Abstract
Objective: To investigate changes in the levels of circulating cytokines with a focus on the Th1/Th2 balance during and after pregnancy in patients with rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA), and ankylosing spondylitis (AS).
Methods: Plasma and serum samples of 34 pregnant patients, 19 with RA, 6 with JIA, and 9 with AS, and of 30 healthy pregnant women, 20 non-pregnant patients, and 10 non-pregnant healthy women were analysed for levels of interferon γ (IFNγ), interleukin (IL) 1ß, IL10, IL1 receptor antagonist (IL1Ra), soluble tumour necrosis factor receptor (sTNFR), and soluble CD30 (sCD30) by ELISA. Clinical assessment and blood sampling in pregnant women was done once in each trimester and 6, 12, and 24 weeks post partum. Disease activity in the patients was evaluated by validated clinical instruments and correlated with circulating levels of cytokines.
Results: Low levels of IL10 were found sporadically, whereas IFNγ and IL1ß were below detection level in the samples tested. Significantly higher concentrations of sTNFR and IL1Ra were measured in pregnant than in non-pregnant subjects. An increase of IL1Ra from the second to the third trimester correlated with improvement of disease activity in patients with RA and AS. Compared with non-pregnant patients and the other pregnant women, patients with RA showed markedly raised levels of sCD30 during pregnancy.
Conclusions: IFNγ and IL10, markers of a Th1 and Th2 response, respectively, were either low or undetectable in the cohorts analysed. The increase of cytokine inhibitors IL1Ra and sTNFR was related to pregnancy and was independent of an underlying disease. These anti-inflammatory mediators seem to affect disease activity.
Full Text
The Full Text of this article is available as a PDF (99.7 KB).
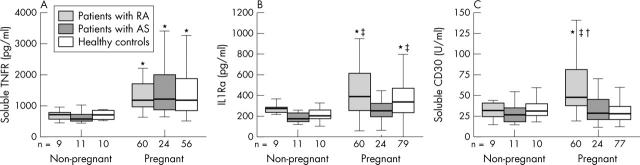
Figure 1.
Levels of (A) sTNFR, (B) IL1Ra, and (C) sCD30 in patients with RA, patients with AS, and healthy women. Values presented in the group of non-pregnant (no pre-pregnancy or postpartum data included) and pregnant (pooled data of first, second, and third trimester) subjects. Horizontal bar within the box marks the median, the boxes represent the range of ±25% around the median (interquartile range). Vertical bars indicate 95% confidence interval. *Significant difference compared with the non-pregnant control group (p<0.05 by Mann-Whitney U test); †significant difference compared with healthy pregnant women (p<0.05 by Mann-Whitney U test); ‡significant difference compared with pregnant patients with AS (p<0.05 by Mann-Whitney U test).
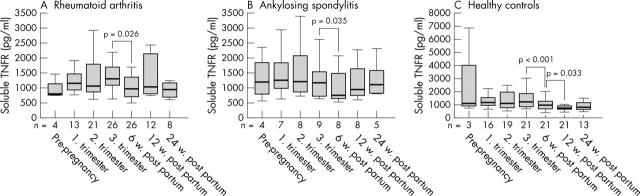
Figure 2.
Levels of sTNFR before, during (first, second, and third trimester), and after pregnancy (6, 12, and 24 weeks post partum) in patients with (A) RA or (B) AS, and (C) in healthy controls. Horizontal bar within the box marks the median, the boxes represent a range of ±25% around the median (interquartile range). Vertical bars indicate 95% confidence interval. Significant changes indicated by p values (Wilcoxon test for paired data).
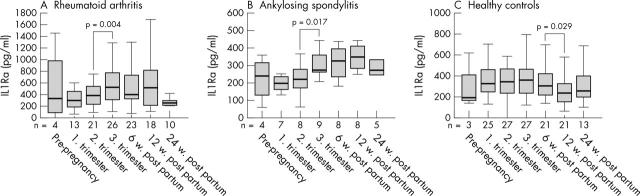
Figure 3.
Levels of IL1Ra before, during (first, second, and third trimester), and after pregnancy (6, 12, and 24 weeks post partum) in patients with (A) RA or (B) AS, and (C) in healthy controls. Horizontal bar within the box marks the median, the boxes represent a range of ±25% around the median (interquartile range). Vertical bars indicate 95% confidence interval. Significant changes indicated by p values (Wilcoxon test for paired data).
