Abstract
Chronic antagonism of melanocortin receptors by the paracrine-acting agouti gene product induces both yellow fur and a maturity-onset obesity syndrome in mice that ubiquitously express wild-type agouti. Functional analysis of agouti mutations in transgenic mice indicate that the cysteine-rich C terminus, signal peptide, and glycosylation site are required for agouti activity in vivo. In contrast, no biological activity has been ascribed to the conserved basic domain. To examine the functional significance of the agouti basic domain, the entire 29-aa region was deleted from the agouti cDNA, and the resulting mutation (agoutiΔbasic) was expressed in transgenic mice under the control of the β-actin promoter (BAPaΔbasic). Three independent lines of BAPaΔbasic transgenic mice all developed some degree of yellow pigment in the fur, indicating that the agoutiΔbasic protein was functional in vivo. However, none of the BAPaΔbasic transgenic mice developed completely yellow fur, obesity, hyperinsulinemia, or hyperglycemia. High levels of agoutiΔbasic expression in relevant tissues exceeded the level of agouti expression in obese viable yellow mice, suggesting that suboptimal activity or synthesis of the agoutiΔbasic protein, rather than insufficient RNA synthesis, accounts for the phenotype of the BAPaΔbasic transgenic mice. These findings implicate a functional role for the agouti basic domain in vivo, possibly influencing the biogenesis of secreted agouti protein or modulating protein–protein interactions that contribute to effective antagonism of melanocortin receptors.
Keywords: pheomelanin, melanocortin receptors
The agouti gene encodes a small secreted factor that normally functions as a paracrine regulator of hair pigmentation in mice and other mammals (refs. 1–3; GenBank accession no. X99692). Mutually exclusive binding (4) of the melanocortin 1 receptor (MC1-R) by the agouti protein or the α-melanocyte stimulating hormone signals hair-bulb melanocytes to synthesize either pheomelanin (yellow-red pigments) or eumelanin (dark pigments), respectively (5). Transient expression of wild-type agouti in the microenvironment of the hair follicle during the hair-growth cycle (1), therefore, gives rise to the characteristic subapical yellow band in individual hairs in the coat of wild-type mice.
Although the mouse agouti gene normally functions only in the skin, ectopically expressed agouti induces significant, pleiotropic effects in multiple tissues (6, 7). Bright yellow fur, maturity-onset obesity, hyperinsulinemia, insulin resistance, hyperglycemia in males, increased body length, and susceptibility to neoplasia all typify the “yellow obese” syndrome that develops in mice carrying dominant agouti alleles (8–11) or in transgenic (Tg) mice that ubiquitously express agouti from the human β-actin promoter (BAPa Tg mice; refs. 12 and 13). Both biochemical evidence and genetic evidence indicate that chronic antagonism of the hypothalamic MC4-R by ectopic agouti protein disrupts normal control of energy homeostasis in yellow obese mice (14–16). MC4-R mutations have now been identified in dominantly inherited forms of human obesity as well (17, 18), highlighting the clinical significance of MC4-R signaling pathways in the regulation of body weight and energy balance.
Characterization of the agouti-related protein (AGRP), a hypothalamic neuropeptide and potent MC4-R antagonist with structural similarity to the agouti protein, strongly suggests that ectopic agouti mimics AGRP to induce obesity (19–22). The region of greatest similarity between AGRP and agouti is the ≈40-aa cysteine-rich C-terminal domain (19, 21) that forms a putative cysteine knot motif stabilized by five disulfide bridges (23, 24). Both agouti and AGRP (25) contain high-affinity melanocortin receptor (MCR)-binding determinants (Arg108 and Arg116-Arg117-Phe118) that are surface-exposed on the cysteine knot (24). The black fur and normal body weight of Tg founder mice expressing mutant agouti cDNAs in which each of the 10 cysteines is individually substituted with serine verify that the structural integrity of the cysteine knot is critical for agouti activity in vivo (26). In addition, the agouti signal peptide and N-terminal glycosylation site are also required for both agouti-induced yellow pigmentation and obesity in Tg founder mice (26), confirming that efficient entry and transit through the secretory pathway are essential for agouti activity in vivo.
The function of another conserved domain of the agouti protein remains enigmatic, however. All identified mammalian agouti genes contain ≈30 aa of predominantly basic residues in the center of the protein (refs. 1, 2, and 27; GenBank accession no. X99692). Trypsin cleavage of recombinant mouse agouti protein in vitro generates a C-terminal fragment (Val83-Cys131) that is equally as potent as full-length agouti in MCR-binding inhibition assays (15, 23), suggesting that the basic domain may provide relevant proteolytic processing sites in vivo. C-terminal fragments of human agouti (28) and AGRP (21, 22) also antagonize MCRs with high affinity. Deletion of part of the basic region of the mouse agouti protein suggests that Arg64-Lys77 is dispensable for the development of yellow fur and obesity in Tg founder mice (26); however, several basic amino acids remain intact both N- and C-terminal to this deletion. Removal of the entire basic domain (agoutiΔbasic; ΔLys57-Arg85) reduces antagonism of MCRs by ≈10-fold in vitro (15), suggesting a potentially direct influence on MCR affinity. To test the biological activity of this mutant in melanogenesis and energy balance, we generated Tg mice that express the agoutiΔbasic cDNA (ΔLys57-Arg85; ref. 15) under the control of the human β-actin promoter (BAPaΔbasic). Three independently derived lines of BAPaΔbasic Tg mice developed partially yellow fur but none of the obesity-related traits that are typically associated with ectopic expression of wild-type agouti. These data provide evidence that the conserved agouti basic domain serves a functionally significant role in vivo.
