Abstract
Background: MMP-13 is one of the most important metalloproteases (MMP) involved in osteoarthritis. Licofelone, a novel dual inhibitor of cyclo-oxygenases (COX) and 5-lipoxygenase (5-LOX), can modulate MMP-13 production in human osteoarthritis chondrocytes.
Objective: To evaluate the impact of licofelone on MMP-13 expression/production, promoter, and major MAP kinase signalling pathways and transcription factors.
Methods: Human osteoarthritis chondrocytes were stimulated by interleukin 1ß (IL1ß) and treated with or without: licofelone (0.3, 1, or 3 µg/ml); NS-398 (10 µM; a specific COX-2 inhibitor); or BayX-1005 (10 µM; a specific 5-LOX inhibitor). MMP-13 synthesis was determined by specific enzyme linked immunosorbent assay, and expression by real time polymerase chain reaction. The effect of licofelone on the MMP-13 promoter was studied through transient transfection; dexamethasone (10–7 M) was used as comparison. The effect on IL1ß induced MMP-13 signalling pathways was determined using specific ELISA for phosphorylated MAP kinases and transcription factors.
Results: Licofelone dose dependently inhibited the IL1ß stimulated production and expression of MMP-13. NS-398 and BayX-1005 had very little effect. Licofelone also inhibited MMP-13 transcription on each of the promoter constructs used. The licofelone inhibition was comparable to that obtained with dexamethasone. Licofelone had no effect on phosphorylated p44/42 or JNK1/2; however, it decreased phosphorylated c-jun and inhibited phosphorylated p38, CREB, and AP-1 activity.
Conclusions: Licofelone inhibited MMP-13 production under proinflammatory conditions on human osteoarthritis chondrocytes, through inhibition of the p38/AP-1 pathway and the transcription factor CREB. This may explain some of the mechanisms whereby licofelone exerts its positive effect on osteoarthritic changes.
