Abstract
Mucolipidosis IV (MLIV) is an autosomal recessive disorder of unknown etiology characterized by severe visual impairment and psychomotor retardation. Recently, there has been considerable interest in positional cloning of the MLIV gene. It is unknown whether MLIV is a genetically homogenous disorder. In this paper, we present experiments that determined whether the MLIV phenotype in fibroblasts could be corrected by fusing normal cells to MLIV cells and fusing fibroblasts from pairs of patients. All of our MLIV patients fulfilled several diagnostic criteria that we developed. In addition, we found high sensitivity to chloroquine in cultured fibroblasts from MLIV patients. We found that normal cells corrected the MLIV phenotype. Fusion products of normal and MLIV fibroblasts, but not MLIV fibroblasts themselves, were relatively protected against chloroquine selection. In addition, 74% of the normal-to-patient fusion products had reduced levels or total loss of MLIV characteristic autofluorescence. However, there was no complementation of the phenotype in fibroblast cultures from any of our MLIV patients, including those of non-Jewish ancestry. In fusion products of MLIV cultures from 24 patients, 90–100% of the cells remained autofluorescent. These results indicate that all of our known MLIV patients, regardless of ancestry or severity of the developmental defect, have a single mutated gene.
Mucolipidosis IV (MLIV) is a metabolic disorder transmitted in an autosomal recessive manner (1). Clinically, the patients suffer visual impairment and psychomotor retardation starting within the first year of life (2, 3). Although most of the patients are of Ashkenazi Jewish origin, MLIV has been described in other ethnic groups (3). Diagnosis of MLIV has been based on clinical and ultramicroscopic parameters (4, 5). Numerous storage bodies of varying size and shape are found throughout many of the tissues by using electron microscopy (6). The gene mutated in the disease has not been identified, and the nature of the primary storage material is also unknown. The prospective longitudinal study on MLIV conducted at the Clinical Center of the National Institutes of Health included more than 20 MLIV patients, most of them of Ashkenazi Jewish ancestry. A few patients are of other ethnic groups, including 3 non-Jewish Caucasians and 1 South American Indian. All suffered hypergastrinemia secondary to achlorohydria (7). Most of them showed a severe thinning of the corpus callosum (8). A characteristic autofluorescence in cultured fibroblasts derived from MLIV patients has been described (9). Although some of the pathological features of these patients are unique to MLIV, the possibility that there is more than one gene involved in the etiology of the disease has not been ruled out. We therefore sought to verify the homogeneity of the MLIV patient population by attempting to correct the phenotype by using cells fused from different patients. We demonstrate that none of the patient cell lines could complement the MLIV phenotype.
MATERIALS AND METHODS
Materials.
Ammonium chloride, chloroquine, quinacrine, and artemisinin were purchased from Sigma. Triton wr1339 was from Supelco. An imidazol analog, dodecylimidazol, was synthesized in our laboratory.
Cell Cultures.
Human skin fibroblasts were grown in Earl’s Modified Eagle’s Medium (EMEM) (Biofluids, Rockville, MD), in 10% fetal bovine serum (HyClone), with penicillin and streptomycin. Normal cultures were obtained under approved National Institutes of Health guidelines from unaffected individuals, up to 28 years of age. They were used between passages 2 and 14. Fibroblast cultures obtained from 24 MLIV patients (ages 3–23) were used between passages 2 and 11. A description of the clinical presentation in each of the patients and their ethnic background are provided in Table 1. One MLIV culture (culture no. GM2527) was obtained from the Human Genetic Mutant Cell Repository (Institute of Medical Research, Camden, NJ). Another MLIV culture (TC95-2754) was kindly provided by A. Hamush (John Hopkins University, Baltimore). Fibroblast cultures from obligate heterozygotes, the parents of patients DMN91.71, and DMN92.24 (passage 2–5), were also tested.
Table 1.
