Abstract
Objective: To compare selected immunohistological features of inflammation in synovial tissue from patients with early and late osteoarthritis (OA).
Methods: Synovial tissue samples were obtained from 10 patients with knee pain, normal radiographs, and arthroscopic manifestations of OA (early OA), and from 15 patients with OA undergoing knee joint arthroplasty (late OA). Conventional immunohistochemical techniques were used to measure microscopic manifestations of inflammation. The inflammatory cell infiltrate, blood vessel formation, and angiogenic factors, NF-κB activation, expression of tumour necrosis factor α (TNFα) and interleukin 1ß (IL1ß), and the presence of cyclo-oxygenase (COX)-1 and COX-2 were quantified. Fibroblast-like synoviocytes (FLS) were isolated from early and late OA tissue samples to compare in vitro production of prostaglandin E2 (PGE2)
Results: Synovial tissue from patients with early OA demonstrated significantly greater CD4+ (p = 0.017) and CD68+ (p<0.001) cell infiltration, blood vessel formation (p = 0.01), vascular endothelial growth factor (p = 0.001), and intercellular adhesion molecule-1 expression (p<0.001). Numbers of cells producing TNFα and IL1ß were also significantly greater in early OA (p<0.001). Manifestations of inflammation in early OA were associated with increased expression of the NF-κB1 (p<0.001) and RelA (p = 0.015) subunits, and with increased COX-2 expression (p = 0.04). Cytokine-induced PGE2 production by cultured FLS was similar in both groups.
Conclusion: Increased mononuclear cell infiltration and overexpression of mediators of inflammation were seen in early OA, compared with late OA. Isolated FLS were functionally similar in both groups, consistent with microenvironmental differences in the synovial tissue during different phases of OA. These observations may have important therapeutic implications for some patients during the early evolution of OA.
Full Text
The Full Text of this article is available as a PDF (146.9 KB).
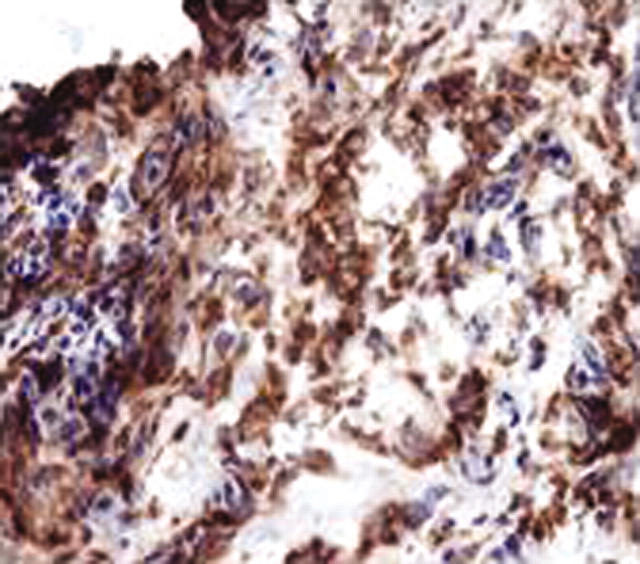
Figure 1.
Photomicrographs of synovial tissue from a patient with early OA, demonstrating infiltration by CD68+ mononuclear cells (magnification x100).
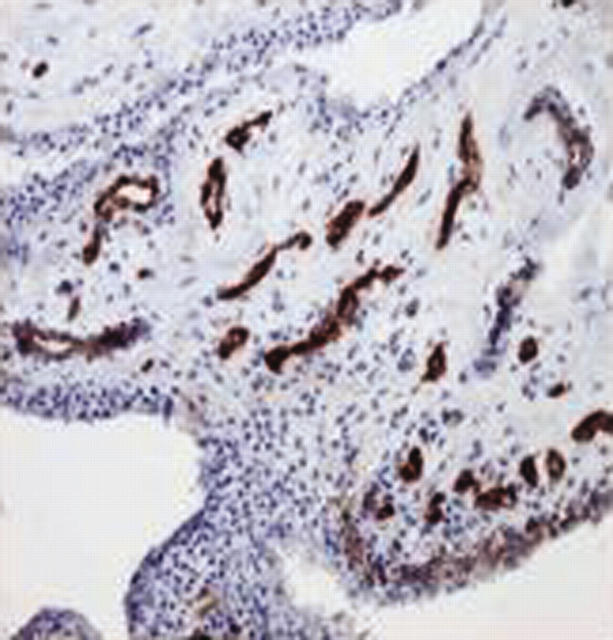
Figure 2.
Photomicrograph of synovial tissue from a patient with early OA, demonstrating factor VIII+ endothelial cells (magnification x100).
