Abstract
Objectives: To define the pathogenesis of pigmented villonodular synovitis (PVNS), by searching for highly expressed genes in primary synovial cells from patients with PVNS.
Methods: A combination of subtraction cloning and Southern colony hybridisation was used to detect highly expressed genes in PVNS in comparison with rheumatoid synovial cells. Northern hybridisation was performed to confirm the differential expression of the humanin gene in PVNS. Expression of the humanin peptide was analysed by western blotting and immunohistochemistry. Electron microscopic immunohistochemistry was performed to investigate the distribution of this peptide within the cell.
Results: 68 highly expressed genes were identified in PVNS. Humanin genes were strongly expressed in diffuse-type PVNS, but were barely detected in nodular-type PVNS, rheumatoid arthritis, or osteoarthritis. Humanin peptide was identified in synovium from diffuse-type PVNS, and most of the positive cells were distributed in the deep layer of the synovial tissue. Double staining with anti-humanin and anti-heat shock protein 60 showed that humanin was expressed mainly in mitochondria. Electron microscopy disclosed immunolocalisation of this peptide, predominantly around dense iron deposits within the siderosome.
Conclusions: Increased expression of the humanin peptide in mitochondria and siderosomes is characteristic of synovial cells from diffuse-type PVNS. Humanin is an anti-apoptotic peptide which is encoded in the mitochondrial genome. Present findings suggest that mitochondrial dysfunction may be the principal factor in pathogenesis of diffuse-type PVNS and that humanin peptide may play a part in the neoplastic process in this form of PVNS.
Full Text
The Full Text of this article is available as a PDF (328.5 KB).
Figure 2.
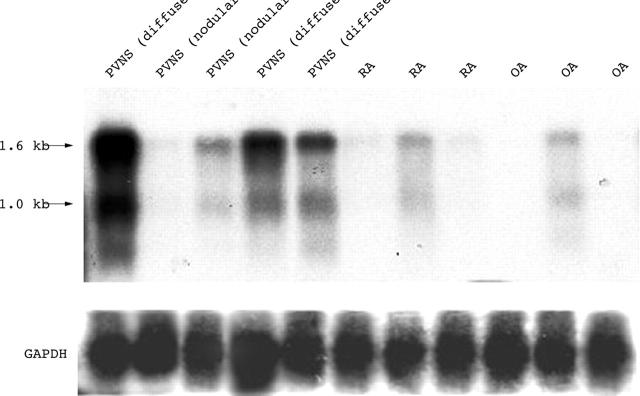
Northern blot analysis of mRNAs expressed by synovial cells from patients with PVNS, RA, and OA. Total RNA (168 ng) was subjected to electrophoresis in a 1.0% agarose gel containing formaldehyde, transferred to a nylon membrane, and probed with [32P]dCTP labelled cDNA (type 9; fig 1). Another cDNA (type 3) encoded in the 16S rRNA region was also used in northern blotting and the expression level and size were same as those using type 9 cDNA (data not shown). Humanin genes were strongly expressed in diffuse-type PVNS, but barely detected in nodular-type PVNS, RA, or OA. The size of the expressed major message was ∼1.6 kb and the other messages were ∼1 kb, which corresponds to the results of a previous report by Hashimoto et al.28
Figure 1.
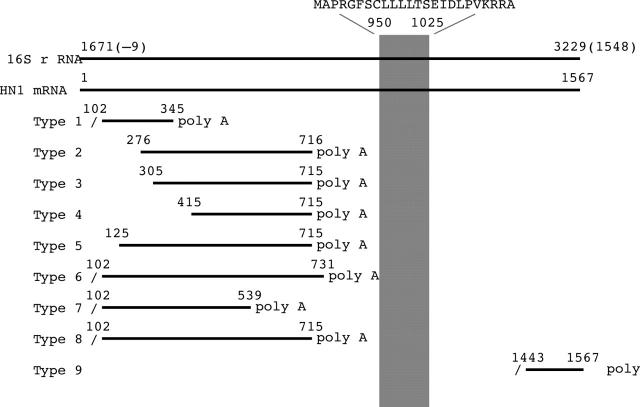
The sequences encoded within the 16S rRNA region with poly A tail. The cDNA fragments were aligned with the 16S rRNA region of the mitochondrial gene and the correlating humanin mRNA sequence. Southern colony hybridisations repeated these sequences in a total of three rounds independently. Oblique bars show the digestion sites by Rsa I and upward diagonal bar shows the region of humanin coding sequences. Although there are nine types of sequences with poly A tail within this region, only the type 9 sequence was identical to the previously reported mRNA encoding humanin peptide.
