Expression of cryopyrin (A), ASC (B), and pyrin (C) mRNA in FLS after stimulation with TNFα (50 ng/ml) at time zero. Results are from real time qPCR studies of three cell lines each and are expressed as GAPDH normalised REU as in fig 1 legend. * p<0.05 from time zero and †p<0.05 between RA and OA, by repeated measures ANOVA. (A) Expression of cryopyrin was transiently raised in both RA and OA FLS. (B) ASC was up regulated in RA but not OA FLS. (C) Pyrin was not detectable at baseline but was induced. (D) As a control, continuous MMP-1 expression demonstrates that FLS were fully activated.
Figure 4.
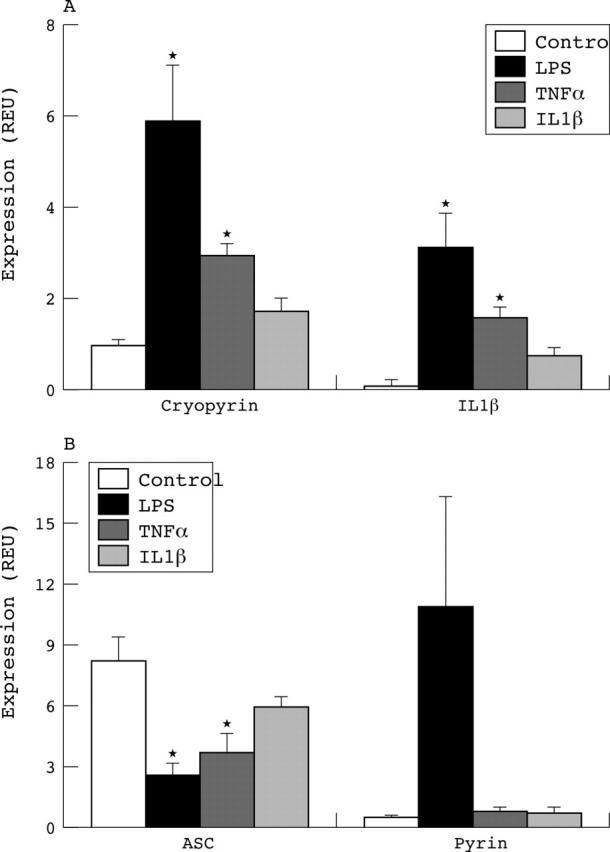
Expression of (A) cryopyrin, (B) ASC and pyrin mRNA in macrophage-like cells differentiated from healthy donor monocytes by 5 day culture in M-CSF and serum. Cells were treated with LPS (10 ng/ml), TNFα (50 ng/ml), or IL1ß (2 ng/ml) for 18 hours before real time qPCR analysis. Results are from three donors and expressed as GAPDH normalised REU as in the legend to fig 1. *p<0.05 compared with control, untreated cells by single ANOVA, and Dunnett's post hoc test. The cryopyrin message was induced by LPS and TNFα, whereas ASC message levels decreased under the same conditions. Pyrin was only raised by LPS but not by either cytokine. The IL1ß message was significantly up regulated by LPS and TNFα (A), whereas IL1ß itself up regulated MMP-9 expression (see text), indicating macrophage activation in all cases.
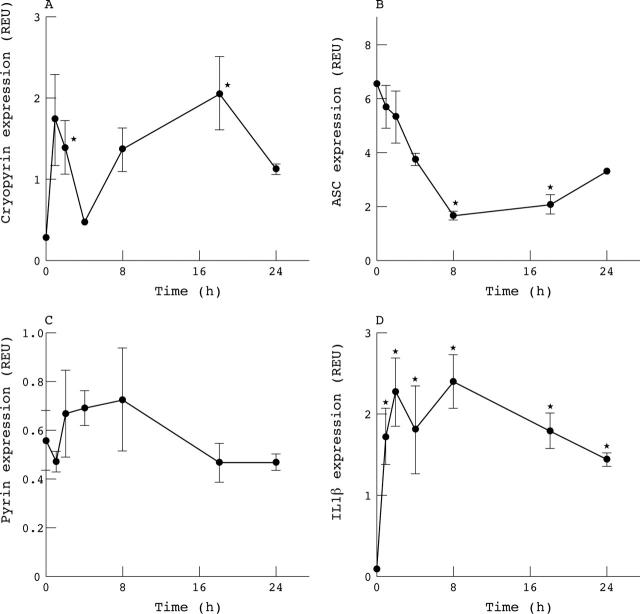
Figure 5.
