Abstract
The chemokine stromal cell-derived factor 1 (SDF-1) stimulates the growth of pre-B cells in vitro, and mice with a disrupted SDF-1 gene have abnormal fetal liver B cell lymphopoiesis. The origin of SDF-1 production has not been determined yet. Using an anti-SDF-1 mAb, we performed immunohistochemical studies in four human embryos and five fetuses to define which cells express the SDF-1 protein at sites of antenatal B cell lymphopoiesis. All mesothelial cells contained SDF-1 at all stages of development, including in the intraembryonic splanchnopleuric mesoderm early into gestation. In fetal lungs and kidneys, SDF-1 was expressed by epithelial cells, and a few B lymphoid precursors, expressing V pre-B chains, were also detected. In the fetal liver, in addition to mesothelial cells, biliary epithelial cells were the only cells to contain SDF-1. Pre-B cells expressing V chains were abundant and exclusively located around the edge of portal spaces, in close contact with biliary ductal plate epithelial cells. They did not colocalize with biliary collecting ducts. Biliary ductal plate epithelial cells and liver B cell lymphopoiesis display a parallel development and disappearance during fetal life. These results indicate that early B cell lymphopoiesis in the splanchnopleura may be triggered by mesothelial cells producing SDF-1. Later into gestation, biliary ductal plate epithelial cells may support B cell lymphopoiesis, thus playing a role similar to that of epithelial cells in the avian bursa of Fabricius, and of thymic epithelial cells for T cell lymphopoiesis.
B cell lymphopoiesis in embryos starts around coelomic cavities: terminal deoxynucleotidyltransferase-positive B cell precursors have been detected in early human fetal mesentery (1), and RAG-1 gene expression in mouse embryos begins in the paraaortic splanchnopleura on day 9 of gestation, 3 days before it starts in the liver (2). Later into gestation, the main site of B cell lymphopoiesis is the liver. The mechanisms initially triggering B cell lymphopoiesis around coelomic cavities and subsequently leading to B cell precursor expansion in the fetal liver remain poorly understood.
Stromal cell-derived factor 1 (SDF-1) is a cytokine with chemoattractant properties for monocytes, subpopulations of B and T lymphocytes, hematopoietic CD34+ stem cells, and B lymphoid precursors (3–7). Studies of mice with disrupted SDF-1 gene have shown that this chemokine plays a key role early in life. SDF-1−/− mice die either in utero or within 1 h of birth (8–10). SDF-1−/− fetuses have impaired liver B cell lymphopoiesis and bone marrow hematopoiesis and abnormal development of mesenteric vessels, cardiac septum, and cerebellum. Some of these abnormalities are presumably related to cell migration defects, but others may result from functional properties of SDF-1 other than chemotaxis. For example, abnormal B cell lymphopoiesis may be related to the ability of SDF-1 to stimulate the proliferation of pre-B lymphocyte precursors (11). CXCR4 is the only known receptor for SDF-1, and SDF-1 is the only known ligand for CXCR4. Mice with disrupted CXCR4 (9, 10, 12) or SDF-1 genes have similar abnormalities, supporting a selective partnership between these two molecules.
SDF-1 differs from other chemokines, as it is extremely conserved between species: murine and human SDF-1 differ by only one amino acid (13). Whereas the production of most other chemokines is associated with inflammation, SDF-1 gene expression, characterized so far at the mRNA level, is constitutive in a number of tissues in adult mice (11, 14, 15), adult humans (4), and mouse embryos (8). However, the nature of the SDF-1-producing cells remains unknown. Using an anti-SDF-1 mAb directed against the NH2 terminus of the chemokine, we performed immunohistochemical studies in humans to identify and characterize cells expressing the SDF-1 protein. Given that SDF-1 gene expression is absolutely required for antenatal B cell lymphopoiesis in mice, this study was focused on the splanchnopleura of embryos and on the liver, omentum, lungs, and kidneys of fetuses. SDF-1 expression was compared with that of the V pre-B chain, a component of the pre-B cell receptor, to define the respective locations of SDF-1-expressing cells and B cell precursors.
MATERIALS AND METHODS
Generation of Anti-SDF-1 mAb.
