Abstract
Background: In primary Sjögren's syndrome (pSS), extraglandular involvement might result from more intense stimulation of autoreactive B cells. Thus markers of B cell activation could be useful in the clinical assessment of this disease.
Objective: To investigate the association of serum B lymphocyte stimulator (BLyS) and ß2 microglobulin with autoantibody production and extraglandular involvement in pSS.
Methods: Serum concentrations of BLyS and ß2 microglobulin were analysed in 177 patients with pSS according to the American–European consensus group criteria. Serum ß2 microglobulin was determined serially in 25 patients.
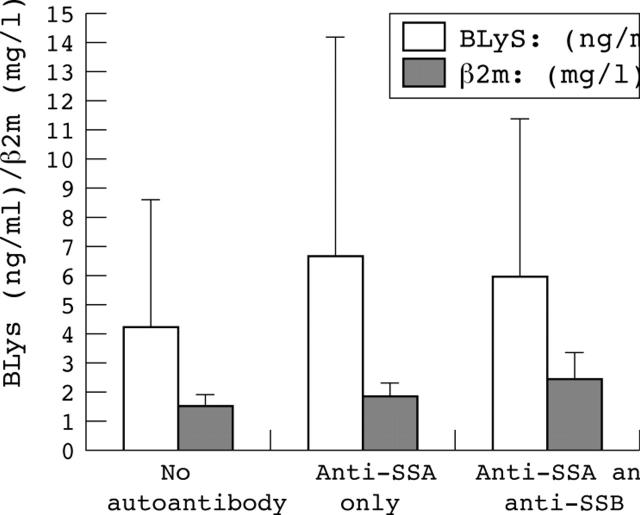
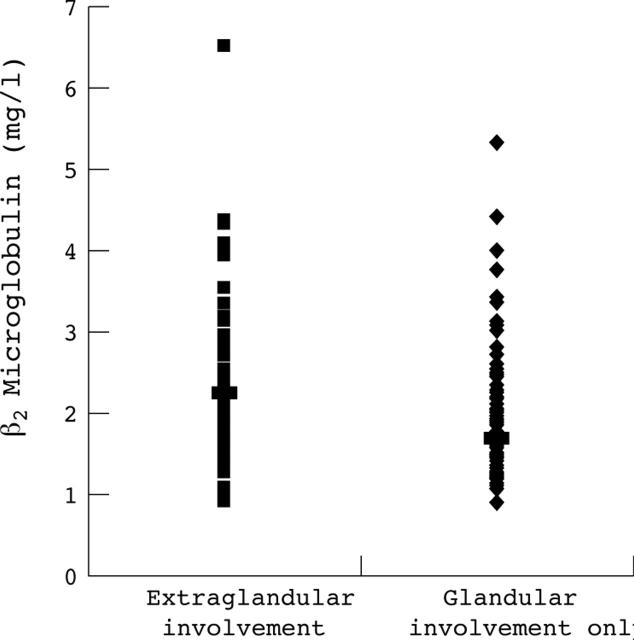
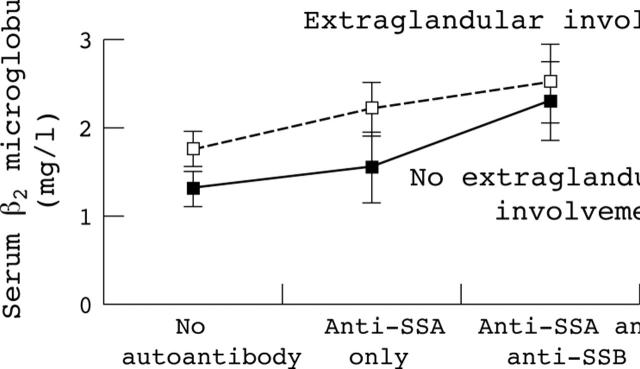
Results: Autoantibody secretion (presence of anti-SSA antibody alone or of both anti-SSA and anti-SSB) was associated with increased serum BLyS and ß2 microglobulin. No correlation was found between BLyS and ß2 microglobulin levels (p = 0.36). Serum concentrations of ß2 microglobulin and C reactive protein and positive anti-SSB antibody results were associated with extraglandular involvement on univariate analysis (p<10–4, p = 0.003, and p = 0.004, respectively). Serum ß2 microglobulin was also significantly increased in patients with extraglandular involvement without autoantibodies (mean (SD): 1.75 (0.7) v 1.39 (0.5) mg/l, p = 0.039). Multivariate analysis showed that extraglandular involvement was associated only with increased serum ß2 microglobulin (p = 0.035, odds ratio = 2.78 (95% confidence interval, 1.07 to 7.22)). Among the 25 patients who had serial determinations of serum ß2 microglobulin, the concentrations were increased in all those with disease flare and decreased in three following treatment. Serum BLyS, gamma globulin, IgG, and rheumatoid factor levels were not associated with features of systemic involvement.