Full Text
The Full Text of this article is available as a PDF (115.8 KB).
Figure 1.
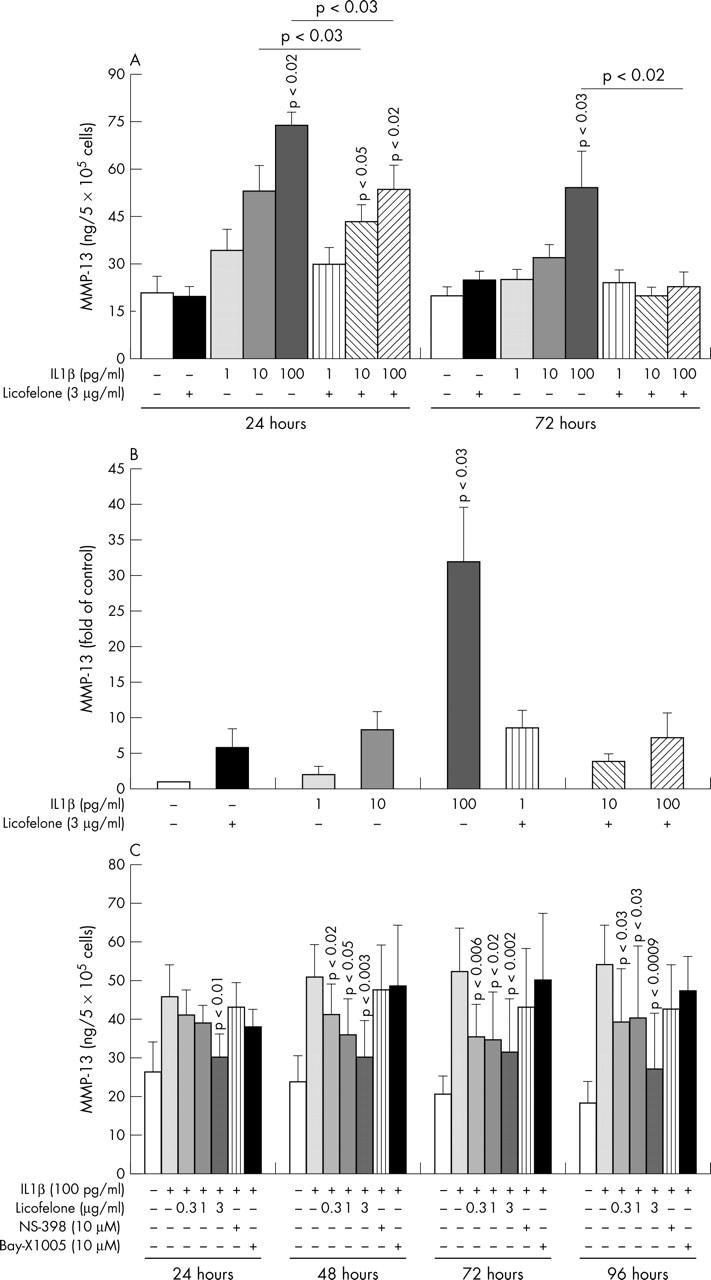
Interleukin (IL) 1ß dose and time response study of matrix metalloprotease 13 (MMP-13) production (A) and expression (B) in the presence or absence of licofelone. Human osteoarthritis chondrocytes were incubated for (A) 24 or 72 hours, or (B) 24 hours with or without 1–100 pg/ml IL1ß or 3 µg/ml licofelone. MMP-13 production was determined in the conditioned medium using a specific enzyme linked immunosorbent assay (ELISA) (A), and MMP-13 expression was determined by real time polymerase chain reaction (B). (C) Licofelone dose and time response study of MMP-13 production. Human osteoarthritis chondrocytes were incubated for 24 to 96 hours in the absence or presence of 100 pg/ml IL1ß and 0.3, 1.0, or 3.0 µg/ml licofelone or NS-398 at 10 µM and Bay-X-1005 at 10 µM. MMP-13 production was determined in the conditioned medium using a specific ELISA. Data are expressed as means of (A) four, (B) six to seven, and (C) four independent experiments. Error bars = SEM; p values indicate significant differences from the respective controls.
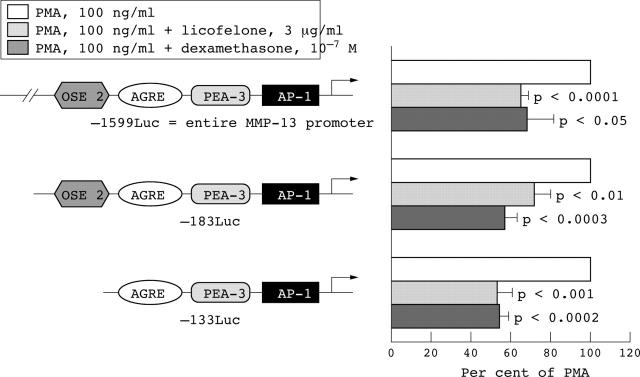
Figure 2.
Functional analysis of the matrix metalloprotease 13 (MMP-13) promoter in human osteoarthritis chondrocytes. Three different constructs of the MMP-13 promoter fused to a luciferase reporter are shown in schematic representation (left). Transfection was carried out in the presence of 100 ng/ml PMA with or without licofelone at 3 µg/ml or dexamethasone at 10–7 M. The cells were also co-transfected with pCMV-ß-galactosidase. Data are expressed as the mean percentage of six to seven independent experiments. Error bars = SEM; p values indicate significant difference from the control.