Anti-SDF-1 mAb K15C‡‡ was produced by inoculating BALB/c mice with the 15-amino acid peptide KPVSLSYRSPSRFFC conjugated via cysteine-15 to bovine serum albumin (BSA). Fusions were carried out as previously described (16). The anti-SDF-1 K15C mAb has been selected from 2,000 hybridoma supernatants tested by an indirect ELISA method. Briefly, 96-well plates (Maxisorp, Nunc) were coated with 100 μl of SDF-1 (1 μg/ml) in 0.05 M carbonate buffer, pH 9.6, for 16 h at 4°C and then saturated with 100 μl of PBS containing 0.1% Tween 20 and 0.5% BSA, for 1 h at 37°C. Then 100 μl of hybridoma supernatant was added to SDF-1-coated plates for 2 h at 37°C. After washing of unbound immunoglobulins, goat anti-mouse IgG (H+L) labeled with horseradish peroxidase (Diagnostic Pasteur, Marnes la Coquettes, France) was used to reveal antigen-bound antibodies, using ortho-phenylenediamine (2%; Sigma) as a substrate for the enzyme. The reaction was stopped with 50 μl of 4 M H2SO4, and optical density at 492 nm was read in a multiscan spectrophotometer. Anti-SDF-1 K15C mAb was determined to be of IgG2a isotype and was purified from bulk culture supernatant by affinity chromatography on a protein A-Sepharose column (Pharmacia Biotech).
Anti-SDF-1 K15C specificity was determined initially by the same ELISA method, in which SDF-1, previously described SDF-1 chimeras (17), or irrelevant chemokines were coated on well-plates. Anti-SDF-1 K15C specificity was further evaluated by competitive ELISA experiments between SDF-1 coated on well-plates and SDF-1, SDF-1 chimera proteins, or irrelevant chemokines (each at 10−5 M) diluted in RMPI medium 1640/10% FCS previously incubated for 1 h at 37°C with the K15C mAb (0.05 μg/ml). Antigen–antibody interactions were quantified by measuring the optical density at 492 nm.
SDF-1 recognition by the K15C mAb was investigated by Western blot experiments. Fifty nanograms of SDF-1α, SDF-1β, SDF-1 chimera (SDF-IP-10), or irrelevant chemokines (IP-10, RANTES, MIP-1β, IL-8) were separated by electrophoresis in SDS/15% polyacrylamide gels, blotted onto nitrocellulose membranes, and probed with the K15C mAb (1 μg/ml). Binding of the antibody was revealed by using a horseradish peroxidase-conjugated anti-mouse Ig antibody and the ECL kit (Amersham).
Tissues.
Four human embryos (nos. 1–4) and five human fetuses (nos. 5–9) were studied. The embryos were from voluntary termination of pregnancy. Their stage of development was determined with the Carnegie classification (18). Embryos 1, 2, 3, and 4 were at the Carnegie stage 12, 14, 16, and 16 of development, respectively, which correspond to days 26, 32, 37, and 37 of pregnancy, respectively. One fetus (no. 5) was from a post-traumatic abortion. Two (nos. 6 and 9) were from abortion carried out because of severe neuropsychiatric disorders of the mother (no. 6) and kidney agenesis (no. 9). Fetuses 7 and 8 were from miscarriages. Abortion occurred on weeks 16, 19.5, 20, 22, and 34 of amenorrhea for fetuses 5, 6, 7, 8, and 9, respectively. Informed written consent was obtained from parents for pathology studies, which were performed in accordance with French law and with the approval of the institutional review board. None of the fetuses displayed organogenesis abnormality at autopsy, except fetus 9 for kidneys. There was no chorioamnionitis or history of sepsis in the mother. Bacterial analysis of fetal lungs and liver showed no infection in any fetus.
Immunohistochemistry.