Clinical phenotype of MLIV patients
| Cell line | Ethnic background | Neurologic motor deficit | Eye pathology | Hypergastri nemia | Head MRI Findings |
|---|---|---|---|---|---|
| DMN98.64 | AJ | Severe | Yes | Yes | a,b,c,d |
| DMN97.55 | NJC | Moderate | Yes | Yes | b,c |
| DMN98.69 | AJ | Moderate | Yes | Yes | a,b,c,d |
| DMN97.98 | AJ | Mild | Yes | Yes | b,c |
| DMN98.52 | NJC | Severe | Yes | Yes | a,b,c,d |
| DMN91.70 | AJ | Severe | Yes | ND | ND |
| DMN97.16 | AJ | Severe | Yes | Yes | a,b,c |
| DMN92.73 | AJ | Moderate | Yes | Yes | a,b,c,d |
| DMN97.13 | AJ | Severe | Yes | Yes | a,b,c |
| TC95-2756 | AJ | ND | ND | ND | ND |
| GM02527 | AJ | ND | ND | ND | ND |
| DMN97.62 | AJ | Severe | Yes | Yes | a,b,c |
| DMN97.33 | AJ | Severe | Yes | Yes | a,b,c |
| DMN97.34 | AJ | Severe | Yes | Yes | a,b,c,d |
| DMN97.22 | AJ | Severe | Yes | Yes | a,b,c,d |
| DMN96.37 | AJ | Moderate | Yes | Yes | a,b,c |
| DMN96.73 | AJ | Severe | Yes | Yes | a,b,c,d |
| DMN96.110 | AJ | Moderate | Yes | Yes | a,b,c,d |
| DMN95.74 | AJ | Moderate | Yes | Yes | a,b,c |
| DMN96.56 | Am Ind | Moderate | Yes | Yes | a,b,c,d |
| DMN95.60 | NJC | Moderate | Yes | Yes | mild a,b,c |
| DMN96.57 | NJC | Moderate | Yes | Yes | a,b,c,d |
| DMN91.85 | AJ | Moderate | Yes | Yes | a,b,c,d |
| DMN92.23 | AJ | Severe | Yes | Yes | a,b,c,d |
AJ, Ashkenazi Jewish; NJC, non-Jewish caucasian; AmInd, South American Indian. Mild motor deficit indicates ability to walk with no or minimal aid; moderate, ability to stand without help or walk with help; severe, ability to sit independently but not stand. Eye pathologies included corneal clouding, retinopathy, and optic atrophy. Head MRI findings included: a, dysplastic corpus callosum; b, white matter abnormalities; c, excess iron in basal ganglia; d, cerebellar atrophy. ND, not determined.
Selection Experiments.
Cells were seeded in 96-well culture dishes at 5 × 105 cells per dish. Cells were incubated with chloroquine in EMEM at pH 7.4 for 6 hr. The chloroquine was removed, and the cells were incubated for an additional 48 hr in growth medium. Under this treatment, MLIV cells die in a dose-dependent fashion, whereas normal cells remain largely intact. After this selection procedure, cell viability was determined by using a colorimetric assay with WST-1 cell proliferation reagent (Boehringer Mannheim) and a plate reader at 450 nm.
Fusion Experiments.
For fusion studies, cells from patients and normal controls were treated for 30 min with either red (orange) or green CellTracker (1 μg/ml medium) (Molecular Probes). The dye stains most cellular organelles, including the cytoplasm and nucleus. Cultures stained differentially were then mixed and seeded together at 4 × 105 cells per culture and fused by using PEG 1500 (10). The following day, cells were passed through a Ficoll step gradient according to the method of Nelson and Carey (11). Dense Ficoll fractions containing multinuclear cells were seeded in 2-chamber glass slides (Lab-Tek). At 3–4 days after gradient enrichment, the cells were fixed with 3% paraformaldehyde in PBS and mounted with p-phenylenediamine for viewing.
Fluorescence Microscopy.
Cells were viewed by using a Zeiss Axioplan microscope equipped with phase and epifluorescence optics and a mercury vapor lamp. Slides were scanned for multinuclear cells under phase ×40 lens, followed by determination of fluorescence in specific cells at three different excitation and emission wavelengths. Autofluorescence was viewed under UV with a filter set: G 365 FT 395 and LP 420 nm. Green and red fluorescence was detected with the filters BP 455, FT 501, LP515–565, and BP 546, FT 580, LP 590 nm, respectively. Cells in which there was a possibility that the nucleus from one of the original cells had been eliminated were discarded from the study.
RESULTS
Chloroquine Selection.