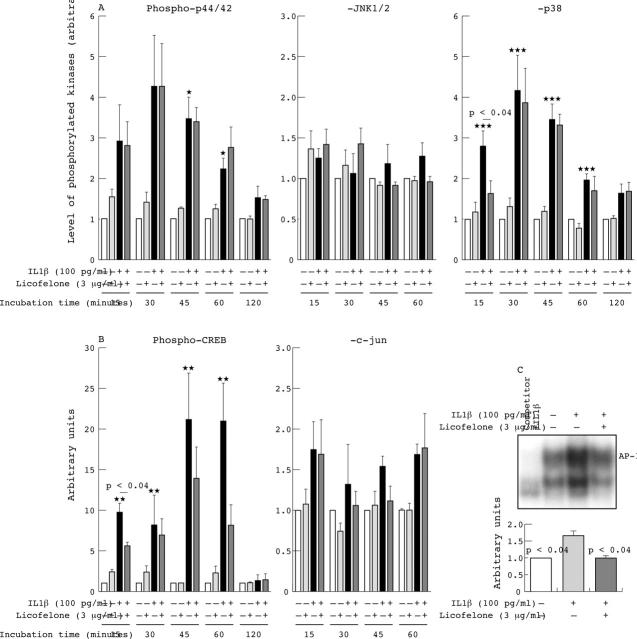
Figure 3.
Effect of licofelone on cell signalling pathways. (A) Phosphorylated forms of p44/42, JNK1/2, and p38. (B) Phosphorylated forms of CREB and c-jun. (C) AP-1 data from the electrophoretic mobility shift assay (EMSA). Specificity of the EMSA binding was assayed through competition of the oligonucleotides with 200-fold of excess unlabelled AP-1 oligonucleotides (competitor) in the presence of the nuclear extract of interleukin (IL) 1ß (100 pg/ml) stimulated chondrocytes. The top panel is a representative EMSA and the lower panel is a histogram of the independent experiments. Human osteoarthritis chondrocytes were incubated for (A) and (B) 15 to 120 minutes or (C) 24 hours with or without 100 pg/ml IL1ß in the presence or absence of 3 µg/ml licofelone. Data are calculated as time-fold expression of the unstimulated control, which was given the arbitrary unit of 1. Data are expressed as the mean of four independent experiments for (A) and (B), and three for (C). Error bars = SEM; p values indicate significant differences. Significant differences between unstimulated and IL1ß stimulated cells were: *p<0.04 for the phosphorylated p44/42 at 45 to 60 minutes; **p<0.03 for phosphorylated CREB at 15 to 60 minutes; and ***p<0.01 for phosphorylated p38 at 15 to 60 minutes. Statistical differences between IL1ß induced phosphorylated p38 and CREB were as indicated in the figure.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman R., Asch E., Bloch D., Bole G., Borenstein D., Brandt K., Christy W., Cooke T. D., Greenwald R., Hochberg M. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986 Aug;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- Angel P., Karin M. Specific members of the Jun protein family regulate collagenase expression in response to various extracellular stimuli. Matrix Suppl. 1992;1:156–164. [PubMed] [Google Scholar]
- Asiedu C. K., Scotto L., Assoian R. K., Ehrlich M. Binding of AP-1/CREB proteins and of MDBP to contiguous sites downstream of the human TGF-beta 1 gene. Biochim Biophys Acta. 1994 Sep 13;1219(1):55–63. doi: 10.1016/0167-4781(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Benbow U., Brinckerhoff C. E. The AP-1 site and MMP gene regulation: what is all the fuss about? Matrix Biol. 1997 Mar;15(8-9):519–526. doi: 10.1016/s0945-053x(97)90026-3. [DOI] [PubMed] [Google Scholar]
- Benderdour Mohamed, Tardif Ginette, Pelletier Jean-Pierre, Di Battista John A., Reboul Pascal, Ranger Pierre, Martel-Pelletier Johanne. Interleukin 17 (IL-17) induces collagenase-3 production in human osteoarthritic chondrocytes via AP-1 dependent activation: differential activation of AP-1 members by IL-17 and IL-1beta. J Rheumatol. 2002 Jun;29(6):1262–1272. [PubMed] [Google Scholar]
- Benderdour Mohamed, Tardif Ginette, Pelletier Jean-Pierre, Dupuis Martine, Geng Changshan, Martel-Pelletier Johanne. A novel negative regulatory element in the human collagenase-3 proximal promoter region. Biochem Biophys Res Commun. 2002 Mar 15;291(5):1151–1159. doi: 10.1006/bbrc.2002.6580. [DOI] [PubMed] [Google Scholar]