The anti-SDF-1 K15C (IgG2a), the anti-CXCR4 mAbs 12G5 (IgG2a)(PharMingen) and 6H8 (IgG1) (19), the CD19 mAb (clone HD37; Dako), and the anti-cytokeratin 7 (IgG1; Dako) mAbs were used at a final concentration of 10 μg/ml. The 4G7 anti-human V pre-B mAb (a mouse IgG1/κ mAb) detects surrogate light (SL) chain at the cell surface of the pro-B (SL+μ−) and pre-B (SL+μ+) cell lines, and it labels both the CD34+CD19+ and CD34−CD19+ normal pro-B and pre-B bone marrow cells (20–22). The anti-V pre-B mAb was used at a final concentration of 13 μg/ml. The isotype-matched mAbs recognized phosphocholine (clone A12, IgG1) and Haemophilus influenzae A (IgG2a, Clonatec, Paris). Binding of mAbs was detected by using biotinylated anti-mouse antibodies and streptavidin conjugated with alkaline phosphatase (Biogenex, San Ramon, CA) and the fast red substrate (Sigma), or streptavidin conjugated with peroxidase (Dako) and the diaminobenzidine substrate (Sigma), as recommended by the manufacturers. The sections were counterstained with Mayer hematoxylin. For technical reasons (necessity of treatment with acid for decalcification), we were unable to perform accurate immunochemical studies on developing bones.
For combined detection of SDF-1 and B lymphoid precursors, V pre-B-expressing cells were first detected with the 4G7 mAb, the EnVision+ System, peroxidase, mouse, diaminobenzidine (Dako). Then SDF-1-containing cells were detected with biotinylated K15C anti-SDF-1 mAb and the EnVision System, alkaline phosphatase, rabbit/mouse (Dako) and the fast red substrate (Sigma).
RESULTS
Specificity of SDF-1 Recognition by the Anti-SDF-1 K15C mAb.
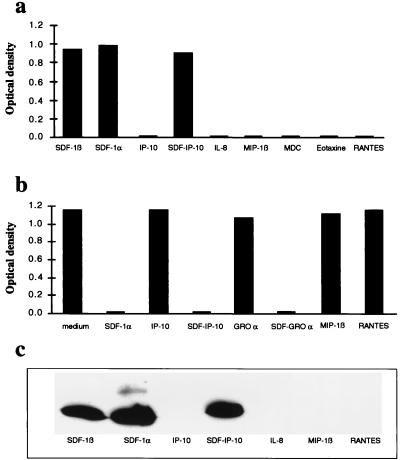
The specificity of SDF1 recognition by the K15C anti-SDF-1 mAb was demonstrated by ELISA and Western blot analysis. Both experiments showed that K15C mAb recognizes the two isoforms (α and β) of SDF-1 but fails to interact with other chemokines such as IP-10, IL-8, MIP-1β, eotaxin, MDC, and RANTES (Fig. 1 a and c). The ability of K15C to react with the SDF-IP-10 chimera expressing the first eight amino acids (KPVSLSRY) of SDF-1 fused to the NH2 terminus of IP-10 (Fig. 1 a and c) shows that this sequence is part of the epitope recognized by the antibody in wild-type SDF-1. This conclusion was confirmed by competitive ELISA studies, which showed that only SDF-1 or chimeras bearing the KPVSLSRY motif of SDF-1 (SDF-IP-10 or SDF-GROα) were able to block the binding of K15C mAb to SDF-1α-coated plates (Fig. 1b).
Figure 1.
Specificity of the anti-SDF-1 K15C mAb. (a) SDF-1α, SDF-1β, SDF-IP10 chimera, and irrelevant CXC and CC chemokines (IP-10, IL-8, MDC, MIP-1β, eotaxin, and RANTES) were coated on well-plates and incubated with anti-SDF-1 mAb K15C (0.05 μg/ml). (b) Competition experiment between SDF-1α coated onto plastic plates and different chemokines or SDF-1 chimeras preincubated with the antibody. Inhibition of antibody–antigen interaction determined by ELISA is shown. (c) Specific recognition of SDF-1 by the K15C mAb in Western blot analysis.
SDF-1 Expression by Mesothelial Cells.
The anti-SDF-1 K15C mAb was used in immunohistochemical experiments to study SDF-1 expression in human embryonic and fetal tissues. In all cases, the specificity of the labeling was confirmed by comparison with the isotype-matched control mAb. No mesenchymal, lymphoid, muscular, nervous, or endothelial cells in any tissue were found to contain SDF-1 (data not shown).
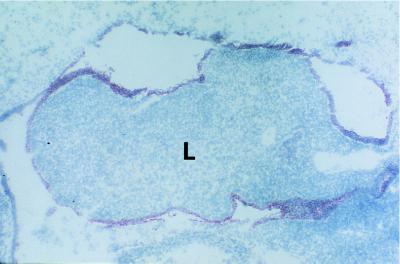
All mesothelial cells were labeled by the anti-SDF-1 K15C mAb, in all tissue sections in which they were present, regardless of their location, and regardless of the stage of development. Cells from the splanchnopleuric mesoderm thus contained SDF-1 in the four embryos (Fig. 2), as well as pleural, pericardial, and peritoneal cells in the five fetuses (Fig. 3b, Fig. 4a, and data not shown).