MATERIALS AND METHODS
Mice.
All mice were maintained at the Oak Ridge National Laboratory. The FVB/N wild-type agouti (A/A) mice were obtained from our closed colony, and C57BL/6J nonagouti (a/a) mice were purchased from The Jackson Laboratory. The viable yellow (Avy/a) mice were obtained from The Jackson Laboratory and maintained as an inbred strain. BAPa20 and BAPaΔbasic Tg mice were maintained on the FVB/N background and fed a diet containing 11% fat by weight (Mouse Diet 5015, Purina). The Avy/a and FVB/N mice used in our maintenance colonies were fed a normal diet containing 4.5% fat (Lab Diet 5002, Purina). Food and water were provided ad libitum.
Agouti Expression Construct.
An EcoRI fragment containing agoutiΔbasic was isolated from the pMT4/agoutiΔbasic plasmid (15) and cloned into the same site of pBluescript (+) (Stratagene) to generate pBS/agoutiΔbasic. The human β-actin promoter-agoutiΔbasic expression construct (pBAPaΔbasic) was generated by replacing the HindIII–BamHI fragment of BAPa (12), which contains the wild-type agouti cDNA, with the HindIII–BamHI fragment of pBS/agoutiΔbasic that contains the agoutiΔbasic cDNA sequence. All constructs were verified by DNA sequencing.
Tg Mice.
Tg mice were derived as described (29) via pronuclear microinjection of one-cell FVB/N embryos with a 5.3-kb ClaI fragment of pBAPaΔbasic. Except for pigmentation analysis, all molecular and physiological data for Tg mice were obtained from F1 generation mice (FVB/N) that were hemizygous [Tg/−] for the transgene. Each line was tested by Southern analysis to insure that only one transgene insertion event was segregating in the F1 progeny. To examine coat pigmentation, Tg mice were backcrossed to the C57BL/6J inbred strain for five generations (N5).
Southern Blot Analysis.
Southern blot hybridizations were performed as described (1, 30) by using DNA prepared from tail biopsy. To determine the genotype of Tg versus non-Tg mice, HindIII- or BamHI-digested genomic DNA was probed with a DNA fragment containing the simian virus 40 polyadenylation sequence. To determine the transgene copy number of each line, BamHI-digested genomic DNA was probed with a DNA fragment containing exon 2 of the wild-type agouti gene. The relative signal intensity of the transgene-specific band versus the endogenous agouti band was determined by using a PhosphorImager (Fujix BAS system, Fuji).
RNase Protection Assay (RPA).
Total RNA was prepared from multiple tissues of adult mice as described (30). RPAs were performed with the RPA II Kit (Ambion, Austin, TX) by using standard conditions described by the manufacturer. Antisense RNA probes were transcribed by T7 RNA polymerase (Promega) and gel-isolated before hybridization with total RNA. A low specific activity 18S rRNA antisense probe was generated by using the Tri-18S template (Ambion). A plasmid containing the wild-type agouti cDNA (pC3Hv2.5) and the T7 promoter was digested with BbsI to generate a transcription template specific for exon 4 of agouti.
Weight Gain and Blood Analysis.
The body weight of mice was measured monthly from 12 to 40 weeks of age. Blood was obtained by retroorbital sinus puncture from anesthetized (Metophane, Schering-Plough), nonfasted mice between 24 and 32 weeks of age. Samples were collected between 9 a.m. and 12 p.m. Glucose levels were determined from freshly collected blood with a One-Touch glucometer (Johnson & Johnson). Plasma insulin levels were measured by RIA (coated tube, ICN) with porcine insulin as a standard. Data analysis was performed by using an unpaired two-group t test with a 95% confidence level (graphpad prism software, GraphPad, San Diego).
RESULTS
Generation of BAPaΔbasic Tg Mice.