- Boileau Christelle, Martel-Pelletier Johanne, Jouzeau Jean-Yves, Netter Patrick, Moldovan Florina, Laufer Stefan, Tries Susanne, Pelletier Jean-Pierre. Licofelone (ML-3000), a dual inhibitor of 5-lipoxygenase and cyclooxygenase, reduces the level of cartilage chondrocyte death in vivo in experimental dog osteoarthritis: inhibition of pro-apoptotic factors. J Rheumatol. 2002 Jul;29(7):1446–1453. [PubMed] [Google Scholar]
- Caron J. P., Tardif G., Martel-Pelletier J., DiBattista J. A., Geng C., Pelletier J. P. Modulation of matrix metalloprotease 13 (collagenase 3) gene expression in equine chondrocytes by interleukin 1 and corticosteroids. Am J Vet Res. 1996 Nov;57(11):1631–1634. [PubMed] [Google Scholar]
- Celotti F., Laufer S. Anti-inflammatory drugs: new multitarget compounds to face an old problem. The dual inhibition concept. Pharmacol Res. 2001 May;43(5):429–436. doi: 10.1006/phrs.2000.0784. [DOI] [PubMed] [Google Scholar]
- Crew T. E., Elder D. J., Paraskeva C. A cyclooxygenase-2 (COX-2) selective non-steroidal anti-inflammatory drug enhances the growth inhibitory effect of butyrate in colorectal carcinoma cells expressing COX-2 protein: regulation of COX-2 by butyrate. Carcinogenesis. 2000 Jan;21(1):69–77. doi: 10.1093/carcin/21.1.69. [DOI] [PubMed] [Google Scholar]
- Diamond M. I., Miner J. N., Yoshinaga S. K., Yamamoto K. R. Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science. 1990 Sep 14;249(4974):1266–1272. doi: 10.1126/science.2119054. [DOI] [PubMed] [Google Scholar]
- Fahmi H., Pelletier J-P, Di Battista J. A., Cheung H. S., Fernandes J. C., Martel-Pelletier J. Peroxisome proliferator-activated receptor gamma activators inhibit MMP-1 production in human synovial fibroblasts likely by reducing the binding of the activator protein 1. Osteoarthritis Cartilage. 2002 Feb;10(2):100–108. doi: 10.1053/joca.2001.0485. [DOI] [PubMed] [Google Scholar]
- Fernandes J. C., Martel-Pelletier J., Lascau-Coman V., Moldovan F., Jovanovic D., Raynauld J. P., Pelletier J. P. Collagenase-1 and collagenase-3 synthesis in normal and early experimental osteoarthritic canine cartilage: an immunohistochemical study. J Rheumatol. 1998 Aug;25(8):1585–1594. [PubMed] [Google Scholar]
- Gay R. E., Neidhart M., Pataky F., Tries S., Laufer S., Gay S. Dual inhibition of 5-lipoxygenase and cyclooxygenases 1 and 2 by ML3000 reduces joint destruction in adjuvant arthritis. J Rheumatol. 2001 Sep;28(9):2060–2065. [PubMed] [Google Scholar]
- He Wendy, Pelletier Jean-Pierre, Martel-Pelletier Johanne, Laufer Stefan, Di Battista John A. Synthesis of interleukin 1beta, tumor necrosis factor-alpha, and interstitial collagenase (MMP-1) is eicosanoid dependent in human osteoarthritis synovial membrane explants: interactions with antiinflammatory cytokines. J Rheumatol. 2002 Mar;29(3):546–553. [PubMed] [Google Scholar]
- Hudson N., Balsitis M., Everitt S., Hawkey C. J. Enhanced gastric mucosal leukotriene B4 synthesis in patients taking non-steroidal anti-inflammatory drugs. Gut. 1993 Jun;34(6):742–747. doi: 10.1136/gut.34.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson N., Ala-aho R., Uitto V., Grénman R., Fusenig N. E., López-Otín C., Kähäri V. M. Expression of collagenase-3 (MMP-13) and collagenase-1 (MMP-1) by transformed keratinocytes is dependent on the activity of p38 mitogen-activated protein kinase. J Cell Sci. 2000 Jan;113(Pt 2):227–235. doi: 10.1242/jcs.113.2.227. [DOI] [PubMed] [Google Scholar]
