Abstract
Background: Monocytes/macrophages have an important and versatile role in joint inflammation and destruction in rheumatoid arthritis (RA).
Objective: To determine the efficiency of monocyte/macrophage elimination by a new drug conjugated antibody (CD64-calicheamicin (CD64-CaMi)) directed to the high affinity receptor for IgG (FcγRI).
Methods: Mononuclear cells from peripheral blood and synovial fluid of patients with RA were cultured in the presence of CD64-CaMi. Cell death of monocytes/macrophages was measured by analysis of phenotypic changes (light scatter patterns, CD14 expression, and FcγRI expression) and nuclear DNA fragmentation. The selectivity of CD64-CaMi was checked by using FcγRI deficient and FcγRI transfected cell lines. In addition, the indirect effect of CD64-CaMi-induced macrophage cell death on arthritogenic T(h1) cell activity was determined.
Results: Inflammatory macrophages from RA synovial fluid, expressing increased FcγRI levels, were efficiently killed by CD64-CaMi through induction of DNA fragmentation. CD64-CaMi-induced cell death of monocytes/macrophages from peripheral blood of patients with RA proved less efficient. Induction of synovial macrophage death by CD64-CaMi was accompanied by efficient inhibition of proinflammatory T(h1) cytokine production.
Conclusion: Together, the presented data suggest that elimination of macrophages through a new FcγRI directed CD64-CaMi is feasible. Because monocytes from peripheral blood are also eliminated by this immunoconjugate, additional experimental studies should validate its potential for local (intra-articular) application in the treatment of RA.
Full Text
The Full Text of this article is available as a PDF (148.7 KB).
Figure 1.
CD64-CaMi induces FcγRI dependent cell death of FcγRI transfected, but not FcαRI transfected, IIA1.6 cells. Proliferation of IIA1.6 cells (2x105/ml) was measured by [3H]thymidine incorporation (proliferation) after 3 days of culture (n = 3). The effects of CD64-CaMi were expressed as percentages of control cultures in the absence of immunoconjugate. *Significant difference of p<0.05.
Figure 2.
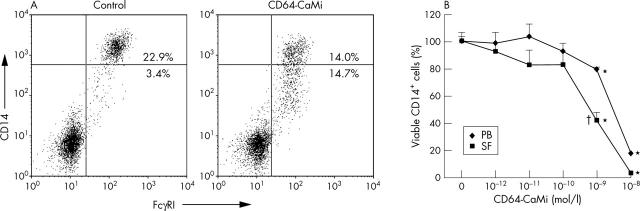
Flow cytometric analysis of the effect of CD64-CaMi on PB monocytes and SF macrophages from patients with RA. (A) Representative FcγRI/CD14 staining of MC from PB and SF cultured for 24 hours with or without CD64-CaMi. CD64-CaMi-induced death of monocytic cells was associated with a reduction in CD14+ monocytic cells from 22.9% to 14.0% (almost 40% reduction). (B) Average of CD64-CaMi-induced monocyte/macrophage cell death from PB and SF (both n = 6) are expressed as a decrease in viable cells compared with controls, measured as a reduction in CD14 expression after 24 hours of culture. *Significant differences of CD64-CaMi-induced cell death compared with control cultures (p<0.05). At a CD64-CaMi concentration of 10–9 mol/l, cell death of macrophages from SF, measured by reduction in CD14+ cells (mean (SD) 57 (7)%), was significantly higher than in PB (19 (3)%; †p<0.05).
Figure 3.
Induction of nuclear DNA fragmentation of CD68+ cells by CD64-CaMi. DNA content of CD68– lymphocytic and CD68+ monocytic cells was stained with PI and measured by flow cytometry (FACS). (A) Representative analysis showing that incubation of SFMC with CD64-CaMi (10–8 mol/l for 24 hours) results in a strong increase in apoptotic CD68+ cells with reduced nuclear DNA content owing to DNA fragmentation (from 3.5% to 92.1% of CD68+ cells). (B) On average (n = 3, 10–8 mol/l for 24 hours), a mean (SD) change from 4.2 (0.2)% apoptotic macrophages in control culture to 57.5 (18.5)% upon culture with CD64-CaMi (p<0.05) was seen. Apoptotic cell death of CD68– lymphocytic cells was not significantly changed (8.2 (4.0)% to 13.1 (8.9)%). *Significant cell death of CD68+ cells (p<0.05).
Figure 4.