Conclusions: Serum ß2 microglobulin and BLyS reflect B cell activation in different ways in pSS. Serum ß2 microglobulin assessment could be helpful as an activity marker in pSS.
Full Text
The Full Text of this article is available as a PDF (95.4 KB).
Figure 1.
Serum ß2 microglobulin (ß2m) and B lymphocyte stimulator (BLys) concentrations according to the presence of anti-SSA/SSB antibodies.
Figure 2.
ß2 microglobulin concentrations in patients with extraglandular involvement and in patients with glandular involvement only.
Figure 3.
Mean serum ß2 microglobulin value in 154 patients with primary Sjögren's syndrome according to the presence of extraglandular involvement and of anti-SSA/SSB antibodies.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander E. L., Arnett F. C., Provost T. T., Stevens M. B. Sjögren's syndrome: association of anti-Ro(SS-A) antibodies with vasculitis, hematologic abnormalities, and serologic hyperreactivity. Ann Intern Med. 1983 Feb;98(2):155–159. doi: 10.7326/0003-4819-98-2-155. [DOI] [PubMed] [Google Scholar]
- Asmussen K. H., Bowman S. J. Outcome measures in Sjögren's syndrome. Rheumatology (Oxford) 2001 Oct;40(10):1085–1088. doi: 10.1093/rheumatology/40.10.1085. [DOI] [PubMed] [Google Scholar]
- Bataille R., Grenier J., Sany J. Beta-2-microglobulin in myeloma: optimal use for staging, prognosis, and treatment--a prospective study of 160 patients. Blood. 1984 Feb;63(2):468–476. [PubMed] [Google Scholar]
- Bianchi C., Donadio C., Tramonti G., Consani C., Lorusso P., Rossi G. Reappraisal of serum beta2-microglobulin as marker of GFR. Ren Fail. 2001 May-Jul;23(3-4):419–429. doi: 10.1081/jdi-100104725. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Parham P. Structure, function, and diversity of class I major histocompatibility complex molecules. Annu Rev Biochem. 1990;59:253–288. doi: 10.1146/annurev.bi.59.070190.001345. [DOI] [PubMed] [Google Scholar]
- Castro José, Jiménez-Alonso Juan, Sabio José Mario, Rivera-Cívico Francisco, Martín-Armada María, Rodríguez Miguel Angel, Jáimez Laura, Castillo María Jesús, Sánchez-Román Julio, Grupo Lupus Virgen de las Nieves Salivary and serum beta2-microglobulin and gamma-glutamyl-transferase in patients with primary Sjögren syndrome and Sjögren syndrome secondary to systemic lupus erythematosus. Clin Chim Acta. 2003 Aug;334(1-2):225–231. doi: 10.1016/s0009-8981(03)00162-1. [DOI] [PubMed] [Google Scholar]
- Cheema G. S., Roschke V., Hilbert D. M., Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum. 2001 Jun;44(6):1313–1319. doi: 10.1002/1529-0131(200106)44:6<1313::AID-ART223>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Chronowski Gregory M., Wilder Richard B., Tucker Susan L., Ha Chul S., Sarris Andreas H., Hagemeister Fredrick B., Barista Ibrahim, Hess Mark A., Cabanillas Fernando, Cox James D. An elevated serum beta-2-microglobulin level is an adverse prognostic factor for overall survival in patients with early-stage Hodgkin disease. Cancer. 2002 Dec 15;95(12):2534–2538. doi: 10.1002/cncr.10998. [DOI] [PubMed] [Google Scholar]