- Johansson N., Saarialho-Kere U., Airola K., Herva R., Nissinen L., Westermarck J., Vuorio E., Heino J., Kähäri V. M. Collagenase-3 (MMP-13) is expressed by hypertrophic chondrocytes, periosteal cells, and osteoblasts during human fetal bone development. Dev Dyn. 1997 Mar;208(3):387–397. doi: 10.1002/(SICI)1097-0177(199703)208:3<387::AID-AJA9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Jovanovic D. V., Fernandes J. C., Martel-Pelletier J., Jolicoeur F. C., Reboul P., Laufer S., Tries S., Pelletier J. P. In vivo dual inhibition of cyclooxygenase and lipoxygenase by ML-3000 reduces the progression of experimental osteoarthritis: suppression of collagenase 1 and interleukin-1beta synthesis. Arthritis Rheum. 2001 Oct;44(10):2320–2330. doi: 10.1002/1529-0131(200110)44:10<2320::aid-art394>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Kageyama Y., Koide Y., Miyamoto S., Yoshida T. O., Inoue T. Leukotrien B4-induced interleukin-1 beta in synovial cells from patients with rheumatoid arthritis. Scand J Rheumatol. 1994;23(3):148–150. doi: 10.3109/03009749409103049. [DOI] [PubMed] [Google Scholar]
- Laufer S., Tries S., Augustin J., Elsässer R., Albrecht W., Guserle R., Algate D. R., Atterson P. R., Munt P. L. Acute and chronic anti-inflammatory properties of [2,2-dimethyl-6-(4- chlorophenyl)-7-phenyl-2,3-dihydro-1H-pyrrolizine-5-yl]-acetic acid. Arzneimittelforschung. 1995 Jan;45(1):27–32. [PubMed] [Google Scholar]
- Laufer S., Zechmeister P., Klein T. Development of an in-vitro test system for the evaluation of cyclooxygenase-2 inhibitors. Inflamm Res. 1999 Mar;48(3):133–138. doi: 10.1007/s000110050436. [DOI] [PubMed] [Google Scholar]
- Mankin H. J., Dorfman H., Lippiello L., Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971 Apr;53(3):523–537. [PubMed] [Google Scholar]
- Martel-Pelletier J., Welsch D. J., Pelletier J. P. Metalloproteases and inhibitors in arthritic diseases. Best Pract Res Clin Rheumatol. 2001 Dec;15(5):805–829. doi: 10.1053/berh.2001.0195. [DOI] [PubMed] [Google Scholar]
- Martel-Pelletier Johanne, Mineau François, Fahmi Hassan, Laufer Stefan, Reboul Pascal, Boileau Christelle, Lavigne Martin, Pelletier Jean-Pierre. Regulation of the expression of 5-lipoxygenase-activating protein/5-lipoxygenase and the synthesis of leukotriene B(4) in osteoarthritic chondrocytes: role of transforming growth factor beta and eicosanoids. Arthritis Rheum. 2004 Dec;50(12):3925–3933. doi: 10.1002/art.20632. [DOI] [PubMed] [Google Scholar]
- Masquilier D., Sassone-Corsi P. Transcriptional cross-talk: nuclear factors CREM and CREB bind to AP-1 sites and inhibit activation by Jun. J Biol Chem. 1992 Nov 5;267(31):22460–22466. [PubMed] [Google Scholar]