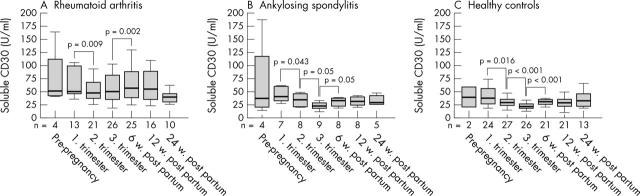
Figure 4.
Levels of sCD30 before, during (first, second, and third trimester), and after pregnancy (6, 12, and 24 weeks post partum) in patients with (A) RA or (B) AS, and (C) in healthy controls. Horizontal bar within the box marks the median, the boxes represent a range of ±25% around the median (interquartile range). Vertical bars indicate 95% confidence interval. Significant changes indicated by p values (Wilcoxon test for paired data).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Annunziato Francesco, Cosmi Lorenzo, Liotta Francesco, Lazzeri Elena, Manetti Roberto, Vanini Vittorio, Romagnani Paola, Maggi Enrico, Romagnani Sergio. Phenotype, localization, and mechanism of suppression of CD4(+)CD25(+) human thymocytes. J Exp Med. 2002 Aug 5;196(3):379–387. doi: 10.1084/jem.20020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend W. P., Dayer J. M. Inhibition of the production and effects of interleukin-1 and tumor necrosis factor alpha in rheumatoid arthritis. Arthritis Rheum. 1995 Feb;38(2):151–160. doi: 10.1002/art.1780380202. [DOI] [PubMed] [Google Scholar]
- Arend W. P., Gabay C. Physiologic role of interleukin-1 receptor antagonist. Arthritis Res. 2000 May 19;2(4):245–248. doi: 10.1186/ar94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Bahar Ahmed M., Ghalib Hashim W., Moosa Riyad A., Zaki Zaki M. S., Thomas Chet, Nabri Osman A. Maternal serum interleukin-6, interleukin-8, tumor necrosis factor-alpha and interferon-gamma in preterm labor. Acta Obstet Gynecol Scand. 2003 Jun;82(6):543–549. [PubMed] [Google Scholar]
- Braun J., Bollow M., Neure L., Seipelt E., Seyrekbasan F., Herbst H., Eggens U., Distler A., Sieper J. Use of immunohistologic and in situ hybridization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arthritis Rheum. 1995 Apr;38(4):499–505. doi: 10.1002/art.1780380407. [DOI] [PubMed] [Google Scholar]
- Caligaris-Cappio F., Bertero M. T., Converso M., Stacchini A., Vinante F., Romagnani S., Pizzolo G. Circulating levels of soluble CD30, a marker of cells producing Th2-type cytokines, are increased in patients with systemic lupus erythematosus and correlate with disease activity. Clin Exp Rheumatol. 1995 May-Jun;13(3):339–343. [PubMed] [Google Scholar]
- Chaouat G., Menu E., Clark D. A., Dy M., Minkowski M., Wegmann T. G. Control of fetal survival in CBA x DBA/2 mice by lymphokine therapy. J Reprod Fertil. 1990 Jul;89(2):447–458. doi: 10.1530/jrf.0.0890447. [DOI] [PubMed] [Google Scholar]
- Chaouat Gérard, Ledée-Bataille Natalie, Dubanchet Sylvie, Zourbas Sandrine, Sandra Olivier, Martal Jacques. TH1/TH2 paradigm in pregnancy: paradigm lost? Cytokines in pregnancy/early abortion: reexamining the TH1/TH2 paradigm. Int Arch Allergy Immunol. 2004 May 17;134(2):93–119. doi: 10.1159/000074300. [DOI] [PubMed] [Google Scholar]
- Del Prete G., De Carli M., Almerigogna F., Daniel C. K., D'Elios M. M., Zancuoghi G., Vinante F., Pizzolo G., Romagnani S. Preferential expression of CD30 by human CD4+ T cells producing Th2-type cytokines. FASEB J. 1995 Jan;9(1):81–86. [PubMed] [Google Scholar]
- Dougados M., van der Linden S., Juhlin R., Huitfeldt B., Amor B., Calin A., Cats A., Dijkmans B., Olivieri I., Pasero G. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991 Oct;34(10):1218–1227. doi: 10.1002/art.1780341003. [DOI] [PubMed] [Google Scholar]
- Ellis J., Wennerholm U. B., Bengtsson A., Lilja H., Pettersson A., Sultan B., Wennergren M., Hagberg H. Levels of dimethylarginines and cytokines in mild and severe preeclampsia. Acta Obstet Gynecol Scand. 2001 Jul;80(7):602–608. [PubMed] [Google Scholar]