Figure 3.
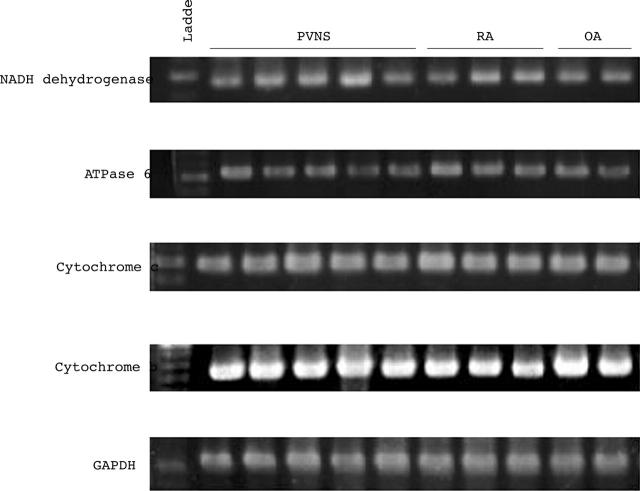
The expression of genes encoded in mitochondria other than humanin genes. Total RNA was extracted from the synovial cells of five patients with PVNS, three with RA, and three with OA, and NADH dehydrogenase, ATPase 6, cytochrome c, cytochrome b, and GAPDH mRNA levels were analysed by semiquantitative RT-PCR. The levels of expression of these genes in PVNS were not increased in other types of arthritis, indicating that the humanin gene was selectively expressed in mitochondrial genes in PVNS.
Figure 4.
Expression of humanin peptide in synovial cells from diffuse-type PVNS. Protein (20 µg) from synovial cell lysates was subjected to SDS-PAGE on a 5–20% gradient gel. Rabbit anti-humanin polyclonal antibody was used for western blotting. Synthesised peptide, which was used as antigen to produce rabbit anti-humanin polyclonal antibody, was used as a standard and rabbit IgG was used as a negative control.
Figure 5.
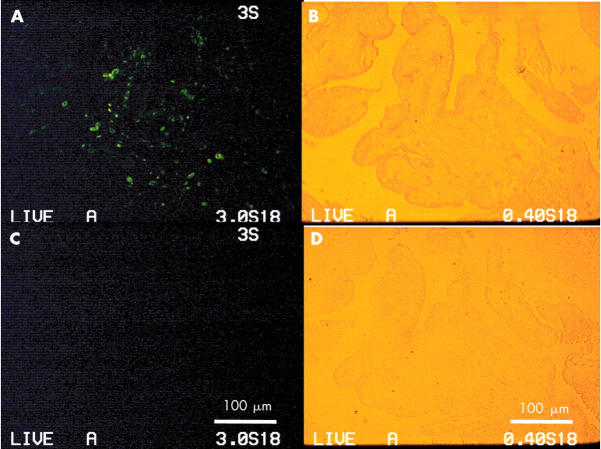
Synovial tissue from diffuse-type PVNS was fixed with 4% formaldehyde in PBS. The specimens were stained with anti-humanin antibody, followed by Alexa 488 goat antirabbit IgG, and photographed with a fluorescent microscope (x40). (A) Most positive cells (green) were distributed in a deep layer with haemosiderin deposit. (C) Negative control of the continuous section. (B, D) Backgrounds for (A) or (C), respectively.
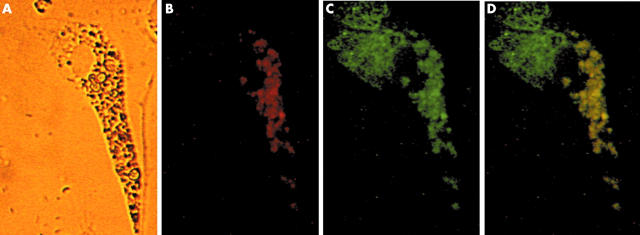
Figure 6.