Chloroquine sensitivity of MLIV cells was discovered when different lysosomotropic agents were screened for their ability to selectively kill MLIV fibroblasts in culture. In the initial screen, we used Triton wr1339, an imidazol analog dodecylimidazol, ammonium chloride, and chloroquine. Only chloroquine was selective for MLIV fibroblasts. In addition, hydrophobic amines, such as stearylamine and sphinganine, did not kill the cells selectively (Table 2). Antimalarial agents with properties similar to chloroquine, such as primaquine or quinacrine, exhibited effects similar to chloroquine, whereas other antimalarial drugs of different chemical structure, such as artemisinin, did not kill fibroblasts even at very high concentrations (Table 2). Selective toxicity toward MLIV cells was evident in experiments using different survival measuring systems. MLIV cell viability was reduced compared with normal cells when measured by protein levels, colony-forming ability, and WST-1 colorimetric assay (Table 3). The mechanism of killing of MLIV cells by chloroquine is unclear. The addition of iron, or its removal by chelation, during the selection experiments did not significantly alter the results (data not shown). Furthermore, decreased viability was unrelated to reduced lysosomal hydrolase activities because cellular viability was unaffected at chloroquine concentrations that produced a 95% reduction in the activity of major lysosomal enzymes (i.e., sphingomyelinase and hexosaminidase) (data not shown). In some experiments, at the lower chloroquine levels, the amount of protein in the cultures increased, probably secondary to inhibition of degradation. Cells of obligate heterozygotes did not differ from normal control cells in terms of chloroquine sensitivity, indicating that one normal chromosome is sufficient to correct the phenotype (data not shown).
Table 2.
Difference in survival of normal and ML4 cells under drug treatment
| Reagent | Concentration | Change in toxicity, % |
|---|---|---|
| Ammonium chloride | 10–200 mM | 0 |
| Triton WR 1339 | 0.25–2.5% | 0 |
| Dodecylimidazol | 50–100 μM | 0 |
| Chloroquine | 50–100 μM | 89–71 |
| Sphinganine | 10–200 μg/ml | 0 |
| Stearylamine | 5–100 μM | 0 |
| Primaquine | 250 μM | 60 |
| Quinarcine | 4–10 μM | 87–70 |
| Artemisinin | 0.1–3 mM | 0 |
Cells were treated with different reagents as described in Materials and Methods with the exception that in these initial experiments, pH was not tightly controlled. The difference in % survival between normal and MLIV cells is presented. Each experiment was repeated at least twice.
Table 3.
Selection results measured by colony-forming units, Lowry protein assay, and WST-1
| Chloroquine, mM | Colonies
|
Protein
|
WST-1
|
|||
|---|---|---|---|---|---|---|
| Normal | MLIV | Normal | MLIV | Normal | MLIV | |
| 0 | 100 | 100 | 100 | 100 | 100 | 100 |
| 0.3 | 75.7 | 5.7 | 79.1 | 55.7 | 109 | 50.6 |
| 0.6 | 62.8 | 4.2 | 66.4 | 24.7 | 76.7 | 23.9 |
| 1.2 | 44.3 | 0.7 | 55.1 | 20.6 | ND | ND |
Cells were grown in 24-well culture dishes for protein and colony-forming ability experiments and in 96-well dishes for WST-1 experiments. For colony-forming ability experiments, cells were treated with chloroquine for 6 hr, and then washed and trypsinized, counted, and seeded in 60-mm dishes at 500 cells per dish. After 14 days, colonies were stained with crystal violet and counted. For protein determination experiments, cells were kept in growth medium for 48 hr after chloroquine treatment, followed by solubilization in 0.5 M NaOH and determination of protein according to Lowry (12). For WST-1 experiments, cells were kept in growth medium for 48 hr after chloroquine treatment followed by addition of the WST-1 reagent, a short incubation, and reading in a plate reader at 450 nm. Each experiment was performed in triplicate. ND, not determined.
Fusion Experiments.