- Mengshol J. A., Vincenti M. P., Coon C. I., Barchowsky A., Brinckerhoff C. E. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 2000 Apr;43(4):801–811. doi: 10.1002/1529-0131(200004)43:4<801::AID-ANR10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Moldovan F., Pelletier J. P., Hambor J., Cloutier J. M., Martel-Pelletier J. Collagenase-3 (matrix metalloprotease 13) is preferentially localized in the deep layer of human arthritic cartilage in situ: in vitro mimicking effect by transforming growth factor beta. Arthritis Rheum. 1997 Sep;40(9):1653–1661. doi: 10.1002/art.1780400915. [DOI] [PubMed] [Google Scholar]
- Németh Zoltán H., Leibovich S. Joseph, Deitch Edwin A., Sperlágh Beáta, Virág László, Vizi E. Sylvester, Szabó Csaba, Haskó György. Adenosine stimulates CREB activation in macrophages via a p38 MAPK-mediated mechanism. Biochem Biophys Res Commun. 2003 Dec 26;312(4):883–888. doi: 10.1016/j.bbrc.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Paredes Yosabeth, Massicotte Frédéric, Pelletier Jean-Pierre, Martel-Pelletier Johanne, Laufer Stefan, Lajeunesse Daniel. Study of the role of leukotriene B()4 in abnormal function of human subchondral osteoarthritis osteoblasts: effects of cyclooxygenase and/or 5-lipoxygenase inhibition. Arthritis Rheum. 2002 Jul;46(7):1804–1812. doi: 10.1002/art.10357. [DOI] [PubMed] [Google Scholar]
- Pelletier J. P., Fernandes J. C., Jovanovic D. V., Reboul P., Martel-Pelletier J. Chondrocyte death in experimental osteoarthritis is mediated by MEK 1/2 and p38 pathways: role of cyclooxygenase-2 and inducible nitric oxide synthase. J Rheumatol. 2001 Nov;28(11):2509–2519. [PubMed] [Google Scholar]
- Pelletier Jean-Pierre, Boileau Christelle, Brunet Julie, Boily Martin, Lajeunesse Daniel, Reboul Pascal, Laufer Stefan, Martel-Pelletier Johanne. The inhibition of subchondral bone resorption in the early phase of experimental dog osteoarthritis by licofelone is associated with a reduction in the synthesis of MMP-13 and cathepsin K. Bone. 2004 Mar;34(3):527–538. doi: 10.1016/j.bone.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Rainsford K. D., Ying C., Smith F. Effects of 5-lipoxygenase inhibitors on interleukin production by human synovial tissues in organ culture: comparison with interleukin-1-synthesis inhibitors. J Pharm Pharmacol. 1996 Jan;48(1):46–52. doi: 10.1111/j.2042-7158.1996.tb05875.x. [DOI] [PubMed] [Google Scholar]
- Rainsford K. D., Ying C., Smith F. Selective effects of some 5-lipoxygenase inhibitors on synovial interleukin-1 (IL-1) production compared with IL-1 synthesis inhibitors. Agents Actions. 1993;39(Spec No):C186–C188. doi: 10.1007/BF01972761. [DOI] [PubMed] [Google Scholar]
- Ravanti L., Häkkinen L., Larjava H., Saarialho-Kere U., Foschi M., Han J., Kähäri V. M. Transforming growth factor-beta induces collagenase-3 expression by human gingival fibroblasts via p38 mitogen-activated protein kinase. J Biol Chem. 1999 Dec 24;274(52):37292–37300. doi: 10.1074/jbc.274.52.37292. [DOI] [PubMed] [Google Scholar]
- Reboul P., Pelletier J. P., Tardif G., Cloutier J. M., Martel-Pelletier J. The new collagenase, collagenase-3, is expressed and synthesized by human chondrocytes but not by synoviocytes. A role in osteoarthritis. J Clin Invest. 1996 May 1;97(9):2011–2019. doi: 10.1172/JCI118636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi Sarah A., Weggen Sascha, Eriksen Jason, Golde Todd E., Koo Edward H. The non-cyclooxygenase targets of non-steroidal anti-inflammatory drugs, lipoxygenases, peroxisome proliferator-activated receptor, inhibitor of kappa B kinase, and NF kappa B, do not reduce amyloid beta 42 production. J Biol Chem. 2003 Jun 12;278(34):31825–31830. doi: 10.1074/jbc.M303588200. [DOI] [PubMed] [Google Scholar]