Relationship between humanin peptide expression and mitochondria. Isolated synovial cells containing haemosiderin were double stained with anti-humanin antibody and anti hsp60 antibody as first antibodies, followed by goat antirabbit IgG and donkey antigoat IgG as second antibodies (x400). (A) Haemosiderin was deposited unequally throughout the cytoplasm. (B) Single anti-humanin antibody staining (red). (C) Single anti-hsp60 antibody staining (mitochondrial staining; green). (D) Humanin was dominantly distributed in the mitochondria around the siderosome (yellow).
Figure 7.
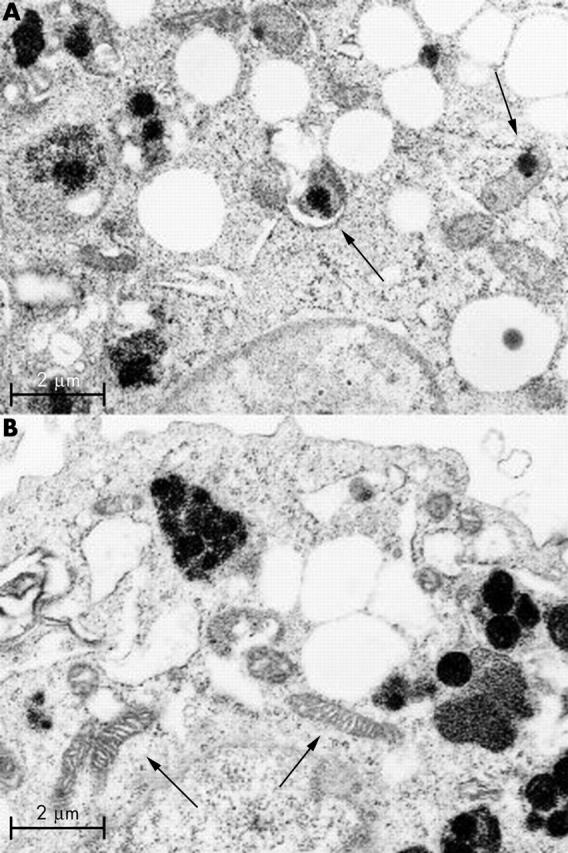
Electron micrograph of synovial cells from diffuse-type PVNS. Most of the electron dense iron deposits were observed within the siderosomes. Some electron dense iron deposits were observed within mitochondria (arrows). (A) Mitochondrial membrane debris with electron dense deposits was observed within the siderosome as an autophagosome (left arrow). (B) Some of the normal mitochondria (arrows) also were scattered throughout the cytoplasm. (Magnification x19 000.)
Figure 8.
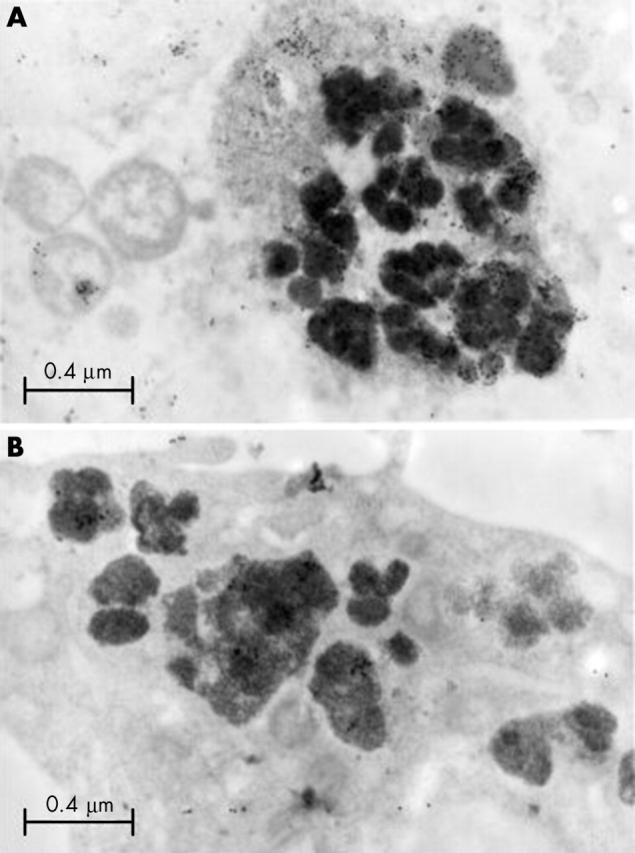
Electron microscopic immunohistochemistry of synovial cells from diffuse-type PVNS. (A) In some of the siderosomes, particles of colloidal gold, were precipitated in the debris adjacent to electron dense iron. These results demonstrate that humanin peptide is present within the debris that is phagocytosed into the siderosome. (B) Negative control for immunohistochemistry. (Magnification x29 000.)