- Felson D. T., Anderson J. J., Boers M., Bombardier C., Chernoff M., Fried B., Furst D., Goldsmith C., Kieszak S., Lightfoot R. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis Rheum. 1993 Jun;36(6):729–740. doi: 10.1002/art.1780360601. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Boyle D. L., Yu C., Paine M. M., Whisenand T. D., Zvaifler N. J., Arend W. P. Synovial interleukin-1 receptor antagonist and interleukin-1 balance in rheumatoid arthritis. Arthritis Rheum. 1994 May;37(5):644–652. doi: 10.1002/art.1780370507. [DOI] [PubMed] [Google Scholar]
- Garrett S., Jenkinson T., Kennedy L. G., Whitelock H., Gaisford P., Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994 Dec;21(12):2286–2291. [PubMed] [Google Scholar]
- Gerli R., Bistoni O., Lunardi C., Giacomelli R., Tomassini C., Biagini P., Pitzalis C. Soluble CD30 in early rheumatoid arthritis as a predictor of good response to second-line therapy. Rheumatology (Oxford) 1999 Dec;38(12):1282–1284. doi: 10.1093/rheumatology/38.12.1282. [DOI] [PubMed] [Google Scholar]
- Gerli R., Lunardi C., Vinante F., Bistoni O., Pizzolo G., Pitzalis C. Role of CD30+ T cells in rheumatoid arthritis: a counter-regulatory paradigm for Th1-driven diseases. Trends Immunol. 2001 Feb;22(2):72–77. doi: 10.1016/s1471-4906(00)01829-9. [DOI] [PubMed] [Google Scholar]
- Giacomelli R., Cipriani P., Lattanzio R., Di Franco M., Locanto M., Parzanese I., Passacantando A., Ciocci A., Tonietti G. Circulating levels of soluble CD30 are increased in patients with systemic sclerosis (SSc) and correlate with serological and clinical features of the disease. Clin Exp Immunol. 1997 Apr;108(1):42–46. doi: 10.1046/j.1365-2249.1997.d01-991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomelli R., Passacantando A., Parzanese I., Vernia P., Klidara N., Cucinelli F., Lattanzio R., Santori E., Cipriani P., Caprilli R. Serum levels of soluble CD30 are increased in ulcerative colitis (UC) but not in Crohn's disease (CD). Clin Exp Immunol. 1998 Mar;111(3):532–535. doi: 10.1046/j.1365-2249.1998.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman Jennifer D., Sack Kenneth E., Davis John C., Jr Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. N Engl J Med. 2002 May 2;346(18):1349–1356. doi: 10.1056/NEJMoa012664. [DOI] [PubMed] [Google Scholar]
- Gran J. T., Ostensen M. Spondyloarthritides in females. Baillieres Clin Rheumatol. 1998 Nov;12(4):695–715. doi: 10.1016/s0950-3579(98)80045-9. [DOI] [PubMed] [Google Scholar]
- Hebisch Gundula, Neumaier-Wagner Peruka M., Huch Renate, von Mandach Ursula. Maternal serum interleukin-1 beta, -6 and -8 levels and potential determinants in pregnancy and peripartum. J Perinat Med. 2004;32(6):475–480. doi: 10.1515/JPM.2004.131. [DOI] [PubMed] [Google Scholar]
- Hoshimoto K., Ohta N., Ohkura T., Inaba N. Changes in plasma soluble CD26 and CD30 during pregnancy: markers of Th1/Th2 balance? Gynecol Obstet Invest. 2000;50(4):260–263. doi: 10.1159/000010328. [DOI] [PubMed] [Google Scholar]
- Huizinga T. W., van der Linden M. W., Deneys-Laporte V., Breedveld F. C. Interleukin-10 as an explanation for pregnancy-induced flare in systemic lupus erythematosus and remission in rheumatoid arthritis. Rheumatology (Oxford) 1999 Jun;38(6):496–498. doi: 10.1093/rheumatology/38.6.496. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Genant H. K., Watt I., Cobby M., Bresnihan B., Aitchison R., McCabe D. A multicenter, double-blind, dose-ranging, randomized, placebo-controlled study of recombinant human interleukin-1 receptor antagonist in patients with rheumatoid arthritis: radiologic progression and correlation of Genant and Larsen scores. Arthritis Rheum. 2000 May;43(5):1001–1009. doi: 10.1002/1529-0131(200005)43:5<1001::AID-ANR7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Joyce D. A., Steer J. H. IL-4, IL-10 and IFN-gamma have distinct, but interacting, effects on differentiation-induced changes in TNF-alpha and TNF receptor release by cultured human monocytes. Cytokine. 1996 Jan;8(1):49–57. doi: 10.1006/cyto.1996.0007. [DOI] [PubMed] [Google Scholar]