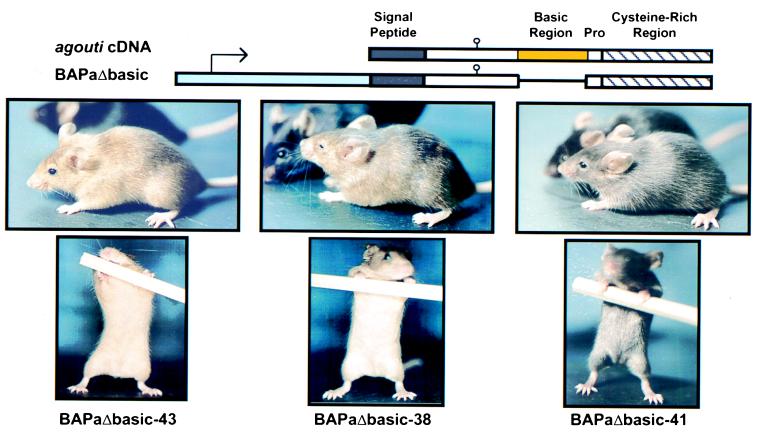
To determine whether the central basic domain of the mouse agouti protein is required for activity in vivo, Tg mice that ectopically express the agoutiΔbasic cDNA were generated as described (15). The human β-actin promoter and enhancer were chosen to drive expression of agoutiΔbasic (Fig. 1), because we previously showed that this promoter drives widespread expression of the wild-type agouti cDNA in BAPa Tg mice, resulting in the yellow obese syndrome (12). Founder BAPaΔbasic mice (FVB/N; n = 7) were generated, and Tg lines were established from each founder by using the FVB/N stock. Founder mice were also outcrossed to C57BL/6J mice for multiple generations to produce Tg lines for pigmentation analysis (Fig. 1). Of the seven Tg lines generated, three lines (BAPaΔbasic-43, -38, and -41) that represented the full range of coat phenotypes and transgene expression levels (data not shown) were characterized further.
Figure 1.
Schematic of the wild-type agouti cDNA, the BAPaΔbasic transgene, and coat phenotype of three independently derived lines of BAPaΔbasic Tg mice (Tg/Tg). Key protein features encoded by the agouti cDNA are denoted: signal peptide (gray box), glycosylation site (stem/loop), basic region (yellow box), polyproline region (open box, Pro), and cysteine-rich region (diagonally striped box). The human β-actin promoter is denoted by the light blue box with an arrow. Deletion of the agouti basic domain in the BAPaΔbasic transgene is denoted by a single black line joining of the N- and C-terminal regions of the agouti cDNA. Tg mice that were 3–4 months old and attained by inbreeding hemizygous Tg mice (Tg/−) after backcrossing to the C57BL/6J strain for five generations are depicted with black non-Tg (a/a) littermates.
Molecular Analysis.
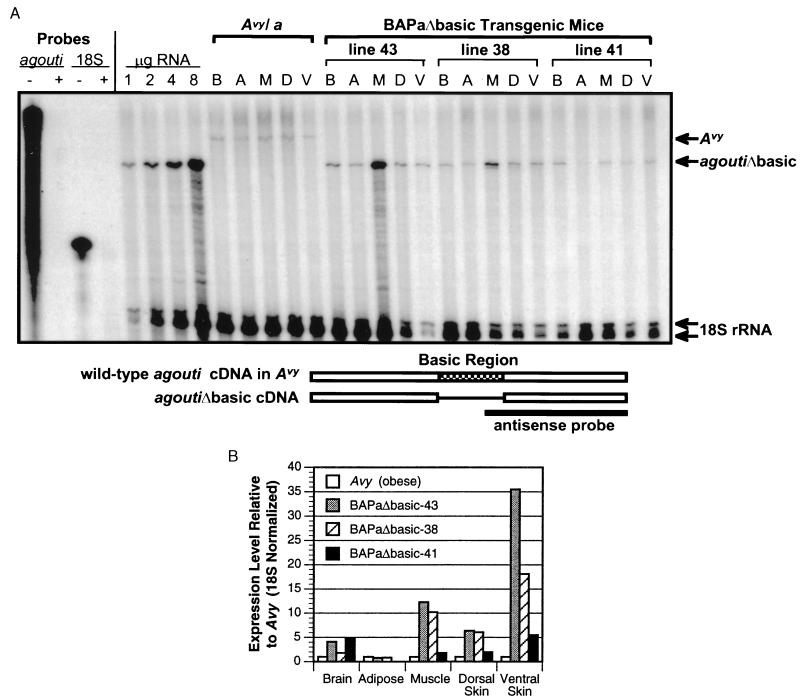
The level of transgene expression was quantified for each Tg line by RPA (Fig. 2). Probe selection included an 18S rRNA antisense probe (Ambion) for the internal control and an agouti exon 4-specific antisense probe that hybridizes specifically to the 3′ region of the agouti gene (Fig. 2A). Because of the deletion in the agoutiΔbasic transgene, the agouti antisense probe protects a shorter-sized transcript in BAPaΔbasic Tg mice compared with Avy/a mice, which ectopically express the wild-type-sized agouti transcript. Non-Tg FVB mice and control C57BL/6J mice express endogenous agouti only in skin during active hair growth (1, 31–33), therefore ectopic expression of agouti from the transgene or the Avy allele was easily detectable by RPA. The tissues analyzed (brain, white adipose tissue, skeletal muscle, and dorsal and ventral skin) were selected based on known or possible agouti-induced effects in these adult tissues (6). As expected, the RPA indicated widespread expression of agoutiΔbasic RNA from the β-actin promoter in every tissue tested and from all three lines of Tg mice, with the single exception of adipose tissue in the BAPaΔbasic-41 line.
Figure 2.