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdul-Karim F. W., el-Naggar A. K., Joyce M. J., Makley J. T., Carter J. R. Diffuse and localized tenosynovial giant cell tumor and pigmented villonodular synovitis: a clinicopathologic and flow cytometric DNA analysis. Hum Pathol. 1992 Jul;23(7):729–735. doi: 10.1016/0046-8177(92)90340-9. [DOI] [PubMed] [Google Scholar]
- Baserga S. J., Linnenbach A. J., Malcolm S., Ghosh P., Malcolm A. D., Takeshita K., Forget B. G., Benz E. J., Jr Polyadenylation of a human mitochondrial ribosomal RNA transcript detected by molecular cloning. Gene. 1985;35(3):305–312. doi: 10.1016/0378-1119(85)90009-5. [DOI] [PubMed] [Google Scholar]
- Brunk Ulf T., Terman Alexei. The mitochondrial-lysosomal axis theory of aging: accumulation of damaged mitochondria as a result of imperfect autophagocytosis. Eur J Biochem. 2002 Apr;269(8):1996–2002. doi: 10.1046/j.1432-1033.2002.02869.x. [DOI] [PubMed] [Google Scholar]
- Byers P. D., Cotton R. E., Deacon O. W., Lowy M., Newman P. H., Sissons H. A., Thomson A. D. The diagnosis and treatment of pigmented villonodular synovitis. J Bone Joint Surg Br. 1968 May;50(2):290–305. [PubMed] [Google Scholar]
- Chamberlain M. A., Petts V., Gollins E. Transport of intravenously-injected ferritin across the guinea-pig synovium. Ann Rheum Dis. 1972 Nov;31(6):493–499. doi: 10.1136/ard.31.6.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Crosby E. B., Inglis A., Bullough P. G. Multiple joint involvement with pigmented villonodular synovitis. Radiology. 1977 Mar;122(3):671–672. doi: 10.1148/122.3.671. [DOI] [PubMed] [Google Scholar]
- Darling J. M., Glimcher L. H., Shortkroff S., Albano B., Gravallese E. M. Expression of metalloproteinases in pigmented villonodular synovitis. Hum Pathol. 1994 Aug;25(8):825–830. doi: 10.1016/0046-8177(94)90254-2. [DOI] [PubMed] [Google Scholar]
- Darling J. M., Goldring S. R., Harada Y., Handel M. L., Glowacki J., Gravallese E. M. Multinucleated cells in pigmented villonodular synovitis and giant cell tumor of tendon sheath express features of osteoclasts. Am J Pathol. 1997 Apr;150(4):1383–1393. [PMC free article] [PubMed] [Google Scholar]
- Docken W. P. Pigmented villonodular synovitis: a review with illustrative case reports. Semin Arthritis Rheum. 1979 Aug;9(1):1–22. doi: 10.1016/0049-0172(79)90001-5. [DOI] [PubMed] [Google Scholar]
- Dorwart R. H., Genant H. K., Johnston W. H., Morris J. M. Pigmented villonodular synovitis of synovial joints: clinical, pathologic, and radiologic features. AJR Am J Roentgenol. 1984 Oct;143(4):877–885. doi: 10.2214/ajr.143.4.877. [DOI] [PubMed] [Google Scholar]
- Fletcher J. A., Henkle C., Atkins L., Rosenberg A. E., Morton C. C. Trisomy 5 and trisomy 7 are nonrandom aberrations in pigmented villonodular synovitis: confirmation of trisomy 7 in uncultured cells. Genes Chromosomes Cancer. 1992 Apr;4(3):264–266. doi: 10.1002/gcc.2870040312. [DOI] [PubMed] [Google Scholar]
- Gehweiler J. A., Wilson J. W. Diffuse biarticular pigmented villonodular synovitis. Radiology. 1969 Oct;93(4):845–851. doi: 10.1148/93.4.845. [DOI] [PubMed] [Google Scholar]