Time course of mRNA expression in monocyte derived, macrophage-like cells in response to treatment with TNFα (50 ng/ml). Results are from three donors and expressed as GAPDH normalised REU as in the legend to fig.1. *p<0.05 from time zero by repeated measures ANOVA. (A) Cryopyrin message was induced in two phases, whereas (B) ASC levels fell. (C) Pyrin remained unaltered. (D) Cells were fully activated as indicated by continuous expression of IL1ß.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agostini Laetitia, Martinon Fabio, Burns Kimberly, McDermott Michael F., Hawkins Philip N., Tschopp Jürg. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004 Mar;20(3):319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- Aksentijevich Ivona, Nowak Miroslawa, Mallah Mustapha, Chae Jae Jin, Watford Wendy T., Hofmann Sigrun R., Stein Leonard, Russo Ricardo, Goldsmith Donald, Dent Peter. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 2002 Dec;46(12):3340–3348. doi: 10.1002/art.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvaro-Gracia J. M., Zvaifler N. J., Brown C. B., Kaushansky K., Firestein G. S. Cytokines in chronic inflammatory arthritis. VI. Analysis of the synovial cells involved in granulocyte-macrophage colony-stimulating factor production and gene expression in rheumatoid arthritis and its regulation by IL-1 and tumor necrosis factor-alpha. J Immunol. 1991 May 15;146(10):3365–3371. [PubMed] [Google Scholar]
- Asahara H., Asanuma M., Ogawa N., Nishibayashi S., Inoue H. High DNA-binding activity of transcription factor NF-kappa B in synovial membranes of patients with rheumatoid arthritis. Biochem Mol Biol Int. 1995 Nov;37(5):827–832. [PubMed] [Google Scholar]
- Aupperle K., Bennett B., Han Z., Boyle D., Manning A., Firestein G. NF-kappa B regulation by I kappa B kinase-2 in rheumatoid arthritis synoviocytes. J Immunol. 2001 Feb 15;166(4):2705–2711. doi: 10.4049/jimmunol.166.4.2705. [DOI] [PubMed] [Google Scholar]
- Bondeson J., Foxwell B., Brennan F., Feldmann M. Defining therapeutic targets by using adenovirus: blocking NF-kappaB inhibits both inflammatory and destructive mechanisms in rheumatoid synovium but spares anti-inflammatory mediators. Proc Natl Acad Sci U S A. 1999 May 11;96(10):5668–5673. doi: 10.1073/pnas.96.10.5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle David L., Rosengren Sanna, Bugbee William, Kavanaugh Arthur, Firestein Gary S. Quantitative biomarker analysis of synovial gene expression by real-time PCR. Arthritis Res Ther. 2003 Oct 8;5(6):R352–R360. doi: 10.1186/ar1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae Jae Jin, Komarow Hirsh D., Cheng Jun, Wood Geryl, Raben Nina, Liu P. Paul, Kastner Daniel L. Targeted disruption of pyrin, the FMF protein, causes heightened sensitivity to endotoxin and a defect in macrophage apoptosis. Mol Cell. 2003 Mar;11(3):591–604. doi: 10.1016/s1097-2765(03)00056-x. [DOI] [PubMed] [Google Scholar]
- Dodé Catherine, Le Dû Nathalie, Cuisset Laurence, Letourneur Frank, Berthelot Jean-Marie, Vaudour Gérard, Meyrier Alain, Watts Richard A., Scott David G. I., Nicholls Anne. New mutations of CIAS1 that are responsible for Muckle-Wells syndrome and familial cold urticaria: a novel mutation underlies both syndromes. Am J Hum Genet. 2002 Apr 25;70(6):1498–1506. doi: 10.1086/340786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowds Theresa A., Masumoto Junya, Chen Felicia F., Ogura Yasunori, Inohara Naohiro, Núez Gabriel. Regulation of cryopyrin/Pypaf1 signaling by pyrin, the familial Mediterranean fever gene product. Biochem Biophys Res Commun. 2003 Mar 14;302(3):575–580. doi: 10.1016/s0006-291x(03)00221-3. [DOI] [PubMed] [Google Scholar]