Analysis of transgene expression by RPA. (A) Quantitative RNase protection was performed with 4 μg of total RNA from brain (B), epididymal adipose (A), skeletal muscle (M), and dorsal (D) and ventral (V) skin of 30- to 40-week-old adult male mice that carry either the Avy/a mutation (solid yellow fur and obese; C57BL/6J strain) or one copy of the BAPaΔbasic transgene (Tg/−; FVB/N stock). Antisense probes specific for agouti exon 4 (165 ng) or 18S rRNA (320 ng) were hybridized separately with yeast tRNA and digested with (+) or without (−) RNase to show complete digestion of the probe in the absence of specific target sequences (lanes 1–4 at left). In all other samples, both probes were hybridized simultaneously with total tissue RNA followed by RNase digestion. Increasing amounts (1–8 μg) of total muscle RNA from BAPaΔbasic-43 Tg mice yielded quantitative protection of both agouti and 18S transcripts, showing a molar excess of each probe relative to the range of specific target sequences used throughout the experiment. The relative positions of protected bands corresponding to ectopic agouti in Avy/a, agoutiΔbasic in the Tg lines and 18S in all samples are indicated by arrows at the right. The region of agouti to which the agouti antisense probe hybridizes is indicated by the schematic at the bottom. (B) The level of agouti-specific RNA detected in the RPA shown in A was normalized to the level of 18S rRNA detected in the same tissue. The 18S-normalized values for agoutiΔbasic expression in BAPaΔbasic Tg mice were then divided by the amount of 18S-normalized ectopic agouti expression in the same tissues of Avy/a mice.
RNA from an obese Avy/a mouse was included in the assay for quantitative comparison. Variable methylation of an intracisternal A particle that inserted in the 5′ end of the wild-type agouti gene in Avy leads to variable expressivity of wild-type agouti mRNA in individual Avy/a mice from the same litter (11, 33, 34). Thus, the phenotype of Avy/a littermates ranges from “pseudoagouti” and nonobese in low-expressing individuals to yellow and obese in individuals that express high levels of ectopic agouti (11, 33). A general correlation between phenotype and RNA expression levels has been observed in other examples of both wild-type and ectopic agouti activity in vivo (10–12, 35), suggesting that agouti activity must accumulate to a threshold level in vivo to induce a yellow obese phenotype. The RPA indicated that the level of transgene expression in each line of BAPaΔbasic Tg mice exceeded the level of ectopic agouti expression in the yellow obese Avy/a mice, with the single exception of adipose tissue in the BAPaΔbasic-41 line (Fig. 2B). These data indicate that the BAPaΔbasic transgene was expressed in biologically relevant tissues and at levels comparable to or in excess of the ectopic threshold that is implied by the variable Avy phenotype.
Coat Phenotype.
Because the BAPaΔbasic Tg mice were generated in an albino strain, each Tg line was outcrossed to the C57BL/6J strain to observe the effects of the transgene on a pigmented, nonagouti (black) coat. Each line of BAPaΔbasic Tg mice produced some degree of yellow pigmentation in the fur, which was slightly more prominent in mice homozygous for the transgene (Fig. 1). The dorsum of BAPaΔbasic-43 and -38 mice were mottled, owing to the intermixture of completely yellow hairs with black-tipped or completely black hairs. The ventral fur of these mice was completely yellow. The BAPaΔbasic-41 Tg mice were barely distinguishable from non-Tg littermates because of a subtle diminution of black pigment that resembles the coat phenotype of the dilute mutation (36). The ventral fur of this line of Tg mice was somewhat lighter than the dorsal fur and was occasionally mottled yellow in some individuals. Collectively, these phenotypes indicate partial activity of the BAPaΔbasic transgene with respect to melanogenesis.
Physiological Analysis.
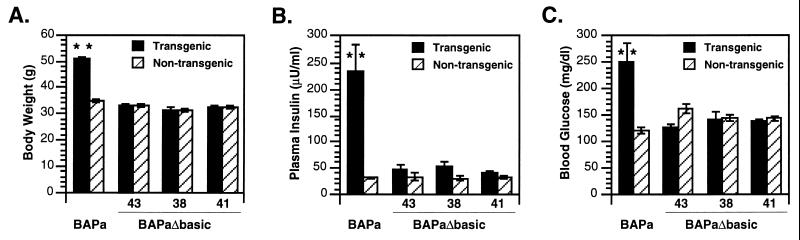
To assess the obesity-related effects of ectopic agoutiΔbasic expression, the BAPaΔbasic Tg mice were aged beyond sexual maturity. A previous study had shown that body weight in BAPa Tg mice (FVB/N), which ectopically express wild-type agouti, is significantly greater than that in non-Tg controls at 24 weeks (37). Hyperinsulinemia and hyperglycemia (in males only) are also prominent in BAPa Tg mice by this age (12). In all three lines of BAPaΔbasic Tg mice, however, body weight (Fig. 3A), plasma insulin levels (Fig. 3B), and blood glucose levels (Fig. 3C) were not significantly elevated over their non-Tg littermates. Up to 48 weeks of age, male or female mice that were either hemizygous or homozygous for the BAPaΔbasic transgene did not have any of the obesity-related traits that are normally associated with ectopic expression of wild-type agouti (data not shown). These findings indicate that ectopic expression of agoutiΔbasic was not sufficient to disrupt central control of body weight and energy balance in the BAPaΔbasic Tg mice.
