Abstract
Objective: To evaluate the effect of tumour necrosis factor α (TNFα), interleukin (IL) 1ß, and their respective inhibitors the p75 TNFα soluble receptor (sTNFR) and the type II sIL1ßR (sIL1RII) on whole muscle and isolated myoblast activation.
Methods: Normal muscle samples were stimulated for 7 days with TNFα alone or in combination with IL1ß, and myoblasts from these samples for 48 hours. IL6 production was measured by ELISA. Nuclear translocation of NF-κB was analysed by immunofluorescent staining and class I MHC expression by FACS.
Results: TNFα and IL1ß induced IL6 production by normal muscle samples and myoblasts, the action of TNFα being more potent on muscle samples. Their soluble receptors (1 µg/ml) decreased this production. Suboptimal concentrations of TNFα and IL1ß induced NF-κB translocation. sTNFR markedly down regulated TNFα-induced translocation while sIL1RII was less potent on IL1ß-induced activation. NF-κB translocation induced by the combination of optimal concentrations of TNFα and IL1ß was completely inhibited by their soluble receptors. TNFα and to a lesser extent IL1ß induced class I MHC expression by myoblasts and this effect was completely inhibited by their respective soluble receptors.
Conclusion: These results suggest that TNFα and IL1ß should be targeted for myositis treatment.
Full Text
The Full Text of this article is available as a PDF (160.8 KB).
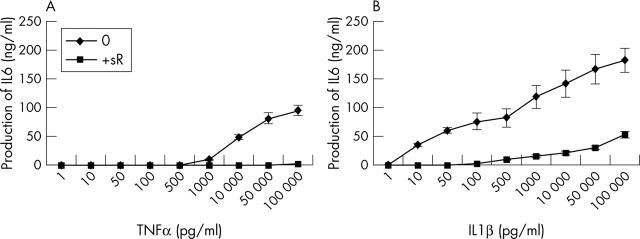
Figure 1.
Dose-response curve of IL6 production by myoblasts stimulated with increasing concentrations of TNFα or IL1ß in the presence or not of a soluble receptor (sR). Myoblasts were incubated with increasing concentrations (pg/ml) of TNFα (A) or IL1ß (B). Myoblasts were also incubated with the same increasing concentrations of cytokine and 1 µg/ml of their respective sR, p75 TNFα sR, and type II IL1ß sR. IL6 levels were measured by ELISA in 48 hour supernatants.
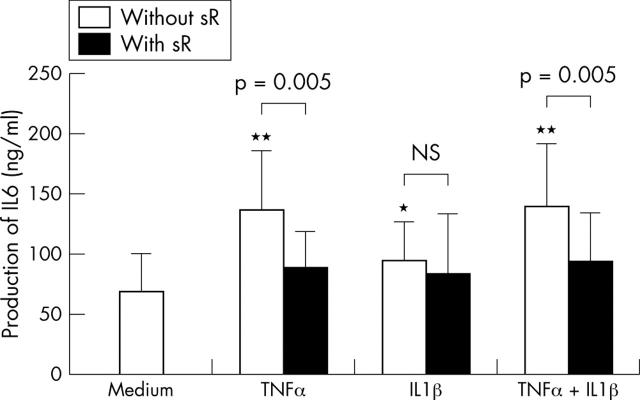
Figure 2.
Effect of TNFα and IL1ß on IL6 production by normal muscle samples in the presence or not of a soluble receptor (sR). Muscle samples from 10 different subjects were incubated for 7 days in the presence of 10 ng/ml TNFα or 10 pg/ml IL1ß alone or in combination, and with or without 1 µg/ml sTNFR or sIL1RII alone or in combination. IL6 levels were measured in supernatants by ELISA.
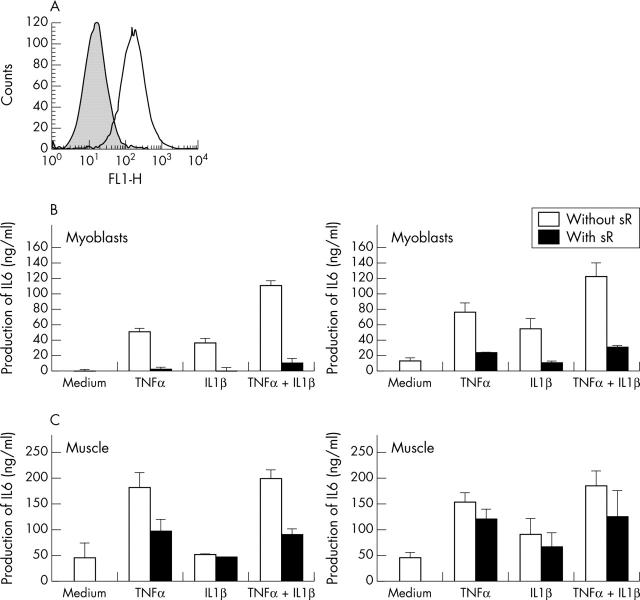
Figure 3.