- Dowds Theresa A., Masumoto Junya, Zhu Li, Inohara Naohiro, Núez Gabriel. Cryopyrin-induced interleukin 1beta secretion in monocytic cells: enhanced activity of disease-associated mutants and requirement for ASC. J Biol Chem. 2004 Mar 12;279(21):21924–21928. doi: 10.1074/jbc.M401178200. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Paine M. M. Stromelysin and tissue inhibitor of metalloproteinases gene expression in rheumatoid arthritis synovium. Am J Pathol. 1992 Jun;140(6):1309–1314. [PMC free article] [PubMed] [Google Scholar]
- Firestein Gary S., Zvaifler Nathan J. How important are T cells in chronic rheumatoid synovitis?: II. T cell-independent mechanisms from beginning to end. Arthritis Rheum. 2002 Feb;46(2):298–308. doi: 10.1002/art.502. [DOI] [PubMed] [Google Scholar]
- Grenier Jill M., Wang Lin, Manji Gulam A., Huang Waan Jeng, Al-Garawi Amal, Kelly Roxanne, Carlson Adam, Merriam Sarah, Lora Jose M., Briskin Michael. Functional screening of five PYPAF family members identifies PYPAF5 as a novel regulator of NF-kappaB and caspase-1. FEBS Lett. 2002 Oct 23;530(1-3):73–78. doi: 10.1016/s0014-5793(02)03416-6. [DOI] [PubMed] [Google Scholar]
- Han Z., Boyle D. L., Manning A. M., Firestein G. S. AP-1 and NF-kappaB regulation in rheumatoid arthritis and murine collagen-induced arthritis. Autoimmunity. 1998;28(4):197–208. doi: 10.3109/08916939808995367. [DOI] [PubMed] [Google Scholar]
- Harton Jonathan A., Linhoff Michael W., Zhang Jinghua, Ting Jenny P-Y. Cutting edge: CATERPILLER: a large family of mammalian genes containing CARD, pyrin, nucleotide-binding, and leucine-rich repeat domains. J Immunol. 2002 Oct 15;169(8):4088–4093. doi: 10.4049/jimmunol.169.8.4088. [DOI] [PubMed] [Google Scholar]
- Hoffman H. M., Mueller J. L., Broide D. H., Wanderer A. A., Kolodner R. D. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001 Nov;29(3):301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman H. M., Wanderer A. A., Broide D. H. Familial cold autoinflammatory syndrome: phenotype and genotype of an autosomal dominant periodic fever. J Allergy Clin Immunol. 2001 Oct;108(4):615–620. doi: 10.1067/mai.2001.118790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull Keith M., Shoham Nitza, Chae Jae Jin, Aksentijevich Ivona, Kastner Daniel L. The expanding spectrum of systemic autoinflammatory disorders and their rheumatic manifestations. Curr Opin Rheumatol. 2003 Jan;15(1):61–69. doi: 10.1097/00002281-200301000-00011. [DOI] [PubMed] [Google Scholar]
- Inohara Naohiro, Nuñez Gabriel. NODs: intracellular proteins involved in inflammation and apoptosis. Nat Rev Immunol. 2003 May;3(5):371–382. doi: 10.1038/nri1086. [DOI] [PubMed] [Google Scholar]
- Manji Gulam A., Wang Lin, Geddes Brad J., Brown Melissa, Merriam Sarah, Al-Garawi Amal, Mak Simona, Lora Jose M., Briskin Michael, Jurman Mark. PYPAF1, a PYRIN-containing Apaf1-like protein that assembles with ASC and regulates activation of NF-kappa B. J Biol Chem. 2002 Jan 10;277(13):11570–11575. doi: 10.1074/jbc.M112208200. [DOI] [PubMed] [Google Scholar]