Figure 3.
Physiological analysis of Tg mice (FVB/N background) that ubiquitously express wild-type agouti (BAPa) or agoutiΔbasic (BAPaΔbasic). The numbers 43, 38, and 41 refer to the three lines of BAPaΔbasic Tg mice. BAPa data were derived from the BAPa20 Tg line (12). All data were obtained from Tg and non-Tg male littermates. The two asterisks (∗∗) indicate that the means for Tg versus non-Tg mice differ significantly (P < 0.01). (A) Mean body weight (in grams) ± SEM at 24 weeks of age. The number of mice in each category are BAPa Tg/− (n = 11), control (n = 53); BAPaΔbasic-43 Tg/− (n = 15), control (n = 35); BAPaΔbasic-38 Tg/− (n = 8), control (n = 8); and BAPaΔbasic-41 Tg/− (n = 18), control (n = 22). (B) Mean insulin levels (in microunits of insulin per milliliter of plasma) ± SEM of mice at least 24 weeks of age. The number of mice in each category are BAPa20 Tg/− (n = 10), control (n = 46); BAPaΔbasic-43 Tg/− (n = 8), control (n = 8); BAPaΔbasic-38 Tg/− (n = 4), control (n = 4); and BAPaΔbasic-41 Tg/− (n = 13), control (n = 8). (C) Mean glucose levels (milligrams of glucose per deciliter of whole blood) ± SEM of mice at least 24 weeks of age. The number of mice in each category are BAPa20 Tg/− (n = 5), control (n = 20); BAPaΔbasic-43 Tg/− (n = 10), control (n = 8); BAPaΔbasic-38 Tg/− (n = 7), control (n = 7); and BAPaΔbasic-41 Tg/− (n = 14), control (n = 8).
DISCUSSION
To assess the biological significance of the agouti basic domain in vivo, we generated and characterized three lines of Tg mice that ubiquitously express an agouti cDNA that lacks the coding sequence for the entire 29-aa basic domain. Analyzing independent lines of mice allowed us to correlate subtle differences in phenotype with quantifiable levels of transgene expression in relevant tissues. Unlike the predominantly yellow fur and obesity-related traits observed in BAPa Tg mice that express the complete wild-type agouti cDNA from the human β-actin promoter (12), the fur of BAPaΔbasic Tg mice was partially yellow or diluted, and body weight, plasma insulin levels, and blood glucose levels were normal. However, significant pheomelanin or markedly reduced eumelanin in the fur of BAPaΔbasic Tg mice, compared with their nonagouti black littermates, nonetheless indicates that the agoutiΔbasic protein was synthesized and was functional in vivo. Because of the dosage-sensitive nature of agouti activity in vivo, (i) insufficient RNA expression, (ii) inhibition of protein synthesis/secretion, (iii) poor interactions with MCRs, or (iv) some combination of these factors may account for the phenotype of the BAPaΔbasic Tg mice.
Manifestation of both wild-type and ectopic agouti phenotypes is dependent on the level of agouti RNA expression in relevant tissues (10–12, 33–35), suggesting that steady-state RNA levels are rate-limiting in the synthesis of functional agouti protein. Accordingly, the degree of pheomelanin in the fur of BAPaΔbasic Tg mice correlated well with the relative level of transgene expression in the skin of these mice (line-43 > line-38 > line-41; Table 1). In contrast, the relatively greater pheomelanin of ventral versus dorsal fur (Fig. 1) did not correlate with consistently higher transgene expression levels in the ventrum (Table 1). Ectopic expression of wild-type or other mutant agouti cDNAs in Tg mice have produced similar results (13, 26), suggesting that an additional factor or factors independently bias the follicular microenvironment in the ventrum toward pheomelanin synthesis.
Table 1.
Comparison of BAPaΔbasic transgene expression levels, transgene copy number, and coat phenotype
| Tg line | Transgene copy number* | Transgene expression in dorsal skin† | Transgene expression in ventral skin† | Dorsal/ventral coat phenotype‡ |
|---|---|---|---|---|
| BAPaΔbasic-43 | 5 ± 0.39 (10) | 13.4 | 28.4 | Mottled/yellow |
| BAPaΔbasic-38 | 18 ± 3.30 (11) | 12.7 | 14.5 | Mottled/yellow |
| BAPaΔbasic-41 | 11 ± 1.22 (18) | 4.1 | 4.4 | Diluted/diluted or mottled |
Mean number of transgene copies inserted into a single site of the genome ± SEM. The numbers of mice analyzed are in parentheses.
Level of transgene expression in dorsal and ventral skin relative to the level of 18S rRNA expression in the same tissue (×100) as determined by RPA.
