Abstract
Objective: To examine the therapeutic activity of hydrophilic glucocorticoid encapsulated in PLGA nanoparticles, which have shown slow release and are targeted to inflamed joints after intravenous administration, in experimental arthritis models.
Methods: Betamethasone sodium phosphate (BSP) encapsulated in PLGA nanoparticles with a size of 100–200 nm (PLGA-nanosteroid) was prepared using a modified oil in water emulsion solvent diffusion method with Zn ions and coated with lecithin. Rats with adjuvant arthritis (AA rats) and mice with anti-type II collagen antibody induced arthritis (AbIA mice) were treated intravenously with PLGA-nanosteroid after the initial sign of arthritis.
Results: In AA rats, a 30% decrease in paw inflammation was obtained in 1 day and maintained for 1 week with a single injection of 100 µg of PLGA-nanosteroid. Soft x ray examination 7 days after this treatment showed decreased soft tissue swelling. Moreover, the PLGA-nanosteroid was also highly effective in AbIA mice. A single injection of 30 µg of the PLGA-nanosteroid resulted in almost complete remission of the inflammatory response after 1 week. In contrast, the same dose of free BSP after three administrations only moderately reduced the severity of inflammation. In addition, a histological examination 7 days after the treatment showed a significant decrease of the inflammatory cells in the joints.
Conclusion: The observed strong therapeutic benefit obtained with PLGA-nanosteroid may be due to the targeting of the inflamed joint and its prolonged release in situ. Targeted drug delivery using a sustained release PLGA-nanosteroid is a successful intervention in experimental arthritis.
Full Text
The Full Text of this article is available as a PDF (152.7 KB).
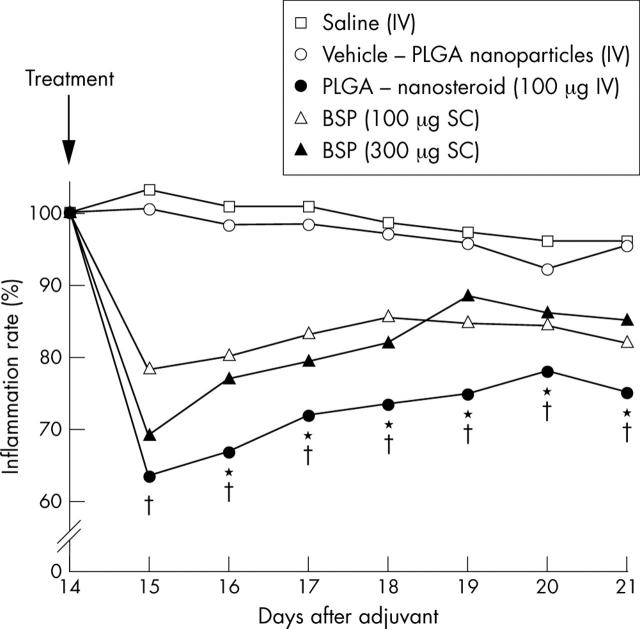
Figure 1.
Paw inflammation rate of AA rats. Arthritis was induced in Lewis rats as described in the "Materials and methods" section. Inflammation rate (%) = (measured leg volume–leg volume without adjuvant)/(leg volume on day 14–leg volume without adjuvant)x100. The average inflammation rate of seven rats from days 14 to 21 in each group is shown. SEM is within 10%. *p<0.05 (PLGA-nanosteroid v 300 µg BSP); †p<0.01 (PLGA-nanosteroid v vehicle-PLGA nanoparticles).
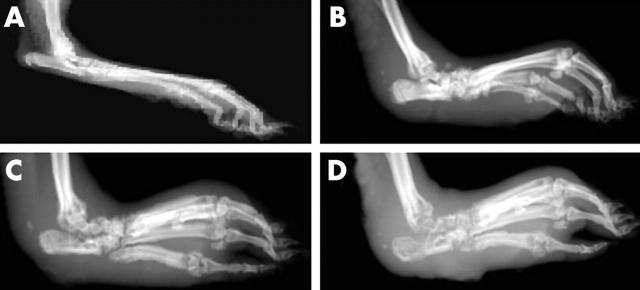
Figure 2.