- Guo Bin, Zhai Dayong, Cabezas Edelmira, Welsh Kate, Nouraini Shahrzad, Satterthwait Arnold C., Reed John C. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature. 2003 May 4;423(6938):456–461. doi: 10.1038/nature01627. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Halliwell B., Treffry A., Harrison P. M., Blake D. Effect of ferritin-containing fractions with different iron loading on lipid peroxidation. Biochem J. 1983 Feb 1;209(2):557–560. doi: 10.1042/bj2090557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y., Ito Y., Niikura T., Shao Z., Hata M., Oyama F., Nishimoto I. Mechanisms of neuroprotection by a novel rescue factor humanin from Swedish mutant amyloid precursor protein. Biochem Biophys Res Commun. 2001 May 4;283(2):460–468. doi: 10.1006/bbrc.2001.4765. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Niikura T., Tajima H., Yasukawa T., Sudo H., Ito Y., Kita Y., Kawasumi M., Kouyama K., Doyu M. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer's disease genes and Abeta. Proc Natl Acad Sci U S A. 2001 May 22;98(11):6336–6341. doi: 10.1073/pnas.101133498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Józsa L. Immunohistochemical characterization of pigmented villonodular synovitis. Zentralbl Pathol. 1992 Apr;138(2):119–123. [PubMed] [Google Scholar]
- Majima H. J., Oberley T. D., Furukawa K., Mattson M. P., Yen H. C., Szweda L. I., St Clair D. K. Prevention of mitochondrial injury by manganese superoxide dismutase reveals a primary mechanism for alkaline-induced cell death. J Biol Chem. 1998 Apr 3;273(14):8217–8224. doi: 10.1074/jbc.273.14.8217. [DOI] [PubMed] [Google Scholar]
- Maximov V., Martynenko A., Hunsmann G., Tarantul V. Mitochondrial 16S rRNA gene encodes a functional peptide, a potential drug for Alzheimer's disease and target for cancer therapy. Med Hypotheses. 2002 Dec;59(6):670–673. doi: 10.1016/s0306-9877(02)00223-2. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Roy R. S. The pathophysiology of superoxide: roles in inflammation and ischemia. Can J Physiol Pharmacol. 1982 Nov;60(11):1346–1352. doi: 10.1139/y82-201. [DOI] [PubMed] [Google Scholar]
- Morris C. J., Blake D. R., Wainwright A. C., Steven M. M. Relationship between iron deposits and tissue damage in the synovium: an ultrastructural study. Ann Rheum Dis. 1986 Jan;45(1):21–26. doi: 10.1136/ard.45.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C. J., Wainwright A. C., Steven M. M., Blake D. R. The nature of iron deposits in haemophilic synovitis. An immunohistochemical, ultrastructural and X-ray microanalytical study. Virchows Arch A Pathol Anat Histopathol. 1984;404(1):75–85. doi: 10.1007/BF00704252. [DOI] [PubMed] [Google Scholar]
- Motoori S., Majima H. J., Ebara M., Kato H., Hirai F., Kakinuma S., Yamaguchi C., Ozawa T., Nagano T., Tsujii H. Overexpression of mitochondrial manganese superoxide dismutase protects against radiation-induced cell death in the human hepatocellular carcinoma cell line HLE. Cancer Res. 2001 Jul 15;61(14):5382–5388. [PubMed] [Google Scholar]
- Muirden K. D. The anaemia of rheumatoid arthritis: the significance of iron deposits in the synovial membrane. Australas Ann Med. 1970 May;19(2):97–104. doi: 10.1111/imj.1970.19.2.97. [DOI] [PubMed] [Google Scholar]
- Nakazawa Yozo, Kamijo Takehiko, Koike Kenichi, Noda Tetsuo. ARF tumor suppressor induces mitochondria-dependent apoptosis by modulation of mitochondrial Bcl-2 family proteins. J Biol Chem. 2003 May 9;278(30):27888–27895. doi: 10.1074/jbc.M300510200. [DOI] [PubMed] [Google Scholar]