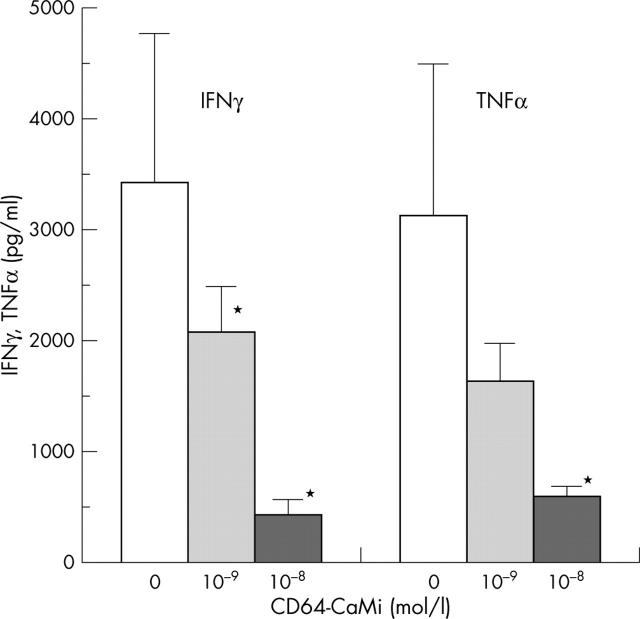
CD64-CaMi-induced macrophage apoptosis in time prevents CD3/CD28-induced T cell cytokine secretion (n = 3). SFMC were cultured with CD64-CaMi at a concentration of 10–8 mol/l and 10–9 mol/l or without CD64-CaMi for 3 days. After this, T cells were costimulated by CD3/CD28 for 24 hours, and IFNγ and TNFα levels were measured. *Significant inhibitions of T cell activity upon (pre-) treatment with CD64-CaMi compared with control cultures (p<0.05).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Barrera P., Blom A., van Lent P. L., van Bloois L., Beijnen J. H., van Rooijen N., de Waal Malefijt M. C., van de Putte L. B., Storm G., van den Berg W. B. Synovial macrophage depletion with clodronate-containing liposomes in rheumatoid arthritis. Arthritis Rheum. 2000 Sep;43(9):1951–1959. doi: 10.1002/1529-0131(200009)43:9<1951::AID-ANR5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Bresnihan B. The safety and efficacy of interleukin-1 receptor antagonist in the treatment of rheumatoid arthritis. Semin Arthritis Rheum. 2001 Apr;30(5 Suppl 2):17–20. doi: 10.1053/sarh.2001.23701. [DOI] [PubMed] [Google Scholar]
- Burmester G. R., Stuhlmüller B., Keyszer G., Kinne R. W. Mononuclear phagocytes and rheumatoid synovitis. Mastermind or workhorse in arthritis? Arthritis Rheum. 1997 Jan;40(1):5–18. doi: 10.1002/art.1780400104. [DOI] [PubMed] [Google Scholar]
- Choy E. H., Panayi G. S. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001 Mar 22;344(12):907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- Deo Y. M., Graziano R. F., Repp R., van de Winkel J. G. Clinical significance of IgG Fc receptors and Fc gamma R-directed immunotherapies. Immunol Today. 1997 Mar;18(3):127–135. doi: 10.1016/s0167-5699(97)01007-4. [DOI] [PubMed] [Google Scholar]
- Elliott M. J., Maini R. N., Feldmann M., Kalden J. R., Antoni C., Smolen J. S., Leeb B., Breedveld F. C., Macfarlane J. D., Bijl H. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994 Oct 22;344(8930):1105–1110. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- Graziano R. F., Tempest P. R., White P., Keler T., Deo Y., Ghebremariam H., Coleman K., Pfefferkorn L. C., Fanger M. W., Guyre P. M. Construction and characterization of a humanized anti-gamma-Ig receptor type I (Fc gamma RI) monoclonal antibody. J Immunol. 1995 Nov 15;155(10):4996–5002. [PubMed] [Google Scholar]
- Guyre P. M., Graziano R. F., Vance B. A., Morganelli P. M., Fanger M. W. Monoclonal antibodies that bind to distinct epitopes on Fc gamma RI are able to trigger receptor function. J Immunol. 1989 Sep 1;143(5):1650–1655. [PubMed] [Google Scholar]
- Hahn G., Stuhlmüller B., Hain N., Kalden J. R., Pfizenmaier K., Burmester G. R. Modulation of monocyte activation in patients with rheumatoid arthritis by leukapheresis therapy. J Clin Invest. 1993 Mar;91(3):862–870. doi: 10.1172/JCI116307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich S. Monocyte CD14: a multifunctional receptor engaged in apoptosis from both sides. J Leukoc Biol. 1999 Jun;65(6):737–743. doi: 10.1002/jlb.65.6.737. [DOI] [PubMed] [Google Scholar]
- Heidenreich S., Schmidt M., August C., Cullen P., Rademaekers A., Pauels H. G. Regulation of human monocyte apoptosis by the CD14 molecule. J Immunol. 1997 Oct 1;159(7):3178–3188. [PubMed] [Google Scholar]