Effect of TNFα and IL1ß on IL6 production by individual myoblasts and muscle samples. (A) Myoblasts were selected by two rounds of affinity purification with an anti-CD56 antibody. One example of staining is shown. (B) Myoblasts from two different subjects (B1 and B2) were incubated for 48 hours in the presence of 10 ng/ml TNFα or 10 pg/ml IL1ß alone or in combination, and with or without 1 µg/ml sTNFR or sIL1RII alone or in combination. (C) The two muscle samples (C1 and C2) from which myoblasts in (B) were obtained, were incubated for 7 days in the presence of 10 ng/ml TNFα or 10 pg/ml IL1ß alone or in combination, with or without 1 µg/ml sTNFR or sIL1RII alone or in combination. IL6 levels were measured in supernatants by ELISA.
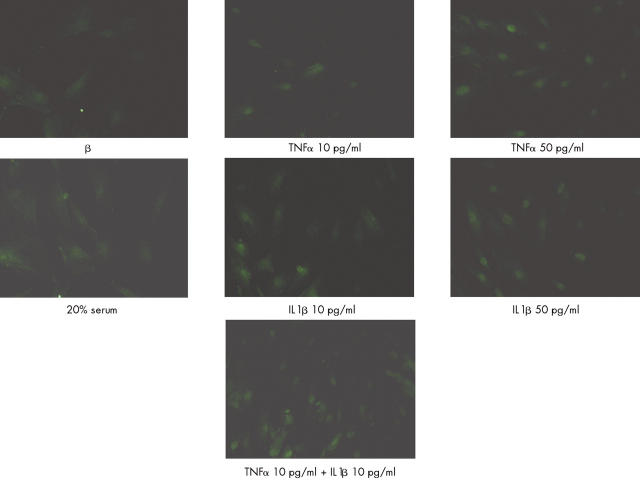
Figure 4.
Effects of TNFα or IL1ß used alone at low and optimal concentrations and in combination on NF-κB nuclear translocation. Myoblasts (104 cells/cm2) were stimulated for 30 minutes with TNFα or IL1ß at the indicated concentrations. Cells were fixed in paraformaldehyde 4% and immunofluorescence staining was performed. Nuclear translocation was evaluated and confirmed by nuclear staining with Hoechst H33258 (data not shown). Incubation with 20% FCS medium was used as a positive control.
Figure 5.
Effect of sTNFR and sIL1RII on NF-κB nuclear translocation induced by TNFα or IL1ß. TNFα (500 pg/ml) or IL1ß (1000 pg/ml) alone or in combination (left column) were incubated for 30 minutes with 1 µg/ml of sTNFR or sIL1RII, either alone or in combination before addition to myoblasts (right columns). Myoblasts (104 cells/cm2) were stimulated for 30 minutes in presence or not of sR (alone or in combination). Cells were fixed in paraformaldehyde 4% and immunofluorescence staining was performed.
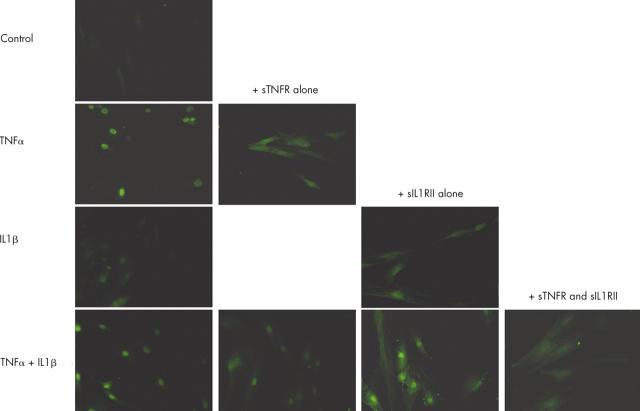
Figure 6.