- Marok R., Winyard P. G., Coumbe A., Kus M. L., Gaffney K., Blades S., Mapp P. I., Morris C. J., Blake D. R., Kaltschmidt C. Activation of the transcription factor nuclear factor-kappaB in human inflamed synovial tissue. Arthritis Rheum. 1996 Apr;39(4):583–591. doi: 10.1002/art.1780390407. [DOI] [PubMed] [Google Scholar]
- Martinon Fabio, Burns Kimberly, Tschopp Jürg. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002 Aug;10(2):417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Masumoto J., Taniguchi S., Nakayama J., Shiohara M., Hidaka E., Katsuyama T., Murase S., Sagara J. Expression of apoptosis-associated speck-like protein containing a caspase recruitment domain, a pyrin N-terminal homology domain-containing protein, in normal human tissues. J Histochem Cytochem. 2001 Oct;49(10):1269–1275. doi: 10.1177/002215540104901009. [DOI] [PubMed] [Google Scholar]
- Masumoto Junya, Dowds Theresa A., Schaner Philip, Chen Felicia F., Ogura Yasunori, Li Mu, Zhu Li, Katsuyama Tsutomu, Sagara Junji, Taniguchi Shun'ichiro. ASC is an activating adaptor for NF-kappa B and caspase-8-dependent apoptosis. Biochem Biophys Res Commun. 2003 Mar 28;303(1):69–73. doi: 10.1016/s0006-291x(03)00309-7. [DOI] [PubMed] [Google Scholar]
- Matzner Y., Abedat S., Shapiro E., Eisenberg S., Bar-Gil-Shitrit A., Stepensky P., Calco S., Azar Y., Urieli-Shoval S. Expression of the familial Mediterranean fever gene and activity of the C5a inhibitor in human primary fibroblast cultures. Blood. 2000 Jul 15;96(2):727–731. [PubMed] [Google Scholar]
- McDermott Michael F. Genetic clues to understanding periodic fevers, and possible therapies. Trends Mol Med. 2002 Dec;8(12):550–554. doi: 10.1016/s1471-4914(02)02425-5. [DOI] [PubMed] [Google Scholar]
- Stehlik Christian, Fiorentino Loredana, Dorfleutner Andrea, Bruey Jean-Marie, Ariza Eugenia M., Sagara Junji, Reed John C. The PAAD/PYRIN-family protein ASC is a dual regulator of a conserved step in nuclear factor kappaB activation pathways. J Exp Med. 2002 Dec 16;196(12):1605–1615. doi: 10.1084/jem.20021552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehlik Christian, Lee Sug Hyung, Dorfleutner Andrea, Stassinopoulos Angela, Sagara Junji, Reed John C. Apoptosis-associated speck-like protein containing a caspase recruitment domain is a regulator of procaspase-1 activation. J Immunol. 2003 Dec 1;171(11):6154–6163. doi: 10.4049/jimmunol.171.11.6154. [DOI] [PubMed] [Google Scholar]
- Tidow N., Chen X., Müller C., Kawano S., Gombart A. F., Fischel-Ghodsian N., Koeffler H. P. Hematopoietic-specific expression of MEFV, the gene mutated in familial Mediterranean fever, and subcellular localization of its corresponding protein, pyrin. Blood. 2000 Feb 15;95(4):1451–1455. [PubMed] [Google Scholar]
- Yamanishi Yuji, Boyle David L., Rosengren Sanna, Green Douglas R., Zvaifler Nathan J., Firestein Gary S. Regional analysis of p53 mutations in rheumatoid arthritis synovium. Proc Natl Acad Sci U S A. 2002 Jul 15;99(15):10025–10030. doi: 10.1073/pnas.152333199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. A., Lowe L. D., Clark S. C. Comparison of the effects of IL-3, granulocyte-macrophage colony-stimulating factor, and macrophage colony-stimulating factor in supporting monocyte differentiation in culture. Analysis of macrophage antibody-dependent cellular cytotoxicity. J Immunol. 1990 Jul 15;145(2):607–615. [PubMed] [Google Scholar]