Results of soft tissue x ray examination of the hind paw of AA rats. The swelling in the paw was assessed by soft tissue x ray examination on day 21 as described in "Materials and methods". (A) Normal rat control; (B) PLGA-nanosteroid (100 µg, intravenously (IV)) treated AA rat; (C) BSP (100 µg) treated AA rat; (D) vehicle-PLGA nanoparticle treated AA rat.
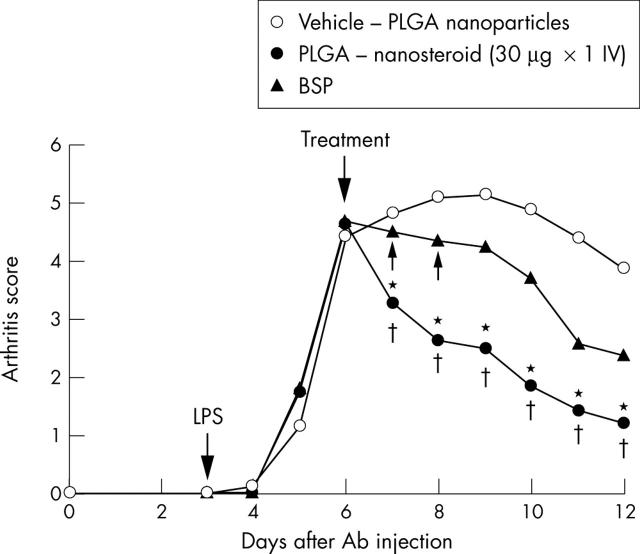
Figure 3.
Paw inflammation score of AbIA mice. Arthritis was induced in Balb/c mice as described in the "Materials and methods" section. The average clinical score is shown. SEM is within 10%. *p<0.05 (PLGA-nanosteroid v BSP) ; †p<0.01 (PLGA-nanosteroid v vehicle-PLGA nanoparticles).
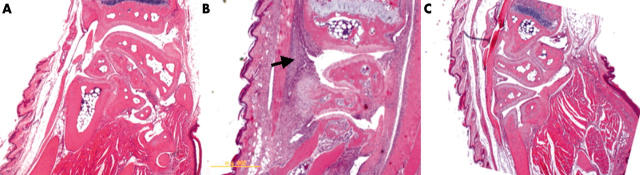
Figure 4.
Representative histopathology of the front paw of AbIA mice. (A) Normal mouse control; (B) vehicle-PLGA nanoparticle treated AbIA mouse; (C) PLGA-nanosteroid (30 µg x1x, IV) treated AbIA mouse. An arrow indicates cellular infiltrations. A yellow bar indicates 5 mm.
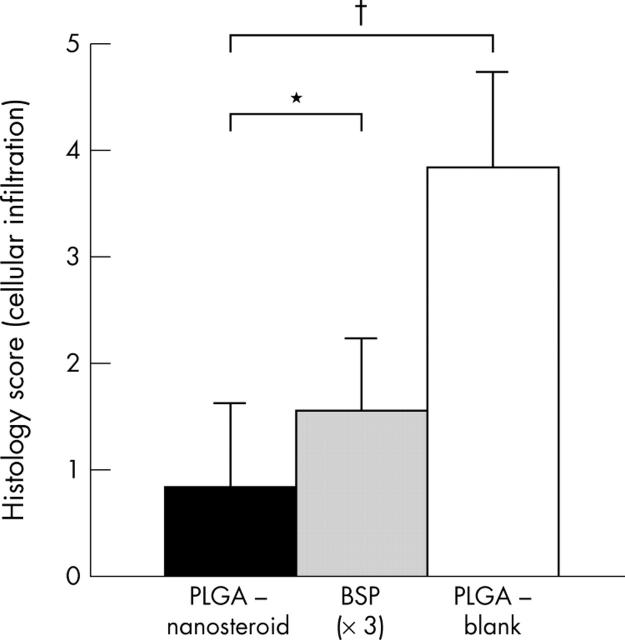
Figure 5.