- Davidson B. K., Kelly C. A., Griffiths I. D. Primary Sjögren's syndrome in the North East of England: a long-term follow-up study. Rheumatology (Oxford) 1999 Mar;38(3):245–253. doi: 10.1093/rheumatology/38.3.245. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Chan E., Benton L., Fong S., Friedlaender M., Howell F. V. Treatment of primary Sjögren's syndrome with hydroxychloroquine. Am J Med. 1988 Oct 14;85(4A):62–67. doi: 10.1016/0002-9343(88)90365-8. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Dixon R., Guarrasi V., Krubel S. Treatment of primary Sjögren's syndrome with hydroxychloroquine: a retrospective, open-label study. Lupus. 1996 Jun;5 (Suppl 1):S31–S36. [PubMed] [Google Scholar]
- Gottenberg Jacques-Eric, Busson Marc, Loiseau Pascale, Cohen-Solal Julien, Lepage Virginia, Charron Dominique, Sibilia Jean, Mariette Xavier. In primary Sjögren's syndrome, HLA class II is associated exclusively with autoantibody production and spreading of the autoimmune response. Arthritis Rheum. 2003 Aug;48(8):2240–2245. doi: 10.1002/art.11103. [DOI] [PubMed] [Google Scholar]
- Harley J. B., Alexander E. L., Bias W. B., Fox O. F., Provost T. T., Reichlin M., Yamagata H., Arnett F. C. Anti-Ro (SS-A) and anti-La (SS-B) in patients with Sjögren's syndrome. Arthritis Rheum. 1986 Feb;29(2):196–206. doi: 10.1002/art.1780290207. [DOI] [PubMed] [Google Scholar]
- Hatron P. Y., Wallaert B., Gosset D., Tonnel A. B., Gosselin B., Voisin C., Devulder B. Subclinical lung inflammation in primary Sjögren's syndrome. Relationship between bronchoalveolar lavage cellular analysis findings and characteristics of the disease. Arthritis Rheum. 1987 Nov;30(11):1226–1231. doi: 10.1002/art.1780301104. [DOI] [PubMed] [Google Scholar]
- Ioannidis John P. A., Vassiliou Vassilios A., Moutsopoulos Haralampos M. Long-term risk of mortality and lymphoproliferative disease and predictive classification of primary Sjögren's syndrome. Arthritis Rheum. 2002 Mar;46(3):741–747. doi: 10.1002/art.10221. [DOI] [PubMed] [Google Scholar]
- Jonsson Roland, Gordon Tom P., Konttinen Yrjö T. Recent advances in understanding molecular mechanisms in the pathogenesis and antibody profile of Sjögren's syndrome. Curr Rheumatol Rep. 2003 Aug;5(4):311–316. doi: 10.1007/s11926-003-0010-z. [DOI] [PubMed] [Google Scholar]
- Kassan S. S., Thomas T. L., Moutsopoulos H. M., Hoover R., Kimberly R. P., Budman D. R., Costa J., Decker J. L., Chused T. M. Increased risk of lymphoma in sicca syndrome. Ann Intern Med. 1978 Dec;89(6):888–892. doi: 10.7326/0003-4819-89-6-888. [DOI] [PubMed] [Google Scholar]
- Lahdensuo A., Korpela M. Pulmonary findings in patients with primary Sjögren's syndrome. Chest. 1995 Aug;108(2):316–319. doi: 10.1378/chest.108.2.316. [DOI] [PubMed] [Google Scholar]
- Lavie Frédéric, Miceli-Richard Corinne, Quillard Jeanine, Roux Sophie, Leclerc Philippe, Mariette Xavier. Expression of BAFF (BLyS) in T cells infiltrating labial salivary glands from patients with Sjögren's syndrome. J Pathol. 2004 Apr;202(4):496–502. doi: 10.1002/path.1533. [DOI] [PubMed] [Google Scholar]
- Lifson A. R., Hessol N. A., Buchbinder S. P., O'Malley P. M., Barnhart L., Segal M., Katz M. H., Holmberg S. D. Serum beta 2-microglobulin and prediction of progression to AIDS in HIV infection. Lancet. 1992 Jun 13;339(8807):1436–1440. doi: 10.1016/0140-6736(92)92030-j. [DOI] [PubMed] [Google Scholar]