Correction of chloroquine sensitivity. To prove that the normal gene could correct the MLIV phenotype by protecting cells in the chloroquine selection assay, we fused normal and MLIV cells and tested the relative sensitivity of fusion products. Normal and MLIV cells stained by green and red CellTracker, respectively, were fused and seeded onto microscopic slides. Cells were treated with chloroquine at different concentrations, and the proportion of normal–MLIV fused cells was compared with that of normal–normal and MLIV–MLIV fused cells. The MLIV–MLIV fusion products dose-dependently disappeared from the culture. In contrast, the normal–MLIV fusion products remained largely intact (Fig. 1). This experiment also demonstrates that coculturing the normal and MLIV fibroblasts is insufficient to correct the MLIV phenotype by a soluble factor and that the only means of correction is the presence of the normal gene product within mutant cells. We could not use chloroquine selection to test for complementation in fused patient cells because in such experiments all the cells are expected to die, which could also indicate an experimental artifact.
Figure 1.
Survival of normal and MLIV fibroblasts fusion products under chloroquine treatment. Normal and MLIV fibroblasts were fused as described in Materials and Methods. After 24 hr, cells were seeded in equal densities in two 2-chamber slides. The following day, the cells were treated with chloroquine for 24 hr and then washed and fixed for microscopy. One hundred surviving cells containing two or more nuclei were counted in each treatment, and the fraction of normal–normal (green), MLIV–MLIV (red), and normal–MLIV (orange) was recorded. The results of a representative experiment are presented. Chloroquine treatment almost eliminated MLIV–MLIV fusion products from the culture. Also, a small fraction of normal–MLIV fusion products disappeared in the higher chloroquine concentrations as indicated by the increased proportion of normal–normal cells.
Correction of autofluorescence.
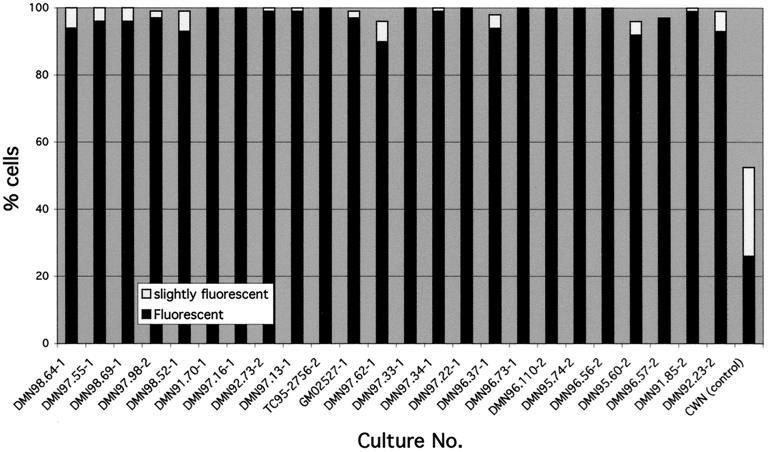
Correcting the MLIV phenotype by normal cell fusion was also detectable by the disappearance of autofluorescence in fusion products (Figs. 2 and 3). It takes more than 48 hours for the autofluorescence to disappear from MLIV cells. Therefore, in most cases, the analysis was done 4–5 days after fusion. In a typical experiment, we counted 100 fusion products containing green and red nuclei and recorded the autofluorescence in each one. In six different experiments, an average 47.5 ± 13.8% of normal-MLIV fusion products had completely lost autofluorescence, and 26.5 ± 7.2% contained reduced levels of autofluorescence. Enhanced correction was obtained when the ratio of normal to MLIV nuclei in the fused cell was ≥1, indicating that the defective gene product may interfere with correction of the phenotype. In a series of experiments, each of the MLIV fibroblast cultures in our laboratory was fused to one of two different index cultures obtained from Ashkenazi Jewish patients to identify complementation groups within the MLIV population. A typical fusion product is shown in Fig. 4. In all of the culture combinations tested, the autofluorescence was very strong after fusion in most cells. The proportion of autofluorescent fusion products carrying nuclei from both cultures in all the experiments was 90–100% (Fig. 3), proving that there are no complementation groups within our cultures. As indicated in Table 1, the cultures were obtained from patients with variable clinical phenotype and ethnic origin.
Figure 2.