Histology score of AbIA mice. The extent of cellular infiltration in AbIA mice treated with PLGA-nanosteroid (30 µg x1, IV), BSP (30 µg, x3, IV), or vehicle-PLGA nanoparticles was determined and graded from 0 to 3 in each paw. Data represent mean (SEM) (seven mice in each group). *p<0.05 (PLGA-nanosteroid v BSP); †p<0.01 (PLGA-nanosteroid v vehicle-PLGA nanoparticles).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chanan-Khan A., Szebeni J., Savay S., Liebes L., Rafique N. M., Alving C. R., Muggia F. M. Complement activation following first exposure to pegylated liposomal doxorubicin (Doxil): possible role in hypersensitivity reactions. Ann Oncol. 2003 Sep;14(9):1430–1437. doi: 10.1093/annonc/mdg374. [DOI] [PubMed] [Google Scholar]
- D'Souza M., DeSouza P. Preparation and testing of cyclosporine microsphere and solution formulations in the treatment of polyarthritis in rats. Drug Dev Ind Pharm. 1998 Sep;24(9):841–852. doi: 10.3109/03639049809088529. [DOI] [PubMed] [Google Scholar]
- Gref R., Minamitake Y., Peracchia M. T., Trubetskoy V., Torchilin V., Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994 Mar 18;263(5153):1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990 May 3;322(18):1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- Horisawa Eijiro, Hirota Tsuyoshi, Kawazoe Satoko, Yamada Jun, Yamamoto Hiromitsu, Takeuchi Hirofumi, Kawashima Yoshiaki. Prolonged anti-inflammatory action of DL-lactide/glycolide copolymer nanospheres containing betamethasone sodium phosphate for an intra-articular delivery system in antigen-induced arthritic rabbit. Pharm Res. 2002 Apr;19(4):403–410. doi: 10.1023/a:1015123024113. [DOI] [PubMed] [Google Scholar]
- Itoh Takeshi, Matsuda Hidetoshi, Tanioka Masatoshi, Kuwabara Kenji, Itohara Shigeyoshi, Suzuki Ryuji. The role of matrix metalloproteinase-2 and matrix metalloproteinase-9 in antibody-induced arthritis. J Immunol. 2002 Sep 1;169(5):2643–2647. doi: 10.4049/jimmunol.169.5.2643. [DOI] [PubMed] [Google Scholar]
- Jamieson T. W., De Smet A. A., Cremer M. A., Kage K. L., Lindsley H. B. Collagen-induced arthritis in rats. Assessment by serial magnification radiography. Invest Radiol. 1985 May-Jun;20(3):324–330. doi: 10.1097/00004424-198505000-00017. [DOI] [PubMed] [Google Scholar]
- Kawashima Y., Yamamoto H., Takeuchi H., Hino T., Niwa T. Properties of a peptide containing DL-lactide/glycolide copolymer nanospheres prepared by novel emulsion solvent diffusion methods. Eur J Pharm Biopharm. 1998 Jan;45(1):41–48. doi: 10.1016/S0939-6411(97)00121-5. [DOI] [PubMed] [Google Scholar]
- Kirwan J. R. The effect of glucocorticoids on joint destruction in rheumatoid arthritis. The Arthritis and Rheumatism Council Low-Dose Glucocorticoid Study Group. N Engl J Med. 1995 Jul 20;333(3):142–146. doi: 10.1056/NEJM199507203330302. [DOI] [PubMed] [Google Scholar]
- Koga T., Pearson C. M. Immunogenicity and arthritogenicity in the rat of an antigen from Mycobacterium tuberculosis wax D. J Immunol. 1973 Aug;111(2):599–608. [PubMed] [Google Scholar]
- Metselaar Josbert M., Wauben Marca H. M., Wagenaar-Hilbers Josee P. A., Boerman Otto C., Storm Gert. Complete remission of experimental arthritis by joint targeting of glucocorticoids with long-circulating liposomes. Arthritis Rheum. 2003 Jul;48(7):2059–2066. doi: 10.1002/art.11140. [DOI] [PubMed] [Google Scholar]