- Heijnen I. A., van Vugt M. J., Fanger N. A., Graziano R. F., de Wit T. P., Hofhuis F. M., Guyre P. M., Capel P. J., Verbeek J. S., van de Winkel J. G. Antigen targeting to myeloid-specific human Fc gamma RI/CD64 triggers enhanced antibody responses in transgenic mice. J Clin Invest. 1996 Jan 15;97(2):331–338. doi: 10.1172/JCI118420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka T., Suzuki K., Matsuki Y., Takamizawa-Matsumoto M., Kataharada K., Ishizuka T., Kawakami M., Nakamura H. Filtration leukocytapheresis therapy in rheumatoid arthritis: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 1999 Mar;42(3):431–437. doi: 10.1002/1529-0131(199904)42:3<431::AID-ANR6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Highton J., Carlisle B., Palmer D. G. Changes in the phenotype of monocytes/macrophages and expression of cytokine mRNA in peripheral blood and synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol. 1995 Dec;102(3):541–546. doi: 10.1111/j.1365-2249.1995.tb03850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilkens C. M., Vermeulen H., van Neerven R. J., Snijdewint F. G., Wierenga E. A., Kapsenberg M. L. Differential modulation of T helper type 1 (Th1) and T helper type 2 (Th2) cytokine secretion by prostaglandin E2 critically depends on interleukin-2. Eur J Immunol. 1995 Jan;25(1):59–63. doi: 10.1002/eji.1830250112. [DOI] [PubMed] [Google Scholar]
- Koester S. K., Schlossman S. F., Zhang C., Decker S. J., Bolton W. E. APO2.7 defines a shared apoptotic-necrotic pathway in a breast tumor hypoxia model. Cytometry. 1998 Nov 1;33(3):324–332. doi: 10.1002/(sici)1097-0320(19981101)33:3<324::aid-cyto6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Liu C., Goldstein J., Graziano R. F., He J., O'Shea J. K., Deo Y., Guyre P. M. F(c)gammaRI-targeted fusion proteins result in efficient presentation by human monocytes of antigenic and antagonist T cell epitopes. J Clin Invest. 1996 Nov 1;98(9):2001–2007. doi: 10.1172/JCI119004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick T. S., Stevens S. R., Kang K. Macrophages and cutaneous inflammation. Nat Biotechnol. 2000 Jan;18(1):25–26. doi: 10.1038/71879. [DOI] [PubMed] [Google Scholar]
- Morita Y., Yamamura M., Kawashima M., Harada S., Tsuji K., Shibuya K., Maruyama K., Makino H. Flow cytometric single-cell analysis of cytokine production by CD4+ T cells in synovial tissue and peripheral blood from patients with rheumatoid arthritis. Arthritis Rheum. 1998 Sep;41(9):1669–1676. doi: 10.1002/1529-0131(199809)41:9<1669::AID-ART19>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Mulherin D., Fitzgerald O., Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996 Jan;39(1):115–124. doi: 10.1002/art.1780390116. [DOI] [PubMed] [Google Scholar]
- Naito K., Takeshita A., Shigeno K., Nakamura S., Fujisawa S., Shinjo K., Yoshida H., Ohnishi K., Mori M., Terakawa S. Calicheamicin-conjugated humanized anti-CD33 monoclonal antibody (gemtuzumab zogamicin, CMA-676) shows cytocidal effect on CD33-positive leukemia cell lines, but is inactive on P-glycoprotein-expressing sublines. Leukemia. 2000 Aug;14(8):1436–1443. doi: 10.1038/sj.leu.2401851. [DOI] [PubMed] [Google Scholar]
- Nicoletti I., Migliorati G., Pagliacci M. C., Grignani F., Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991 Jun 3;139(2):271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- Perlman H., Pagliari L. J., Liu H., Koch A. E., Haines G. K., 3rd, Pope R. M. Rheumatoid arthritis synovial macrophages express the Fas-associated death domain-like interleukin-1beta-converting enzyme-inhibitory protein and are refractory to Fas-mediated apoptosis. Arthritis Rheum. 2001 Jan;44(1):21–30. doi: 10.1002/1529-0131(200101)44:1<21::AID-ANR4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Reterink T. J., van Zandbergen G., van Egmond M., Klar-Mohamad N., Morton C. H., van de Winkel J. G., Daha M. R. Size-dependent effect of IgA on the IgA Fc receptor (CD89). Eur J Immunol. 1997 Sep;27(9):2219–2224. doi: 10.1002/eji.1830270915. [DOI] [PubMed] [Google Scholar]