Elimination of autofluorescence in fusion products of normal and MLIV fibroblasts. Normal (stained green) and MLIV (stained red) fibroblasts were fused as described in Materials and Methods. Four days after seeding on slides, cells were fixed and photographed. (A) A phase micrograph showing a normal cell (nucleus marked N), a normal–MLIV fusion product (nuclei marked N, M), and an MLIV cell (nucleus marked M). The large arrow points to the phase-dense material. (B) Fluorescence in the UV channel showing autofluorescence in MLIV cell and not in the fusion product. The autofluorescence colocalizes with the phase-dense material. (C) Fluorescence in the green wavelength. In the MLIV cell, only autofluorescent material appears green in that channel. (D) Fluorescence of the same cells in the red channel. The green nucleus is highly visible in the fused cell, whereas the red nucleus is less apparent because of postlabeling diffusion of the red dye.
Figure 3.
Complementation of autofluorescence in MLIV fibroblast cultures. Cells from patients were fused with either one of the two index MLIV cultures, DMN96.73 (labeled 1) and DMN96.37 (labeled 2), as described in Materials and Methods. The results of counting 100 fusion products in each experiment are presented. The right bar presents the average results of fusing normal control and six different MLIV cell lines.
Figure 4.
No complementation of phenotype by fusion of two MLIV fibroblasts lines. Two MLIV cultures (stained green and red) were fused as described in Materials and Methods. Four days after seeding on slides, cells were fixed and photographed under the microscope. (A) A phase micrograph showing a red cell (nucleus marked R) and a green and red fusion product (nuclei marked G and R); large arrows point to phase-dense material. (B) Autofluorescence shown in the UV channel. In the fusion product, fluorescence intensity seems even stronger than in the single nuclear cell. (C) The same cells at green wavelength. (D) The same cells at the red channel.
DISCUSSION
In this study, we found that the clinical criteria we used to diagnose MLIV identified a homogenous population of patients. Introduction of tests for fibroblast autofluorescence and blood gastrin level as an indicator for achlorohydria (7, 13) will increase the confidence in diagnosis of MLIV. This is especially important in cases that fail to be properly diagnosed because of heterogeneity of the clinical phenotype. Our past experience indicates that there are MLIV patients in the pool of children diagnosed with cerebral palsy. Because MLIV is a genetic disease, it would be extremely important for parents to obtain correct diagnoses in such cases.
Increased sensitivity of MLIV fibroblasts to chloroquine can also assist in diagnosis. Initially, we speculated that MLIV fibroblasts bearing abnormal lysosomes would be more sensitive to lysosomotropic drugs than normal cells. Dodecylimidazol was previously found to be more toxic to normal cells than mucolipidosis II cells (I cell disease) (14). Other lysosomotropic drugs, which were found to inhibit lysosomal enzymatic processing (15–17), were also tested. Chloroquine and several other antimalarial quinolines were the only lysosomotropic drugs to selectively affect MLIV fibroblasts in our experiments. Although the mechanism of chloroquine toxicity is unknown, it has been suggested that chloroquine may induce iron toxicity to the malaria plasmodium or abolish lysosomal enzyme activity (18, 19). We ruled out both possibilities in our selection experiments. In addition, the malaria plasmodium is sensitive to chloroquine concentrations that are two to three orders of magnitude lower than that required to kill MLIV fibroblasts, suggesting that the mechanism of action is different in each case.
Determination of phenotypic correction by screening multinuclear cells for autofluorescence was greatly enhanced by labeling the cultures first with CellTracker. About half of the multinuclear cells in the culture result from fusion of cells from only one of the cultures. Differential labeling of the cultures to be fused allowed us to count only cells containing nuclei from both cultures. In addition, when MLIV cells were fused with normal cells, there was always a portion of the cells that remained fluorescent. In cultures from several patients, a significant fraction of the cells was not autofluorescent; therefore, scanning fusion products labeled by both markers was the only way to prove that there was no complementation of the phenotype. The fact that normal nuclei did not eliminate the fluorescence in all of the cells may reflect the ratio of normal to faulty gene products in the fused cells. However, the fact that in all patient–patient fusion experiments, at least 90% of the cells remained autofluorescent proves beyond a doubt that MLIV is a single complementation group. The possibility remains that another complementation group may be found in non-Jewish patients once a large population of candidates is screened. The currently described screening method increases our confidence that it will be possible to reliably determine the incidence and distribution of MLIV. These results will also permit the integration of non-Jewish families into the chromosomal linkage study and enhance the physical mapping of the MLIV gene.