- Ståhle-Bäckdahl M., Sandstedt B., Bruce K., Lindahl A., Jiménez M. G., Vega J. A., López-Otín C. Collagenase-3 (MMP-13) is expressed during human fetal ossification and re-expressed in postnatal bone remodeling and in rheumatoid arthritis. Lab Invest. 1997 May;76(5):717–728. [PubMed] [Google Scholar]
- Tardif G., Pelletier J. P., Dupuis M., Geng C., Cloutier J. M., Martel-Pelletier J. Collagenase 3 production by human osteoarthritic chondrocytes in response to growth factors and cytokines is a function of the physiologic state of the cells. Arthritis Rheum. 1999 Jun;42(6):1147–1158. doi: 10.1002/1529-0131(199906)42:6<1147::AID-ANR11>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Tardif G., Pelletier J. P., Dupuis M., Hambor J. E., Martel-Pelletier J. Cloning, sequencing and characterization of the 5'-flanking region of the human collagenase-3 gene. Biochem J. 1997 Apr 1;323(Pt 1):13–16. doi: 10.1042/bj3230013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif Ginette, Hum David, Pelletier Jean-Pierre, Boileau Christelle, Ranger Pierre, Martel-Pelletier Johanne. Differential gene expression and regulation of the bone morphogenetic protein antagonists follistatin and gremlin in normal and osteoarthritic human chondrocytes and synovial fibroblasts. Arthritis Rheum. 2004 Aug;50(8):2521–2530. doi: 10.1002/art.20441. [DOI] [PubMed] [Google Scholar]
- Tegeder I., Niederberger E., Israr E., Gühring H., Brune K., Euchenhofer C., Grösch S., Geisslinger G. Inhibition of NF-kappaB and AP-1 activation by R- and S-flurbiprofen. FASEB J. 2001 Mar;15(3):595–597. doi: 10.1096/fasebj.15.3.595. [DOI] [PubMed] [Google Scholar]
- Tetlow L. C., Adlam D. J., Woolley D. E. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001 Mar;44(3):585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Tries S., Neupert W., Laufer S. The mechanism of action of the new antiinflammatory compound ML3000: inhibition of 5-LOX and COX-1/2. Inflamm Res. 2002 Mar;51(3):135–143. doi: 10.1007/pl00000285. [DOI] [PubMed] [Google Scholar]
- Vincenti Matthew P., Brinckerhoff Constance E. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2001 Nov 23;4(3):157–164. doi: 10.1186/ar401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg R. H., Willburger R. E., Kleemeyer K. S., Peskar B. A. In vitro release of prostaglandins and leukotrienes from synovial tissue, cartilage, and bone in degenerative joint diseases. Arthritis Rheum. 1993 Oct;36(10):1444–1450. doi: 10.1002/art.1780361017. [DOI] [PubMed] [Google Scholar]
- Yoon Joo-Byoung, Kim Song-Ja, Hwang Sang-Gu, Chang Sunghoe, Kang Shin-Sung, Chun Jang-Soo. Non-steroidal anti-inflammatory drugs inhibit nitric oxide-induced apoptosis and dedifferentiation of articular chondrocytes independent of cyclooxygenase activity. J Biol Chem. 2003 Feb 14;278(17):15319–15325. doi: 10.1074/jbc.M212520200. [DOI] [PubMed] [Google Scholar]