- Mizushima Y., Hamano T., Yokoyama K. Tissue distribution and anti-inflammatory activity of corticosteroids incorporated in lipid emulsion. Ann Rheum Dis. 1982 Jun;41(3):263–267. doi: 10.1136/ard.41.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima Y., Hamano T., Yokoyama K. Use of a lipid emulsion as a novel carrier for corticosteroids. J Pharm Pharmacol. 1982 Jan;34(1):49–50. doi: 10.1111/j.2042-7158.1982.tb04678.x. [DOI] [PubMed] [Google Scholar]
- Okada H. One- and three-month release injectable microspheres of the LH-RH superagonist leuprorelin acetate. Adv Drug Deliv Rev. 1997 Oct 13;28(1):43–70. doi: 10.1016/s0169-409x(97)00050-1. [DOI] [PubMed] [Google Scholar]
- Oku N., Namba Y. Long-circulating liposomes. Crit Rev Ther Drug Carrier Syst. 1994;11(4):231–270. [PubMed] [Google Scholar]
- Pelegrí C., Franch A., Castellote C., Castell M. Immunohistochemical changes in synovial tissue during the course of adjuvant arthritis. J Rheumatol. 1995 Jan;22(1):124–132. [PubMed] [Google Scholar]
- Saag Kenneth G. Glucocorticoid use in rheumatoid arthritis. Curr Rheumatol Rep. 2002 Jun;4(3):218–225. doi: 10.1007/s11926-002-0068-z. [DOI] [PubMed] [Google Scholar]
- Schwendeman Steven P. Recent advances in the stabilization of proteins encapsulated in injectable PLGA delivery systems. Crit Rev Ther Drug Carrier Syst. 2002;19(1):73–98. doi: 10.1615/critrevtherdrugcarriersyst.v19.i1.20. [DOI] [PubMed] [Google Scholar]
- Taurog J. D., Argentieri D. C., McReynolds R. A. Adjuvant arthritis. Methods Enzymol. 1988;162:339–355. doi: 10.1016/0076-6879(88)62089-1. [DOI] [PubMed] [Google Scholar]
- Terato K., Harper D. S., Griffiths M. M., Hasty D. L., Ye X. J., Cremer M. A., Seyer J. M. Collagen-induced arthritis in mice: synergistic effect of E. coli lipopolysaccharide bypasses epitope specificity in the induction of arthritis with monoclonal antibodies to type II collagen. Autoimmunity. 1995;22(3):137–147. doi: 10.3109/08916939508995311. [DOI] [PubMed] [Google Scholar]
- Wallace P. M., MacMaster J. F., Rouleau K. A., Brown T. J., Loy J. K., Donaldson K. L., Wahl A. F. Regulation of inflammatory responses by oncostatin M. J Immunol. 1999 May 1;162(9):5547–5555. [PubMed] [Google Scholar]
- Woodle M. C., Lasic D. D. Sterically stabilized liposomes. Biochim Biophys Acta. 1992 Aug 14;1113(2):171–199. doi: 10.1016/0304-4157(92)90038-c. [DOI] [PubMed] [Google Scholar]
- Wooley P. H., Whalen J. D., Chapman D. L., Berger A. E., Richard K. A., Aspar D. G., Staite N. D. The effect of an interleukin-1 receptor antagonist protein on type II collagen-induced arthritis and antigen-induced arthritis in mice. Arthritis Rheum. 1993 Sep;36(9):1305–1314. doi: 10.1002/art.1780360915. [DOI] [PubMed] [Google Scholar]
- Zhu G., Mallery S. R., Schwendeman S. P. Stabilization of proteins encapsulated in injectable poly (lactide- co-glycolide) Nat Biotechnol. 2000 Jan;18(1):52–57. doi: 10.1038/71916. [DOI] [PubMed] [Google Scholar]
- de Silva M., Hazleman B. L., Thomas D. P., Wraight P. Liposomes in arthritis: a new approach. Lancet. 1979 Jun 23;1(8130):1320–1322. doi: 10.1016/s0140-6736(79)91951-2. [DOI] [PubMed] [Google Scholar]