- Kawanaka Norikuni, Yamamura Masahiro, Aita Tetsushi, Morita Yoshitaka, Okamoto Akira, Kawashima Masanori, Iwahashi Mitsuhiro, Ueno Akiko, Ohmoto Yasukazu, Makino Hirofumi. CD14+,CD16+ blood monocytes and joint inflammation in rheumatoid arthritis. Arthritis Rheum. 2002 Oct;46(10):2578–2586. doi: 10.1002/art.10545. [DOI] [PubMed] [Google Scholar]
- Kay J., Calabrese L. The role of interleukin-1 in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 2004 Jun;43 (Suppl 3):iii2–iii9. doi: 10.1093/rheumatology/keh201. [DOI] [PubMed] [Google Scholar]
- Keller C., Webb A., Davis J. Cytokines in the seronegative spondyloarthropathies and their modification by TNF blockade: a brief report and literature review. Ann Rheum Dis. 2003 Dec;62(12):1128–1132. doi: 10.1136/ard.2003.011023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferminc M. J., Peaceman A. M., Aderka D., Wallach D., Peyser M. R., Lessing J. B., Socol M. L. Soluble tumor necrosis factor receptors in maternal plasma and second-trimester amniotic fluid. Am J Obstet Gynecol. 1995 Sep;173(3 Pt 1):900–905. doi: 10.1016/0002-9378(95)90363-1. [DOI] [PubMed] [Google Scholar]
- Lard L. R., van Gaalen F. A., Schonkeren J. J. M., Pieterman E. J., Stoeken G., Vos K., Nelissen R. G. H. H., Westendorp R. G. J., Hoeben R. C., Breedveld F. C. Association of the -2849 interleukin-10 promoter polymorphism with autoantibody production and joint destruction in rheumatoid arthritis. Arthritis Rheum. 2003 Jul;48(7):1841–1848. doi: 10.1002/art.11160. [DOI] [PubMed] [Google Scholar]
- Makhseed M., Raghupathy R., Azizieh F., Farhat R., Hassan N., Bandar A. Circulating cytokines and CD30 in normal human pregnancy and recurrent spontaneous abortions. Hum Reprod. 2000 Sep;15(9):2011–2017. doi: 10.1093/humrep/15.9.2011. [DOI] [PubMed] [Google Scholar]
- Moreland L. W., Schiff M. H., Baumgartner S. W., Tindall E. A., Fleischmann R. M., Bulpitt K. J., Weaver A. L., Keystone E. C., Furst D. E., Mease P. J. Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med. 1999 Mar 16;130(6):478–486. doi: 10.7326/0003-4819-130-6-199903160-00004. [DOI] [PubMed] [Google Scholar]
- Mulherin D., Fitzgerald O., Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996 Jan;39(1):115–124. doi: 10.1002/art.1780390116. [DOI] [PubMed] [Google Scholar]
- Muñoz-Valle J. F., Vázquez-Del Mercado M., García-Iglesias T., Orozco-Barocio G., Bernard-Medina G., Martínez-Bonilla G., Bastidas-Ramírez B. E., Navarro A. D., Bueno M., Martínez-López E. T(H)1/T(H)2 cytokine profile, metalloprotease-9 activity and hormonal status in pregnant rheumatoid arthritis and systemic lupus erythematosus patients. Clin Exp Immunol. 2003 Feb;131(2):377–384. doi: 10.1046/j.1365-2249.2003.02059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto Akira, Yamamura Masahiro, Iwahashi Mitsuhiro, Aita Tetsushi, Ueno Akiko, Kawashima Masanori, Yamana Jiro, Kagawa Hidetoshi, Makino Hirofumi. Pathophysiological functions of CD30+ CD4+ T cells in rheumatoid arthritis. Acta Med Okayama. 2003 Dec;57(6):267–277. doi: 10.18926/AMO/32814. [DOI] [PubMed] [Google Scholar]
- Opsjłn S. L., Wathen N. C., Tingulstad S., Wiedswang G., Sundan A., Waage A., Austgulen R. Tumor necrosis factor, interleukin-1, and interleukin-6 in normal human pregnancy. Am J Obstet Gynecol. 1993 Aug;169(2 Pt 1):397–404. doi: 10.1016/0002-9378(93)90096-2. [DOI] [PubMed] [Google Scholar]