Figure 2.
SDF-1 expression in mesodermal cells from the splanchnopleura. Cells containing SDF-1 were labeled by immunochemistry with the K15C anti-SDF-1 mAb. In the four embryos studied, mesodermal cells from the splanchnopleura contained SDF-1. Results shown are from embryo 4 (day 37 of gestation). Note that at this stage of development, the primitive liver (L) contains neither SDF-1-expressing cells nor portal spaces and biliary epithelial cells. (Original magnification: 40×.)
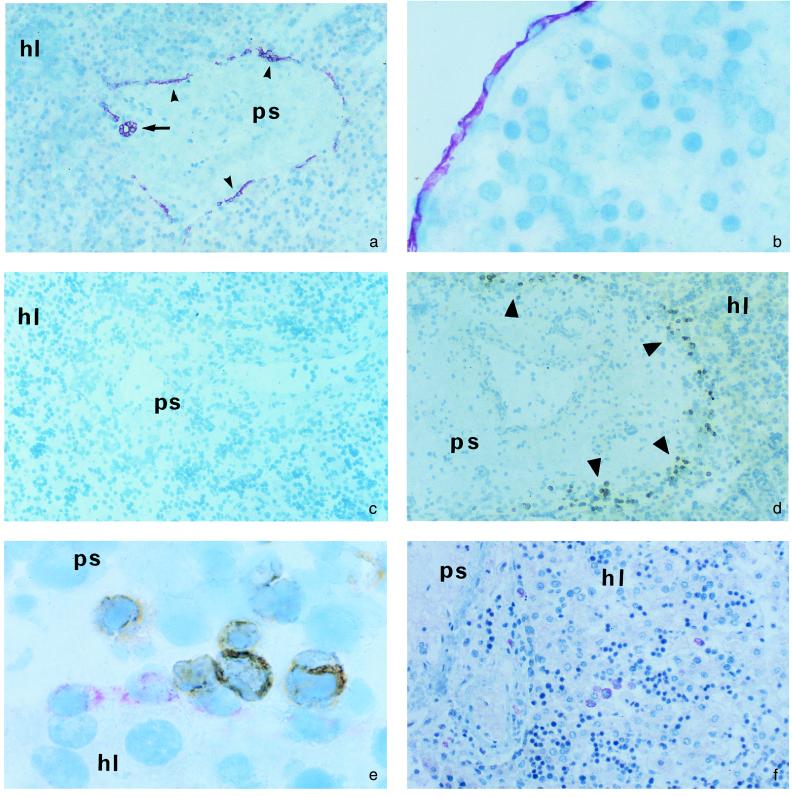
Figure 3.
Immunohistochemical studies of fetal liver. Presence of cells expressing SDF-1 (a and b), the V pre-B chain (d), or CXCR4 (f) was analyzed by immunohistochemistry. SDF-1 labeling was abolished by neutralization of the anti-SDF-1 mAb with an excess of SDF-1 before incubation with tissues sections (c). (e) Combined analysis of SDF-1- and V pre-B-expressing cells; SDF-1-expressing cells appear in red and V pre-B-expressing cells in brown. ps, Portal spaces; hl, hepatic lobules; small arrowhead, ductal plates; arrow, biliary duct; large arrowheads, V pre-B cells. (Original magnification: 100× in a and c; 200× in d and f; 400× in b; and 1000× in e.)