- Thepen T., van Vuuren A. J., Kiekens R. C., Damen C. A., Vooijs W. C., van De Winkel J. G. Resolution of cutaneous inflammation after local elimination of macrophages. Nat Biotechnol. 2000 Jan;18(1):48–51. doi: 10.1038/71908. [DOI] [PubMed] [Google Scholar]
- Thrush G. R., Lark L. R., Clinchy B. C., Vitetta E. S. Immunotoxins: an update. Annu Rev Immunol. 1996;14:49–71. doi: 10.1146/annurev.immunol.14.1.49. [DOI] [PubMed] [Google Scholar]
- Trail P. A., Bianchi A. B. Monoclonal antibody drug conjugates in the treatment of cancer. Curr Opin Immunol. 1999 Oct;11(5):584–588. doi: 10.1016/s0952-7915(99)00012-6. [DOI] [PubMed] [Google Scholar]
- Van Vugt M. J., Van den Herik-Oudijk I. E., Van de Winkel J. G. FcgammaRIa-gamma-chain complexes trigger antibody-dependent cell-mediated cytotoxicity (ADCC) in CD5+ B cell/macrophage IIA1.6 cells. Clin Exp Immunol. 1998 Sep;113(3):415–422. doi: 10.1046/j.1365-2249.1998.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoef C. M., Van Roon J. A., Vianen M. E., Glaudemans C. A., Lafeber F. P., Bijlsma J. W. Lymphocyte stimulation by CD3-CD28 enables detection of low T cell interferon-gamma and interleukin-4 production in rheumatoid arthritis. Scand J Immunol. 1999 Oct;50(4):427–432. doi: 10.1046/j.1365-3083.1999.00617.x. [DOI] [PubMed] [Google Scholar]
- Verhoef C. M., van Roon J. A., Vianen M. E., Bijlsma J. W., Lafeber F. P. Interleukin 10 (IL-10), not IL-4 or interferon-gamma production, correlates with progression of joint destruction in rheumatoid arthritis. J Rheumatol. 2001 Sep;28(9):1960–1966. [PubMed] [Google Scholar]
- Wijngaarden S., van Roon J. A. G., Bijlsma J. W. J., van de Winkel J. G. J., Lafeber F. P. J. G. Fcgamma receptor expression levels on monocytes are elevated in rheumatoid arthritis patients with high erythrocyte sedimentation rate who do not use anti-rheumatic drugs. Rheumatology (Oxford) 2003 May;42(5):681–688. doi: 10.1093/rheumatology/keg174. [DOI] [PubMed] [Google Scholar]
- Yudoh K., Matsuno H., Nakazawa F., Yonezawa T., Kimura T. Reduced expression of the regulatory CD4+ T cell subset is related to Th1/Th2 balance and disease severity in rheumatoid arthritis. Arthritis Rheum. 2000 Mar;43(3):617–627. doi: 10.1002/1529-0131(200003)43:3<617::AID-ANR19>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Zein N., Sinha A. M., McGahren W. J., Ellestad G. A. Calicheamicin gamma 1I: an antitumor antibiotic that cleaves double-stranded DNA site specifically. Science. 1988 May 27;240(4856):1198–1201. doi: 10.1126/science.3240341. [DOI] [PubMed] [Google Scholar]
- van Roon J. A., van Eden W., van Roy J. L., Lafeber F. J., Bijlsma J. W. Stimulation of suppressive T cell responses by human but not bacterial 60-kD heat-shock protein in synovial fluid of patients with rheumatoid arthritis. J Clin Invest. 1997 Jul 15;100(2):459–463. doi: 10.1172/JCI119553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Roon J. A., van Roy J. L., Duits A., Lafeber F. P., Bijlsma J. W. Proinflammatory cytokine production and cartilage damage due to rheumatoid synovial T helper-1 activation is inhibited by interleukin-4. Ann Rheum Dis. 1995 Oct;54(10):836–840. doi: 10.1136/ard.54.10.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Roon Joel A. G., van Vuuren Anneke J., Wijngaarden Siska, Jacobs Kim M. G., Bijlsma Johannes W. J., Lafeber Floris P. J. G., Thepen Theo, van de Winkel Jan G. J. Selective elimination of synovial inflammatory macrophages in rheumatoid arthritis by an Fcgamma receptor I-directed immunotoxin. Arthritis Rheum. 2003 May;48(5):1229–1238. doi: 10.1002/art.10940. [DOI] [PubMed] [Google Scholar]