- O'Connell J. X., Fanburg J. C., Rosenberg A. E. Giant cell tumor of tendon sheath and pigmented villonodular synovitis: immunophenotype suggests a synovial cell origin. Hum Pathol. 1995 Jul;26(7):771–775. doi: 10.1016/0046-8177(95)90226-0. [DOI] [PubMed] [Google Scholar]
- Panduri Vijayalakshmi, Weitzman Sigmund A., Chandel Navdeep, Kamp David W. The mitochondria-regulated death pathway mediates asbestos-induced alveolar epithelial cell apoptosis. Am J Respir Cell Mol Biol. 2003 Feb;28(2):241–248. doi: 10.1165/rcmb.4903. [DOI] [PubMed] [Google Scholar]
- Peng G., Taylor J. D., Tchen T. T. Increased mitochondrial activities in pigmented (melanized) fish cells and nucleotide sequence of mitochondrial large rRNA. Biochem Biophys Res Commun. 1992 Nov 30;189(1):445–449. doi: 10.1016/0006-291x(92)91578-e. [DOI] [PubMed] [Google Scholar]
- Penta J. S., Johnson F. M., Wachsman J. T., Copeland W. C. Mitochondrial DNA in human malignancy. Mutat Res. 2001 May;488(2):119–133. doi: 10.1016/s1383-5742(01)00053-9. [DOI] [PubMed] [Google Scholar]
- Schumacher H. R., Lotke P., Athreya B., Rothfuss S. Pigmented villonodular synovitis: light and electron microscopic studies. Semin Arthritis Rheum. 1982 Aug;12(1):32–43. doi: 10.1016/0049-0172(82)90021-x. [DOI] [PubMed] [Google Scholar]
- Singh R., Grewal D. S., Chakravarti R. N. Experimental production of pigmented villonodular synovitis in the knee and ankle joints of rhesus monkeys. J Pathol. 1969 Jun;98(2):137–142. doi: 10.1002/path.1710980207. [DOI] [PubMed] [Google Scholar]
- Somerhausen N. S., Fletcher C. D. Diffuse-type giant cell tumor: clinicopathologic and immunohistochemical analysis of 50 cases with extraarticular disease. Am J Surg Pathol. 2000 Apr;24(4):479–492. doi: 10.1097/00000478-200004000-00002. [DOI] [PubMed] [Google Scholar]
- Tarantul V., Nikolaev A., Hannig H., Kalmyrzaev B., Muchoyan I., Maximov V., Nenasheva V., Dubovaya V., Hunsmann G., Bodemer W. Detection of abundantly transcribed genes and gene translocation in human immunodeficiency virus-associated non-Hodgkin's lymphoma. Neoplasia. 2001 Mar-Apr;3(2):132–142. doi: 10.1038/sj.neo.7900137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M. L., Spjut H. J., Dutton R. V., Glassman A. L., Askew J. B. Polyarticular pigmented villonodular synovitis. AJR Am J Roentgenol. 1981 Apr;136(4):821–823. doi: 10.2214/ajr.136.4.821. [DOI] [PubMed] [Google Scholar]
- Wixom R. L., Prutkin L., Munro H. N. Hemosiderin: nature, formation, and significance. Int Rev Exp Pathol. 1980;22:193–225. [PubMed] [Google Scholar]
- Wyllie J. C. The stromal cell reaction of pigmented villonodular synovitis: an electron microscopic study. Arthritis Rheum. 1969 Jun;12(3):205–214. doi: 10.1002/art.1780120307. [DOI] [PubMed] [Google Scholar]
- YOUNG J. M., HUDACEK A. G. Experimental production of pigmented villonodular synovitis in dogs. Am J Pathol. 1954 Jul-Aug;30(4):799–811. [PMC free article] [PubMed] [Google Scholar]
- Yu Weiping, Sanders Bob G., Kline Kimberly. RRR-alpha-tocopheryl succinate-induced apoptosis of human breast cancer cells involves Bax translocation to mitochondria. Cancer Res. 2003 May 15;63(10):2483–2491. [PubMed] [Google Scholar]