Coat phenotype of BAPaΔbasic Tg mice (Tg/− or Tg/Tg), which are nonagouti at the agouti locus. Yellow indicates that individual hairs are completely yellow. Mottled indicates that a mixture of both yellow and black hairs are present. The entire coat of line 43 is generally more yellow than line 38, although both are mottled. Diluted indicates a general reduction of eumelanin in individual hairs, which appear neither black nor yellow but greyish or dusty instead. Occasional individuals of line 41 have hairs that are partially yellow, especially at the base of hairs and in the ventrum.
The kinetics of MCR antagonism by agouti (15, 38) are consistent with the range of phenotypes among Avy/a littermates that express wild-type agouti at variable levels (33, 34). Completely yellow fur and obesity-related traits seem to correspond with maximal antagonism of MCRs by extremely high levels of ectopic agouti in the skin and hypothalamus, respectively, whereas partially yellow fur and normal body weight may correlate with lower levels of MCR antagonism because of relatively less Avy expression in vivo. Increased pheomelanin may coincide with normal energy balance in some Avy/a mice that express lower levels of wild-type agouti because of the greater affinity of MC1-R for its native antagonist, compared with the affinity of MC4-R for a nonnative antagonist (15). These comparisons further suggest that the mottled/diluted fur and normal body weight of BAPaΔbasic Tg mice might also result from suboptimal MCR antagonism in vivo. However, unlike the mottled, nonobese Avy/a mice, low transgene expression levels are less likely to account for the phenotype of the BAPaΔbasic Tg mice; the level of agoutiΔbasic RNA in all three Tg lines exceeded the level of wild-type agouti expression in relevant tissues of yellow obese Avy/a mice by 2- to 35-fold. Although we cannot formally rule out the influence of genetic background differences between the Tg (FVB/N or [C57BL/6J × FVB/N]N5) and Avy/a mice (C57BL/6J), these data imply that excess transgene RNA expression was insufficient to compensate for a qualitative and/or quantitative deficiency in agoutiΔbasic protein activity in vivo. Despite several attempts with different antisera (data not shown), we were unable to estimate agoutiΔbasic or wild-type agouti protein levels in relevant tissues of BAPaΔbasic or BAPa Tg mice, respectively, including the skin where biological activity was visually apparent (Fig. 1). However, the findings of MCR-binding inhibition assays in vitro independently suggest that MCRs have decreased affinity (≈10-fold) for agoutiΔbasic compared with wild-type agouti (15). Collectively, these data suggest that diminished affinity of MCRs for the agoutiΔbasic protein or low levels of agoutiΔbasic protein synthesis/stability—or perhaps both—may account for the mottled/dilute fur color and lack of obesity-related traits in the BAPaΔbasic Tg mice.
At the molecular level, the Δbasic mutation may compromise agouti biogenesis in vivo (protein folding, processing, and secretion rate) or may physically hinder agouti–MCR interactions. The basic domain may contain sequence determinants that functionally interact with MCRs, because the agouti Val83-Cys131 fragment that is equipotent to full length agouti in melanocortin-binding inhibition assays (15, 23, 28) actually contains 3 aa of the basic domain; in addition, mutation of these residues increases the binding inhibition constants (i.e., reduces affinity) at mouse MC1-R and MC4-R by as much as 13- and 5.7-fold, respectively (25). In addition, synthetic peptides (≤15 aa) from the agouti Ser59-Pro91 region, which covers the basic domain and polyproline region, also reduce tyrosinase mRNA expression in cultured melan-a melanocytes to about the same degree as recombinant wild-type agouti (V. M. Virador and V. J. Hearing, personal communication). Interestingly, the C-terminal 5 aa of the basic domain were especially important for this activity (V. M. Virador and V. J. Hearing, personal communication), and these residues were left intact in the agouti ΔArg65-Lys77 mutation that produced yellow obese Tg founder mice in a previous mutagenesis study (26). These findings collectively suggest that the basic domain contributes to MCR affinity for the agouti protein, perhaps via a direct electrostatic interaction with MCRs. Alternatively, the basic domain may interact directly with an accessory factor that contributes to MCR antagonism, such as the proteoglycan-like receptor encoded by the mahogany locus (39, 40), which is known genetically to facilitate agouti function through both MC1-R and MC4-R (41).
Our analysis of BAPaΔbasic Tg mice has established a functional role for the agouti basic domain in vivo. Our findings are consistent with conservation of the agouti basic domain across several mammalian species (refs. 1, 2, and 27; GenBank accession no. X 99692) and with data from MCR binding inhibition assays in vitro (15, 25). Although our analysis could not delineate between a quantitative and/or qualitative effect of the Δbasic mutation on agouti activity in vivo, future studies into agouti biogenesis, agouti–MCR interactions, and the molecular function of additional genes in the agouti signaling pathway will aid in determining the precise mechanism by which the basic domain contributes to wild-type and ectopic agouti activity in vivo.