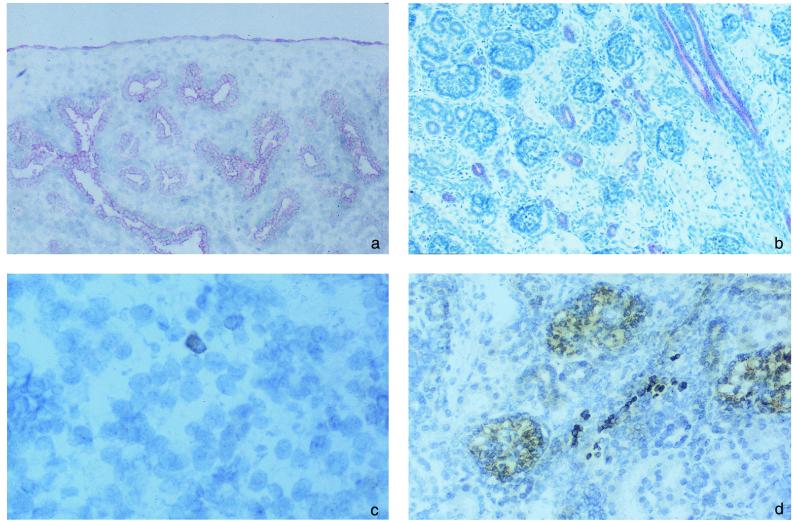
Figure 4.
SDF-1 expression and presence of V pre-B+ cells in fetal lungs and kidneys. Presence of cells expressing SDF-1 (a and b) or V pre-B (c and d) was analyzed by immunohistochemistry in fetal lung (a and c) and kidney (b and d). Results shown are from fetus 4. (Original magnification: 200×.)
Expression of SDF-1 by Biliary Epithelial Cells in the Fetal Liver.
The pattern of SDF-1 expression in fetal livers was also analyzed. In the center of portal spaces, in close contact with portal vessels, biliary duct epithelial cells were strongly labeled with the anti-SDF-1 K15C mAb. Epithelial cells from the biliary ductal plates were also labeled, forming a layer of SDF-1-producing cells at the border between the portal spaces and hepatic lobules (Fig. 3a). This pattern of SDF-1 expression was identical to that of cytokeratin 7, a specific marker of biliary epithelial cells (23) (data not shown). SDF-1 was also detected in peritoneal cells, which formed a continuous layer separating the liver parenchyma from the peritoneal cavity (Fig. 3b). Similar findings were observed for the four 16- to 22-week fetuses. In the 34-week fetus, SDF-1-expressing ductal plates were less abundant, consistent with the progressive decline of ductal plates from week 25 of gestation (23). No SDF-1 was detected in the primitive liver in embryos (Fig. 2), consistent with the absence of biliary epithelial cells at this stage of development.
In addition to isotype-matched controls, we used other controls to assess the specificity of SDF-1 detection in the liver. We neutralized the anti-SDF-1 K15C mAb with a 100-fold molar excess of recombinant SDF-1 before incubation with tissue sections. Neutralization of the antibody abolished labeling of both biliary epithelial cells (Fig. 3c) and peritoneal cells. In contrast, an identical molar excess (100-fold) of SDF-1 mutant protein (SDF-1 9–67), lacking the first 8 amino acids, which contain the epitope recognized by the K15C mAb, failed to inhibit labeling of biliary epithelial cells and peritoneal cells by K15C (data not shown).
Colocalization of SDF-1-Expressing Biliary Ductal Plate Cells and B Lymphoid Precursors in Human Fetal Liver.
We studied in detail the distributions of SDF-1-expressing cells and B lymphoid precursors in the fetal liver. We hypothesized that areas containing SDF-1-expressing cells could represent foci of B cell lymphopoiesis.
Identification of B lymphoid precursors was performed by immunohistochemical experiments using an anti-V pre-B mAb. Cells producing the V pre-B chain were abundant in the four 16- to 22-week fetuses but rare in the 34-week fetus. In all cases, positive cells were exclusively located around the edge of the portal spaces (Fig. 3d). In the two fetuses (nos. 3 and 4) studied for CD19 expression, CD19+ cells and V pre-B+ cells had a similar distribution in the liver (data not shown). Therefore, B lymphoid precursors were concentrated in the areas of the fetal liver containing the biliary ductal plate epithelial cells. No B lymphoid precursors were detected in the liver in contact with intrahepatic biliary ducts or with peritoneal cells.
Direct evidence for colocalization of B lymphoid precursors and SDF-1-expressing biliary ductal plate cells was obtained by immunohistochemical experiments combining the anti-SDF-1 and the anti-V pre-B mAbs. These experiments unambiguously showed that most B lymphoid precursors were in close contact with SDF-1-containing biliary ductal plates (Fig. 3e).