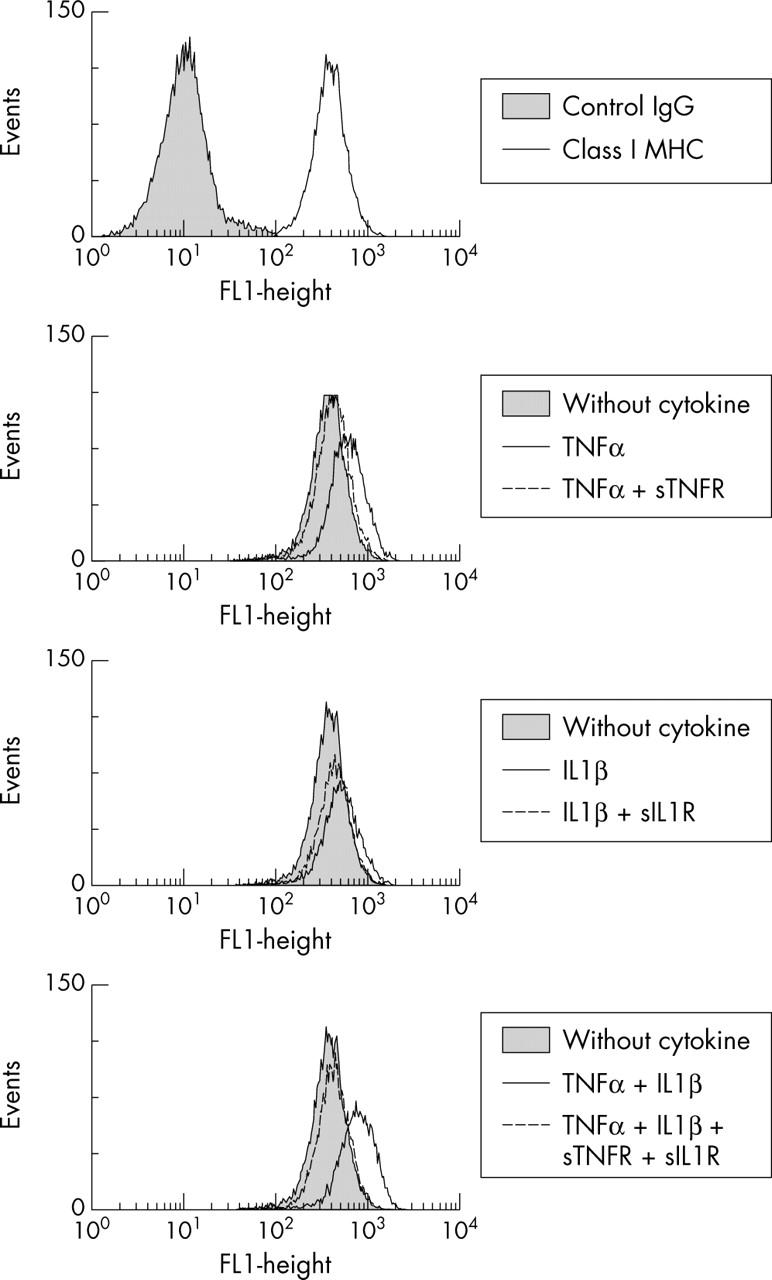
Effect of sTNFR and sIL1RII on class I MHC class I expression induced by TNFα or IL1ß. Myoblasts were incubated with 100 pg/ml TNFα or IL1ß alone or in combination and 1 µg/ml of their respective sR, p75 TNFα sR and type II IL1ß sR. Class I MHC expression was analysed after 48 hours of incubation. Constitutive expression of MHC class I antigen is shown at the top of the figure.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams Volker, Nehrhoff Britta, Späte Ulrike, Linke Axel, Schulze Paul C., Baur Angelika, Gielen Stephan, Hambrecht Rainer, Schuler Gerhard. Induction of iNOS expression in skeletal muscle by IL-1beta and NFkappaB activation: an in vitro and in vivo study. Cardiovasc Res. 2002 Apr;54(1):95–104. doi: 10.1016/s0008-6363(02)00228-6. [DOI] [PubMed] [Google Scholar]
- Arend William P. The mode of action of cytokine inhibitors. J Rheumatol Suppl. 2002 Sep;65:16–21. [PubMed] [Google Scholar]
- Chabaud M., Fossiez F., Taupin J. L., Miossec P. Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokines. J Immunol. 1998 Jul 1;161(1):409–414. [PubMed] [Google Scholar]
- Chabaud M., Miossec P. The combination of tumor necrosis factor alpha blockade with interleukin-1 and interleukin-17 blockade is more effective for controlling synovial inflammation and bone resorption in an ex vivo model. Arthritis Rheum. 2001 Jun;44(6):1293–1303. doi: 10.1002/1529-0131(200106)44:6<1293::AID-ART221>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Chabaud M., Page G., Miossec P. Enhancing effect of IL-1, IL-17, and TNF-alpha on macrophage inflammatory protein-3alpha production in rheumatoid arthritis: regulation by soluble receptors and Th2 cytokines. J Immunol. 2001 Nov 15;167(10):6015–6020. doi: 10.4049/jimmunol.167.10.6015. [DOI] [PubMed] [Google Scholar]
- Chevrel G., Page G., Granet C., Streichenberger N., Varennes A., Miossec P. Interleukin-17 increases the effects of IL-1 beta on muscle cells: arguments for the role of T cells in the pathogenesis of myositis. J Neuroimmunol. 2003 Apr;137(1-2):125–133. doi: 10.1016/s0165-5728(03)00032-8. [DOI] [PubMed] [Google Scholar]
- Dalakas M. C. Polymyositis, dermatomyositis and inclusion-body myositis. N Engl J Med. 1991 Nov 21;325(21):1487–1498. doi: 10.1056/NEJM199111213252107. [DOI] [PubMed] [Google Scholar]