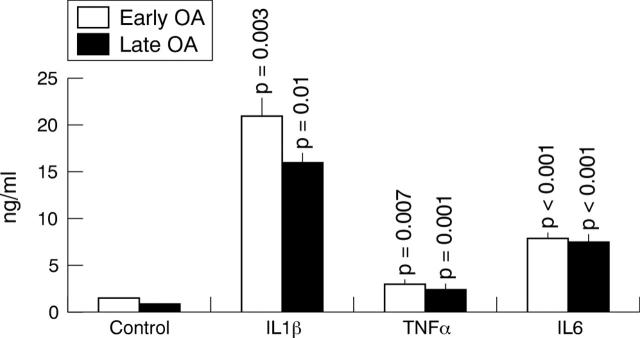
Figure 3.
PGE2 production by isolated synoviocytes from patients with early and late OA. PGE2 levels were measured after stimulation of synoviocytes by IL1ß, TNFα, and IL6, and were compared with unstimulated control cultures. The results represent the mean (SE) values from four experiments.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman R., Asch E., Bloch D., Bole G., Borenstein D., Brandt K., Christy W., Cooke T. D., Greenwald R., Hochberg M. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986 Aug;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- Ayral X., Ravaud P., Bonvarlet J. P., Simonnet J., Lecurieux R., Nguyen M., Sauvage E., Dougados M. Arthroscopic evaluation of post-traumatic patellofemoral chondropathy. J Rheumatol. 1999 May;26(5):1140–1147. [PubMed] [Google Scholar]
- Ballara S., Taylor P. C., Reusch P., Marmé D., Feldmann M., Maini R. N., Paleolog E. M. Raised serum vascular endothelial growth factor levels are associated with destructive change in inflammatory arthritis. Arthritis Rheum. 2001 Sep;44(9):2055–2064. doi: 10.1002/1529-0131(200109)44:9<2055::AID-ART355>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Benito Maria J., Murphy Eithne, Murphy Evelyn P., van den Berg Wim B., FitzGerald Oliver, Bresnihan Barry. Increased synovial tissue NF-kappa B1 expression at sites adjacent to the cartilage-pannus junction in rheumatoid arthritis. Arthritis Rheum. 2004 Jun;50(6):1781–1787. doi: 10.1002/art.20260. [DOI] [PubMed] [Google Scholar]
- Bresnihan B., Cunnane G., Youssef P., Yanni G., Fitzgerald O., Mulherin D. Microscopic measurement of synovial membrane inflammation in rheumatoid arthritis: proposals for the evaluation of tissue samples by quantitative analysis. Br J Rheumatol. 1998 Jun;37(6):636–642. doi: 10.1093/rheumatology/37.6.636. [DOI] [PubMed] [Google Scholar]
- Cunnane G., Bjork L., Ulfgren A. K., Lindblad S., FitzGerald O., Bresnihan B., Andersson U. Quantitative analysis of synovial membrane inflammation: a comparison between automated and conventional microscopic measurements. Ann Rheum Dis. 1999 Aug;58(8):493–499. doi: 10.1136/ard.58.8.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolhain R. J., Ter Haar N. T., De Kuiper R., Nieuwenhuis I. G., Zwinderman A. H., Breedveld F. C., Miltenburg A. M. Distribution of T cells and signs of T-cell activation in the rheumatoid joint: implications for semiquantitative comparative histology. Br J Rheumatol. 1998 Mar;37(3):324–330. doi: 10.1093/rheumatology/37.3.324. [DOI] [PubMed] [Google Scholar]
- Farahat M. N., Yanni G., Poston R., Panayi G. S. Cytokine expression in synovial membranes of patients with rheumatoid arthritis and osteoarthritis. Ann Rheum Dis. 1993 Dec;52(12):870–875. doi: 10.1136/ard.52.12.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felson D. T., Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum. 1998 Aug;41(8):1343–1355. doi: 10.1002/1529-0131(199808)41:8<1343::AID-ART3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Goldenberg D. L., Egan M. S., Cohen A. S. Inflammatory synovitis in degenerative joint disease. J Rheumatol. 1982 Mar-Apr;9(2):204–209. [PubMed] [Google Scholar]
- Haywood L., McWilliams D. F., Pearson C. I., Gill S. E., Ganesan A., Wilson D., Walsh D. A. Inflammation and angiogenesis in osteoarthritis. Arthritis Rheum. 2003 Aug;48(8):2173–2177. doi: 10.1002/art.11094. [DOI] [PubMed] [Google Scholar]
- Iagnocco A., Coari G. Usefulness of high resolution US in the evaluation of effusion in osteoarthritic first carpometacarpal joint. Scand J Rheumatol. 2000;29(3):170–173. doi: 10.1080/030097400750002049. [DOI] [PubMed] [Google Scholar]
- Johnson B. A., Haines G. K., Harlow L. A., Koch A. E. Adhesion molecule expression in human synovial tissue. Arthritis Rheum. 1993 Feb;36(2):137–146. doi: 10.1002/art.1780360203. [DOI] [PubMed] [Google Scholar]