We then tested by immunohistochemistry with an anti-CXCR4 mAb whether cell targets of SDF-1 other than B lymphoid precursors had a similar distribution in the liver. CXCR4-expressing cells all had the morphological features of hematopoietic cells. They were clustered, but these clusters were randomly distributed throughout the livers, in both the hepatic lobules and portal spaces, with no enrichment at the periphery of portal spaces (Fig. 3f).
Thus, the close association between SDF-1-expressing biliary ductal plates and B lymphoid precursors is a specific feature. It is not observed with SDF-1-expressing biliary duct cells. It is also not observed with other CXCR4-positive cells of the hematopoietic lineage.
SDF-1 Expression and B Lymphoid Precursors in Fetal Lungs, Kidneys, and Omentum.
We analyzed the expression of SDF-1 in the lungs and kidneys. In the lungs, in addition to pleural cells, all bronchial and alveolar epithelial cells were labeled (Fig. 4a). In the kidneys, the distal tubules, the Bellini collecting ducts, and the urothelium were also labeled with the anti-SDF-1 mAb. Not all epithelial cells expressed SDF-1, as the glomerular epithelial cells and the proximal tubules were negative (Fig. 4b). In the omentum, SDF-1 was expressed only by peritoneal cells (data not shown).
We also looked for the presence of B cell precursors in these tissues. This search was done in two fetuses (nos. 3 and 4). In the lungs, a few V pre-B+ cells were found scattered within the interstitium (Fig. 4c). In the kidneys, a few positive cells were also found in the interstitium, but only in the renal cortex (Fig. 4d). There was no V pre-B+ cells in the medulla. Both in lungs and in the renal cortex, V pre-B+ cells were close to epithelial cells, but considering the large number of epithelial cells in these organs and the absence of tissue compartmentalization, it was not possible to determine whether this reflected a selective colocalization or a random distribution of B cell precursors. In the omentum, a few V pre-B+ cells were also found. They were clustered in the connective tissue and were all distant from peritoneal cells (data not shown).
DISCUSSION
SDF-1 was originally identified as a growth factor for pre-B lymphocyte precursors in vitro (11), and studies of mice with disrupted SDF-1 genes have confirmed the critical role played by this chemokine in B cell lymphopoiesis in vivo during the fetal life (8–10). To extend these previous studies, we analyzed the expression of the SDF-1 protein in human embryos and fetuses, to define which type of cells support B cell lymphopoiesis. We focused our study on the splanchnopleura in the embryos and on the liver, lungs, kidneys, and omentum in the fetuses. We found that mesothelial cells and epithelial cells were the only cells containing SDF-1 at these sites of antenatal B cell lymphopoiesis. However, not all epithelial cells expressed SDF-1, as hepatocytes and glomerular and proximal tubule cells did not.
In contrast to epithelial cells, all mesothelial cells examined contained SDF-1. This was true for mesothelial cells covering the splanchnopleuric mesoderm in embryos and for pleural, pericardial, and peritoneal cells in the fetuses. Our results are consistent with a role of SDF-1 and mesothelial cells in the initial triggering of B cell lymphopoiesis around coelomic cavities, the first site of B cell lymphopoiesis in humans (1) and in mice (2). As SDF-1 was detected as early as on week 5 of gestation, its expression precedes the initiation of B cell lymphopoiesis. In adult mice, there is a B lymphocyte subpopulation, the B1 cells, which are closely associated with the peritoneal cavity and the omentum, where they proliferate (24). SDF-1 production by mesothelial cells, which persists in adults (data not shown), may account for the ability of B1 cells to target themselves and to divide at this site.
Fetal liver hematopoiesis is decreased but not abolished in SDF-1−/− or CXCR4−/− mice (8–10). The different distributions of CXCR4-positive hematopoietic cells and SDF-1 expression that we observed in the liver are consistent with such a limited role of SDF-1 in fetal liver hematopoiesis. Therefore, although SDF-1 acts as a chemoattractant in vitro for CD34+ hematopoietic stem cells (6), other chemoattractants substitute for it in vivo, at least in the fetal liver. In contrast, SDF-1 production is clearly a key event for the commitment to and differentiation of hematopoietic progenitors of the B lymphocyte lineage in this organ.