Acknowledgments
We thank Carmen Foster and Anne Chang for technical assistance. R.J.M. was supported jointly by National Institute on Environmental Health Sciences Interagency Agreement Grant 1-Y01-ES-50318-00 and by an appointment to the Alexander Hollaender Distinguished Postdoctoral Fellowship Program, which is sponsored by the U.S. Office of Health and Environmental Research (Department of Energy) and administered by Oak Ridge Institute for Science and Engineering. R.L.M. was supported by funding from Glaxo Wellcome. Research was supported by a grant to B.D.B. from the Cell Biology Program at the National Science Foundation and by funding to E.J.M. from the Department of Energy at Oak Ridge National Laboratory, managed by Lockheed Martin Energy Research Corporation for the U.S. Department of Energy under Contract DE-AC05-96OR22464.
ABBREVIATIONS
- MCR
melanocortin receptor
- MC1-R
melanocortin 1 receptor
- MC4-R
melanocortin 4 receptor
- BAPa
β-actin promoter-agouti transgene
- agoutiΔbasic
mutation of the agouti cDNA with deletion of the 29-aa basic domain
- BAPaΔbasic
β-actin promoter-agoutiΔbasic transgene
- AGRP
agouti-related protein
- RPA
RNase protection assay
- a
nonagouti
- Avy
viable yellow agouti
- Tg
transgenic
References
- 1.Bultman S J, Michaud E J, Woychik R P. Cell. 1992;71:1195–1204. doi: 10.1016/s0092-8674(05)80067-4. [DOI] [PubMed] [Google Scholar]
- 2.Vage D I, Lu D, Klungland H, Lien S, Adalsteinsson S, Cone R D. Nat Genet. 1997;15:311–315. doi: 10.1038/ng0397-311. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Westby C A, Johansen M, Marshall D M, Granholm N. Pigment Cell Res. 1998;11:155–157. doi: 10.1111/j.1600-0749.1998.tb00726.x. [DOI] [PubMed] [Google Scholar]
- 4.Ollmann M M, Lamoreux M L, Wilson B D, Barsh G S. Genes Dev. 1998;12:316–330. doi: 10.1101/gad.12.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson I J. Annu Rev Genet. 1994;28:189–217. doi: 10.1146/annurev.ge.28.120194.001201. [DOI] [PubMed] [Google Scholar]
- 6.Miltenberger R J, Mynatt R L, Wilkinson J E, Woychik R P. J Nutr. 1997;127:1902S–1907S. doi: 10.1093/jn/127.9.1902S. [DOI] [PubMed] [Google Scholar]
- 7.Michaud E J, Mynatt R L, Miltenberger R J, Klebig M L, Wilkinson J E, Zemel M B, Wilkison W O, Woychik R P. J Endocrinol. 1997;155:207–209. doi: 10.1677/joe.0.1550207. [DOI] [PubMed] [Google Scholar]
- 8.Michaud E J, Bultman S J, Stubbs L J, Woychik R P. Genes Dev. 1993;7:1203–1213. doi: 10.1101/gad.7.7a.1203. [DOI] [PubMed] [Google Scholar]
- 9.Duhl D M, Stevens M E, Vrieling H, Saxon P J, Miller M W, Epstein C J, Barsh G S. Development (Cambridge, UK) 1994;120:1695–1708. doi: 10.1242/dev.120.6.1695. [DOI] [PubMed] [Google Scholar]
- 10.Michaud E J, van Vugt M J, Bultman S J, Sweet H O, Davisson M T, Woychik R P. Genes Dev. 1994;8:1463–1472. doi: 10.1101/gad.8.12.1463. [DOI] [PubMed] [Google Scholar]
- 11.Duhl D M, Vrieling H, Miller K A, Wolff G L, Barsh G S. Nat Genet. 1994;8:59–65. doi: 10.1038/ng0994-59. [DOI] [PubMed] [Google Scholar]
- 12.Klebig M L, Wilkinson J E, Geisler J G, Woychik R P. Proc Natl Acad Sci USA. 1995;92:4728–4732. doi: 10.1073/pnas.92.11.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perry W L, Hustad C M, Swing D A, Jenkins N A, Copeland N G. Genetics. 1995;140:267–274. doi: 10.1093/genetics/140.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan W, Boston B A, Kesterson R A, Hruby V J, Cone R D. Nature (London) 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 15.Kiefer L L, Ittoop O R, Bunce K, Truesdale A T, Willard D H, Nichols J S, Blanchard S G, Mountjoy K, Chen W J, Wilkison W O. Biochemistry. 1997;36:2084–2090. doi: 10.1021/bi962647v. [DOI] [PubMed] [Google Scholar]
- 16.Huszar D, Lynch C A, Fairchild-Huntress V, Dunmore J H, Fang Q, Berkemeier L R, Gu W, Kesterson R A, Boston B A, Cone R D, et al. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 17.Yeo G S, Farooqi I S, Aminian S, Halsall D J, Stanhope R G, O’Rahilly S. Nat Genet. 1998;20:111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- 18.Vaisse C, Clement K, Guy-Grand B, Froguel P. Nat Genet. 1998;20:113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- 19.Shutter J R, Graham M, Kinsey A C, Scully S, Luthy R, Stark K L. Genes Dev. 1997;11:593–602. doi: 10.1101/gad.11.5.593. [DOI] [PubMed] [Google Scholar]