Acknowledgments
We thank Devera G. Schoenberg, M.S., for editorial assistance and Leonid Kopylev for statistical advice. The work was supported in part by the Mucolipidosis IV Foundation.
ABBREVIATION
- MLIV
mucolipidosis IV
References
- 1.Berman E R, Livni N, Shapira E, Merin S, Levij I S. J Pediatr (Berlin) 1974;84:519–526. doi: 10.1016/s0022-3476(74)80671-2. [DOI] [PubMed] [Google Scholar]
- 2.Amir N, Zlotogora J, Bach G. Pediatrics. 1987;79:953–959. [PubMed] [Google Scholar]
- 3.O’Brien J S. In: The Metabolic Basis of Inherited Disease. 6th Ed. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw–Hill; 1989. pp. 1797–1806. [Google Scholar]
- 4.Livni N, Legum C. Exp Cell Biol. 1976;44:1–11. doi: 10.1159/000162848. [DOI] [PubMed] [Google Scholar]
- 5.Kohn G, Livni N, Ornoy A, Sekeles E, Beyth Y, Legum C, Bach G, Cohen M M. J Pediatr (Berlin) 1977;90:62–66. doi: 10.1016/s0022-3476(77)80765-8. [DOI] [PubMed] [Google Scholar]
- 6.Tellez-Nagel I, Rapin I, Iwamoto T, Johnson A B, Norton W T, Nitowsky H. Arch Neurol. 1976;33:828–835. doi: 10.1001/archneur.1976.00500120032005. [DOI] [PubMed] [Google Scholar]
- 7.Schiffmann R, Dwyer N K, Lubensky I A, Tsokos M, Sutliff V E, Latimer J S, Frei K P, Brady R O, Barton N W, Blanchette-Mackie E J, Goldin E. Proc Natl Acad Sci USA. 1998;95:1207–1212. doi: 10.1073/pnas.95.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frei K P, Patronas N J, Cruchfield K E, Altarescu G, Schiffmann R. Neurology. 1998;51:565–569. doi: 10.1212/wnl.51.2.565. [DOI] [PubMed] [Google Scholar]
- 9.Goldin E, Blanchette-Makie E J, Dwyer N K, Pentchev P G, Brady R O. Pediatr Res. 1995;37:687–690. doi: 10.1203/00006450-199506000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Brul S, Westerveld A, Strijland A, Wanders R J A, Schram A W, Heymans H S A, Schutgens R B H, Van den Bosch H, Tager J M. J Clin Invest. 1988;81:1710–1715. doi: 10.1172/JCI113510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson P V, Carey W F. J Inherited Metab Dis. 1985;8:95–99. doi: 10.1007/BF01819286. [DOI] [PubMed] [Google Scholar]
- 12.Lowry O H, Rosebrough N J, Farr A L, Randall R j. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 13.Magee D F. J Gastroenterol. 1996;31:758–763. doi: 10.1007/BF02347632. [DOI] [PubMed] [Google Scholar]
- 14.Wilson P D, Firestone R A, Lenard J. J Cell Biol. 1987;104:1223–1229. doi: 10.1083/jcb.104.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Duve C, De Barsy T, Poole B, Trouet A, Tulkens P, Van Hoof F. Biochem Pharmacol. 1974;23:2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein J L, Brunschede G Y, Brown M S. J Biol Chem. 1975;250:7854–7862. [PubMed] [Google Scholar]
- 17.Roff C F, Goldin E, Comly M E, Cooney A, Brown A, Vanier M T, Miller S P, Brady R O, Pentchev P G. Dev Neurosci. 1991;13:315–319. doi: 10.1159/000112179. [DOI] [PubMed] [Google Scholar]
- 18.Ginsburg H, Geary T G. Biochem Pharmacol. 1987;36:1567–1576. doi: 10.1016/0006-2952(87)90038-4. [DOI] [PubMed] [Google Scholar]
- 19.Slater A F G. Pharmacol Ther. 1993;57:203–235. doi: 10.1016/0163-7258(93)90056-j. [DOI] [PubMed] [Google Scholar]