- Gallucci S., Provenzano C., Mazzarelli P., Scuderi F., Bartoccioni E. Myoblasts produce IL-6 in response to inflammatory stimuli. Int Immunol. 1998 Mar;10(3):267–273. doi: 10.1093/intimm/10.3.267. [DOI] [PubMed] [Google Scholar]
- Granet Corinne, Maslinski Wova, Miossec Pierre. Increased AP-1 and NF-kappaB activation and recruitment with the combination of the proinflammatory cytokines IL-1beta, tumor necrosis factor alpha and IL-17 in rheumatoid synoviocytes. Arthritis Res Ther. 2004 Feb 26;6(3):R190–R198. doi: 10.1186/ar1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengstman G. J. D., van den Hoogen F. H. J., van Engelen B. G. M. Treatment of dermatomyositis and polymyositis with anti-tumor necrosis factor-alpha: long-term follow-up. Eur Neurol. 2004 Jul 5;52(1):61–63. doi: 10.1159/000079547. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Engel A. G. The immunobiology of muscle. Immunol Today. 1994 Jun;15(6):269–274. doi: 10.1016/0167-5699(94)90006-X. [DOI] [PubMed] [Google Scholar]
- Kuru S., Inukai A., Liang Y., Doyu M., Takano A., Sobue G. Tumor necrosis factor-alpha expression in muscles of polymyositis and dermatomyositis. Acta Neuropathol. 2000 May;99(5):585–588. doi: 10.1007/s004010051165. [DOI] [PubMed] [Google Scholar]
- Langen R. C., Schols A. M., Kelders M. C., Wouters E. F., Janssen-Heininger Y. M. Inflammatory cytokines inhibit myogenic differentiation through activation of nuclear factor-kappaB. FASEB J. 2001 May;15(7):1169–1180. doi: 10.1096/fj.00-0463. [DOI] [PubMed] [Google Scholar]
- Lundberg I., Brengman J. M., Engel A. G. Analysis of cytokine expression in muscle in inflammatory myopathies, Duchenne dystrophy, and non-weak controls. J Neuroimmunol. 1995 Dec;63(1):9–16. doi: 10.1016/0165-5728(95)00122-0. [DOI] [PubMed] [Google Scholar]
- Lundberg I., Ulfgren A. K., Nyberg P., Andersson U., Klareskog L. Cytokine production in muscle tissue of patients with idiopathic inflammatory myopathies. Arthritis Rheum. 1997 May;40(5):865–874. doi: 10.1002/art.1780400514. [DOI] [PubMed] [Google Scholar]
- Sprott H., Glatzel M., Michel B. A. Treatment of myositis with etanercept (Enbrel), a recombinant human soluble fusion protein of TNF-alpha type II receptor and IgG1. Rheumatology (Oxford) 2004 Apr;43(4):524–526. doi: 10.1093/rheumatology/keh062. [DOI] [PubMed] [Google Scholar]