- Mackay F., Woodcock S. A., Lawton P., Ambrose C., Baetscher M., Schneider P., Tschopp J., Browning J. L. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999 Dec 6;190(11):1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay Fabienne, Browning Jeffrey L. BAFF: a fundamental survival factor for B cells. Nat Rev Immunol. 2002 Jul;2(7):465–475. doi: 10.1038/nri844. [DOI] [PubMed] [Google Scholar]
- Maddali Bongi S., Campana G., D'Agata A., Palermo C., Bianucci G. The diagnosis value of beta 2-microglobulin and immunoglobulins in primary Sjögren's syndrome. Clin Rheumatol. 1995 Mar;14(2):151–156. doi: 10.1007/BF02214934. [DOI] [PubMed] [Google Scholar]
- Mariette X., Roux S., Zhang J., Bengoufa D., Lavie F., Zhou T., Kimberly R. The level of BLyS (BAFF) correlates with the titre of autoantibodies in human Sjögren's syndrome. Ann Rheum Dis. 2003 Feb;62(2):168–171. doi: 10.1136/ard.62.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T., Weber J. C., Levallois H., Labouret N., Soley A., Koenig S., Korganow A. S., Pasquali J. L. Salivary gland lymphomas in patients with Sjögren's syndrome may frequently develop from rheumatoid factor B cells. Arthritis Rheum. 2000 Apr;43(4):908–916. doi: 10.1002/1529-0131(200004)43:4<908::AID-ANR24>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Michalski J. P., Daniels T. E., Talal N., Grey H. M. Beta2 microglobulin and lymphocytic infiltration in Sjögren's syndrome. N Engl J Med. 1975 Dec 11;293(24):1228–1231. doi: 10.1056/NEJM197512112932404. [DOI] [PubMed] [Google Scholar]
- Moore P. A., Belvedere O., Orr A., Pieri K., LaFleur D. W., Feng P., Soppet D., Charters M., Gentz R., Parmelee D. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999 Jul 9;285(5425):260–263. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- Morfeldt-Månson J., Julander I., von Stedingk L. V., Wasserman J., Nilsson B. Elevated serum beta-2-microglobulin--a prognostic marker for development of AIDS among patients with persistent generalized lymphadenopathy. Infection. 1988 Mar-Apr;16(2):109–110. doi: 10.1007/BF01644315. [DOI] [PubMed] [Google Scholar]
- Mori M., Terui Y., Tanaka M., Tomizuka H., Mishima Y., Ikeda M., Kasahara T., Uwai M., Ueda M., Inoue R. Antitumor effect of beta2-microglobulin in leukemic cell-bearing mice via apoptosis-inducing activity: activation of caspase-3 and nuclear factor-kappaB. Cancer Res. 2001 Jun 1;61(11):4414–4417. [PubMed] [Google Scholar]
- Oxholm P. Primary Sjögren's syndrome--clinical and laboratory markers of disease activity. Semin Arthritis Rheum. 1992 Oct;22(2):114–126. doi: 10.1016/0049-0172(92)90005-x. [DOI] [PubMed] [Google Scholar]
- Pertovaara M., Korpela M., Pasternack A. Factors predictive of renal involvement in patients with primary Sjögren's syndrome. Clin Nephrol. 2001 Jul;56(1):10–18. [PubMed] [Google Scholar]
- Pertovaara M., Pukkala E., Laippala P., Miettinen A., Pasternack A. A longitudinal cohort study of Finnish patients with primary Sjögren's syndrome: clinical, immunological, and epidemiological aspects. Ann Rheum Dis. 2001 May;60(5):467–472. doi: 10.1136/ard.60.5.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmbeck R., Thoma S., Reimann J. Processing of exogenous hepatitis B surface antigen particles for Ld-restricted epitope presentation depends on exogenous beta2-microglobulin. Eur J Immunol. 1997 Dec;27(12):3471–3484. doi: 10.1002/eji.1830271248. [DOI] [PubMed] [Google Scholar]