- Ostensen M., Husby G. A prospective clinical study of the effect of pregnancy on rheumatoid arthritis and ankylosing spondylitis. Arthritis Rheum. 1983 Sep;26(9):1155–1159. doi: 10.1002/art.1780260915. [DOI] [PubMed] [Google Scholar]
- Piccinni M. P., Giudizi M. G., Biagiotti R., Beloni L., Giannarini L., Sampognaro S., Parronchi P., Manetti R., Annunziato F., Livi C. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J Immunol. 1995 Jul 1;155(1):128–133. [PubMed] [Google Scholar]
- Smith C. A., Davis T., Anderson D., Solam L., Beckmann M. P., Jerzy R., Dower S. K., Cosman D., Goodwin R. G. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science. 1990 May 25;248(4958):1019–1023. doi: 10.1126/science.2160731. [DOI] [PubMed] [Google Scholar]
- Spinozzi F., Agea E., Bistoni O., Forenza N., Monaco A., Falini B., Bassotti G., De Benedictis F., Grignani F., Bertotto A. Local expansion of allergen-specific CD30+Th2-type gamma delta T cells in bronchial asthma. Mol Med. 1995 Nov;1(7):821–826. [PMC free article] [PubMed] [Google Scholar]
- Stucki G., Liang M. H., Stucki S., Brühlmann P., Michel B. A. A self-administered rheumatoid arthritis disease activity index (RADAI) for epidemiologic research. Psychometric properties and correlation with parameters of disease activity. Arthritis Rheum. 1995 Jun;38(6):795–798. doi: 10.1002/art.1780380612. [DOI] [PubMed] [Google Scholar]
- Tan A. L., Marzo-Ortega H., O'Connor P., Fraser A., Emery P., McGonagle D. Efficacy of anakinra in active ankylosing spondylitis: a clinical and magnetic resonance imaging study. Ann Rheum Dis. 2004 Apr 5;63(9):1041–1045. doi: 10.1136/ard.2004.020800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannier E., Miller L. C., Dinarello C. A. Coordinated antiinflammatory effects of interleukin 4: interleukin 4 suppresses interleukin 1 production but up-regulates gene expression and synthesis of interleukin 1 receptor antagonist. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4076–4080. doi: 10.1073/pnas.89.9.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Del Mercado Monica, Garcia-Gonzalez Araceli, Muñoz-Valle José Francisco, Garcia-Iglesias Trinidad, Martinez-Bonilla Gloria, Bernard-Medina Guislaine, Sanchez-Ortiz Adriana, Ornelas-Aguirre José M., Salazar-Paramo Mario, Gamez-Nava Jorge I. Interleukin 1beta (IL-1beta), IL-10, tumor necrosis factor-alpha, and cellular proliferation index in peripheral blood mononuclear cells in patients with ankylosing spondylitis. J Rheumatol. 2002 Mar;29(3):522–526. [PubMed] [Google Scholar]
- Wais Thomas, Fierz Walter, Stoll Thomas, Villiger Peter M. Subclinical disease activity in systemic lupus erythematosus: immunoinflammatory markers do not normalize in clinical remission. J Rheumatol. 2003 Oct;30(10):2133–2139. [PubMed] [Google Scholar]
- Wegmann T. G., Lin H., Guilbert L., Mosmann T. R. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993 Jul;14(7):353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- Williams R. O., Feldmann M., Maini R. N. Cartilage destruction and bone erosion in arthritis: the role of tumour necrosis factor alpha. Ann Rheum Dis. 2000 Nov;59 (Suppl 1):i75–i80. doi: 10.1136/ard.59.suppl_1.i75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Roon J. A. G., Bijlsma J. W. J. Th2 mediated regulation in RA and the spondyloarthropathies. Ann Rheum Dis. 2002 Nov;61(11):951–954. doi: 10.1136/ard.61.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Roon Joel, Wijngaarden Siska, Lafeber Floris P. J. G., Damen Cora, van de Winkel Jan, Bijlsma Johannes W. J. Interleukin 10 treatment of patients with rheumatoid arthritis enhances Fc gamma receptor expression on monocytes and responsiveness to immune complex stimulation. J Rheumatol. 2003 Apr;30(4):648–651. [PubMed] [Google Scholar]