- 20.Fong T M, Mao C, MacNeil T, Kalyani R, Smith T, Weinberg D, Tota M R, Van der Ploeg L H. Biochem Biophys Res Commun. 1997;237:629–631. doi: 10.1006/bbrc.1997.7200. [DOI] [PubMed] [Google Scholar]
- 21.Ollmann M M, Wilson B D, Yang Y K, Kerns J A, Chen Y, Gantz I, Barsh G S. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 22.Rossi M, Kim M S, Morgan D G, Small C J, Edwards C M, Sunter D, Abusnana S, Goldstone A P, Russell S H, Stanley S A, et al. Endocrinology. 1998;139:4428–4431. doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- 23.Willard D H, Bodnar W, Harris C, Kiefer L, Nichols J S, Blanchard S, Hoffman C, Moyer M, Burkhart W, Weiel J, et al. Biochemistry. 1995;34:12341–12346. doi: 10.1021/bi00038a030. [DOI] [PubMed] [Google Scholar]
- 24.Pallaghy P K, Nielsen K J, Craik D J, Norton R S. Protein Sci. 1994;3:1833–1839. doi: 10.1002/pro.5560031022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiefer L L, Veal J M, Mountjoy K G, Wilkison W O. Biochemistry. 1998;37:991–997. doi: 10.1021/bi971913h. [DOI] [PubMed] [Google Scholar]
- 26.Perry W L, Nakamura T, Swing D A, Secrest L, Eagleson B, Hustad C M, Copeland N G, Jenkins N A. Genetics. 1996;144:255–264. doi: 10.1093/genetics/144.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon H Y, Bultman S J, Loffler C, Chen W J, Furdon P J, Powell J G, Usala A L, Wilkison W, Hansmann I, Woychik R P. Proc Natl Acad Sci USA. 1994;91:9760–9764. doi: 10.1073/pnas.91.21.9760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quillan J M, Sadee W, Wei E T, Jimenez C, Ji L, Chang J K. FEBS Lett. 1998;428:59–62. doi: 10.1016/s0014-5793(98)00487-6. [DOI] [PubMed] [Google Scholar]
- 29.Hogan B, Costantini F, Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1986. [Google Scholar]
- 30.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 31.Miller M W, Duhl D M, Vrieling H, Cordes S P, Ollmann M M, Winkes B M, Barsh G S. Genes Dev. 1993;7:454–467. doi: 10.1101/gad.7.3.454. [DOI] [PubMed] [Google Scholar]
- 32.Bultman S J, Klebig M L, Michaud E J, Sweet H O, Davisson M T, Woychik R P. Genes Dev. 1994;8:481–490. doi: 10.1101/gad.8.4.481. [DOI] [PubMed] [Google Scholar]
- 33.Zemel M B, Kim J H, Woychik R P, Michaud E J, Kadwell S H, Patel I R, Wilkison W O. Proc Natl Acad Sci USA. 1995;92:4733–4737. doi: 10.1073/pnas.92.11.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yen T T, Gill A M, Frigeri L G, Barsh G S, Wolff G L. FASEB J. 1994;8:479–488. doi: 10.1096/fasebj.8.8.8181666. [DOI] [PubMed] [Google Scholar]
- 35.Hustad C M, Perry W L, Siracusa L D, Rasberry C, Cobb L, Cattanach B M, Kovatch R, Copeland N G, Jenkins N A. Genetics. 1995;140:255–265. doi: 10.1093/genetics/140.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Green M C. In: Genetic Variants and Strains of the Laboratory Mouse. Lyon M F, Searle A G, editors. Oxford: Oxford Univ. Press; 1989. pp. 82–83. [Google Scholar]
- 37.Mynatt R L, Miltenberger R J, Klebig M L, Zemel M B, Wilkinson J E, Wilkinson W O, Woychik R P. Proc Natl Acad Sci USA. 1997;94:919–922. doi: 10.1073/pnas.94.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu D, Willard D, Patel I R, Kadwell S, Overton L, Kost T, Luther M, Chen W, Woychik R P, Wilkison W O. Nature (London) 1994;371:799–802. doi: 10.1038/371799a0. [DOI] [PubMed] [Google Scholar]
- 39.Nagle D L, McGrail S H, Vitale J, Woolf E A, Dussault B J, Jr, DiRocco L, Holmgren L, Montagno J, Bork P, Huszar D, et al. Nature (London) 1999;398:148–152. doi: 10.1038/18210. [DOI] [PubMed] [Google Scholar]
- 40.Gunn T M, Miller K A, He L, Hyman R W, Davis R W, Azarani A, Schlossman S F, Duke-Cohan J S, Barsh G S. Nature (London) 1999;398:152–156. doi: 10.1038/18217. [DOI] [PubMed] [Google Scholar]
- 41.Miller K A, Gunn T M, Carrasquillo M M, Lamoreux M L, Galbraith D B, Barsh G S. Genetics. 1997;146:1407–1415. doi: 10.1093/genetics/146.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]