- Schneider P., MacKay F., Steiner V., Hofmann K., Bodmer J. L., Holler N., Ambrose C., Lawton P., Bixler S., Acha-Orbea H. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999 Jun 7;189(11):1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour John F., Pro Barbara, Fuller Lillian M., Manning John T., Hagemeister Fredrick B., Romaguera Jorge, Rodriguez Maria A., Ha Chul S., Smith Terry L., Ayala Ana. Long-term follow-up of a prospective study of combined modality therapy for stage I-II indolent non-Hodgkin's lymphoma. J Clin Oncol. 2003 Jun 1;21(11):2115–2122. doi: 10.1200/JCO.2003.07.111. [DOI] [PubMed] [Google Scholar]
- Shields M. J., Kubota R., Hodgson W., Jacobson S., Biddison W. E., Ribaudo R. K. The effect of human beta2-microglobulin on major histocompatibility complex I peptide loading and the engineering of a high affinity variant. Implications for peptide-based vaccines. J Biol Chem. 1998 Oct 23;273(43):28010–28018. doi: 10.1074/jbc.273.43.28010. [DOI] [PubMed] [Google Scholar]
- Stohl William, Metyas Samy, Tan Soon-Min, Cheema Gurtej S., Oamar Bonifacia, Xu Dong, Roschke Viktor, Wu Youmei, Baker Kevin P., Hilbert David M. B lymphocyte stimulator overexpression in patients with systemic lupus erythematosus: longitudinal observations. Arthritis Rheum. 2003 Dec;48(12):3475–3486. doi: 10.1002/art.11354. [DOI] [PubMed] [Google Scholar]
- Talal N., Grey H. M., Zvaifler N., Michalski J. P., Daniels T. E. Elevated salivary and synovial fluid beta2-microglobulin in Sjogren's syndrome and rheumatoid arthritis. Science. 1975 Mar 28;187(4182):1196–1198. doi: 10.1126/science.46621. [DOI] [PubMed] [Google Scholar]
- Tishler M., Yaron I., Shirazi I., Yaron M. Hydroxychloroquine treatment for primary Sjögren's syndrome: its effect on salivary and serum inflammatory markers. Ann Rheum Dis. 1999 Apr;58(4):253–256. doi: 10.1136/ard.58.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitali C., Bombardieri S., Jonsson R., Moutsopoulos H. M., Alexander E. L., Carsons S. E., Daniels T. E., Fox P. C., Fox R. I., Kassan S. S. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002 Jun;61(6):554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren M., Tengnér P., Gunnarsson I., Lundberg I., Hedfors E., Ringertz N. R., Pettersson I. Ro/SS-A and La/SS-B antibody level variation in patients with Sjögren's syndrome and systemic lupus erythematosus. J Autoimmun. 1998 Feb;11(1):29–38. doi: 10.1006/jaut.1997.0173. [DOI] [PubMed] [Google Scholar]
- Xie Jin, Wang Ying, Freeman Muta E., 3rd, Barlogie Bart, Yi Qing. Beta 2-microglobulin as a negative regulator of the immune system: high concentrations of the protein inhibit in vitro generation of functional dendritic cells. Blood. 2003 Jan 16;101(10):4005–4012. doi: 10.1182/blood-2002-11-3368. [DOI] [PubMed] [Google Scholar]
- Xie Jin, Yi Qing. Beta2-microglobulin as a potential initiator of inflammatory responses. Trends Immunol. 2003 May;24(5):228–230. doi: 10.1016/s1471-4906(03)00076-0. [DOI] [PubMed] [Google Scholar]