- Kane D., Veale D. J., FitzGerald O., Reece R. Survey of arthroscopy performed by rheumatologists. Rheumatology (Oxford) 2002 Feb;41(2):210–215. doi: 10.1093/rheumatology/41.2.210. [DOI] [PubMed] [Google Scholar]
- Murphy E. P., McEvoy A., Conneely O. M., Bresnihan B., FitzGerald O. Involvement of the nuclear orphan receptor NURR1 in the regulation of corticotropin-releasing hormone expression and actions in human inflammatory arthritis. Arthritis Rheum. 2001 Apr;44(4):782–793. doi: 10.1002/1529-0131(200104)44:4<782::AID-ANR134>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Myers S. L., Brandt K. D., Ehlich J. W., Braunstein E. M., Shelbourne K. D., Heck D. A., Kalasinski L. A. Synovial inflammation in patients with early osteoarthritis of the knee. J Rheumatol. 1990 Dec;17(12):1662–1669. [PubMed] [Google Scholar]
- Peat G., McCarney R., Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis. 2001 Feb;60(2):91–97. doi: 10.1136/ard.60.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Mikami Y., Kurasono Y. [Expression of adhesion molecules, intercellular molecule (ICAM)-1 and lymphocyte function associated antigen (LFA)-1 in the synovial tissues of rheumatoid arthritis (RA)]. Ryumachi. 1994 Oct;34(5):854–862. [PubMed] [Google Scholar]
- Sharif M., Elson C. J., Dieppe P. A., Kirwan J. R. Elevated serum C-reactive protein levels in osteoarthritis. Br J Rheumatol. 1997 Jan;36(1):140–141. doi: 10.1093/rheumatology/36.1.140. [DOI] [PubMed] [Google Scholar]
- Shibakawa A., Aoki H., Masuko-Hongo K., Kato T., Tanaka M., Nishioka K., Nakamura H. Presence of pannus-like tissue on osteoarthritic cartilage and its histological character. Osteoarthritis Cartilage. 2003 Feb;11(2):133–140. doi: 10.1053/joca.2002.0871. [DOI] [PubMed] [Google Scholar]
- Smith M. D., Triantafillou S., Parker A., Youssef P. P., Coleman M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol. 1997 Feb;24(2):365–371. [PubMed] [Google Scholar]
- Soren A., Cooper N. S., Waugh T. R. The nature and designation of osteoarthritis determined by its histopathology. Clin Exp Rheumatol. 1988 Jan-Mar;6(1):41–46. [PubMed] [Google Scholar]
- Sowers MaryFran, Jannausch Mary, Stein Evan, Jamadar David, Hochberg Marc, Lachance Laurie. C-reactive protein as a biomarker of emergent osteoarthritis. Osteoarthritis Cartilage. 2002 Aug;10(8):595–601. doi: 10.1053/joca.2002.0800. [DOI] [PubMed] [Google Scholar]
- Spector T. D., Hart D. J., Nandra D., Doyle D. V., Mackillop N., Gallimore J. R., Pepys M. B. Low-level increases in serum C-reactive protein are present in early osteoarthritis of the knee and predict progressive disease. Arthritis Rheum. 1997 Apr;40(4):723–727. doi: 10.1002/art.1780400419. [DOI] [PubMed] [Google Scholar]
- Veale D. J. The role of arthroscopy in early arthritis. Clin Exp Rheumatol. 1999 Jan-Feb;17(1):37–38. [PubMed] [Google Scholar]
- Young L., Katrib A., Cuello C., Vollmer-Conna U., Bertouch J. V., Roberts-Thomson P. J., Ahern M. J., Smith M. D., Youssef P. P. Effects of intraarticular glucocorticoids on macrophage infiltration and mediators of joint damage in osteoarthritis synovial membranes: findings in a double-blind, placebo-controlled study. Arthritis Rheum. 2001 Feb;44(2):343–350. doi: 10.1002/1529-0131(200102)44:2<343::AID-ANR52>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Youssef P. P., Kraan M., Breedveld F., Bresnihan B., Cassidy N., Cunnane G., Emery P., Fitzgerald O., Kane D., Lindblad S. Quantitative microscopic analysis of inflammation in rheumatoid arthritis synovial membrane samples selected at arthroscopy compared with samples obtained blindly by needle biopsy. Arthritis Rheum. 1998 Apr;41(4):663–669. doi: 10.1002/1529-0131(199804)41:4<663::AID-ART13>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]