In addition to the liver and the bone marrow, lungs, kidneys, and omentum support B cell lymphopoiesis in fetuses (25, 26). We indeed observed a few pre-B V+ cells at these sites. Interestingly, SDF-1 is expressed by epithelial cells in lungs and kidneys, suggesting that it may contribute to the local B cell lymphopoiesis. In the kidneys, despite the presence of SDF-1-expressing cells in the medulla, no B cell precursors were found at this site, indicating that SDF-1 expression per se may not be sufficient to trigger B cell lymphopoiesis. A similar finding was observed in the liver. Although biliary duct cells expressed SDF-1 in the center of portal spaces, B cell precursors were located exclusively at the edge of portal spaces, in direct contact with SDF-1-expressing ductal plates.
The expression of SDF-1 by biliary ductal plate cells may provide insight into the role of the liver in the development of the B lymphocyte lineage. B lymphopoiesis in the liver starts before week 8 of gestation in humans (27), and it persists until a few weeks after birth. It is, however, less intense beyond week 34 of gestation than earlier (28). The development of liver B cell lymphopoiesis follows a pattern similar to that of biliary ductal plates. Biliary ductal plates differentiate from primitive hepatocytes 6 to 9 weeks into gestation, first appearing around large portal vein branches close to the hilum, and subsequently extending to more peripheral branches. They gradually disappear from week 25 of gestation onwards, and on week 40, only the smallest portal spaces are still surrounded by discontinuous ductal plates (23).
The similar distributions of liver B lymphoid precursors and SDF-1-expressing ductal plates, and their parallel development in the fetus and regression around birth, indicate that biliary ductal plates may have a key role in fetal B cell lymphopoiesis. The close contact between the two cell populations is consistent with SDF-1 attracting early B lymphocyte precursors (5). B lymphocyte precursors also require SDF-1 for their commitment and/or proliferation (11). Factors other than SDF-1 production are likely to be required for liver B cell lymphopoiesis, such as production of other growth factors or membrane interactions. It is unknown whether biliary ductal plates or other liver cells provide these additional signals, but absence of such signals may explain why close colocalization with B cell precursors is not observed for all SDF-1-expressing cells.
There is in the avian bursa of Fabricius a close association between B lymphocyte progenitors and digestive epithelial cells, which are required for B lymphocyte development (29). Our data suggest that there is in the fetal liver of mammals a parallel partnership between epithelial cells and B lymphoid precursors, involving biliary ductal plate epithelial cells. If this is the case, then the involvement of epithelial cells in mammalian lymphocyte development, already demonstrated for T lymphocytes in the thymus and digestive tract, would also apply to B lymphocytes in the fetal liver. This interaction could be based largely on the ability of SDF-1 expressed by biliary ductal plates to attract and stimulate B lymphocyte precursors.
Acknowledgments
We thank Françoise Baleux for her gift of recombinant SDF-1 and Ian Clark-Lewis for his gift of SDF-1 9–67 and SDF-1 chimera. Pascale Laurent is acknowledged for technical assistance. This work was supported by the Agence Nationale de Recherche sur le SIDA (ANRS), by the Association Claude Bernard, and by the Université Paris-Sud. A.A. and A.F. were supported by fellowships from the ANRS.
ABBREVIATION
- SDF-1
stromal cell-derived factor 1
Footnotes
Correspondence and requests concerning the anti-SDF-1 K15C mAb should be addressed to F.A.-S. e-mail: farenzan@pasteur.fr.
References
- 1.Catoretti G, Parravicini C, Bonati A A, Buscaglio M, Zuliani G, Plebani A, Delia D, Rilke F. Eur J Immunol. 1989;19:493–498. doi: 10.1002/eji.1830190313. [DOI] [PubMed] [Google Scholar]
- 2.Marcos A R, Godin I, Cumano A, Morales S, Garcia-Porrero J A, Dieterlen-Lièvre F, Gaspar M L. Immunol Rev. 1994;137:155–171. doi: 10.1111/j.1600-065x.1994.tb00663.x. [DOI] [PubMed] [Google Scholar]
- 3.Bleul C, Fuhlbrigge C, Casanovas J M, Aiuti A, Springer T A. J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bleul C, Schultze J L, Springer T A. J Exp Med. 1998;187:753–762. doi: 10.1084/jem.187.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Apuzzo M, Rolink A, Loestscher M, Hoxie J A, Clark-Lewis I, Melchers F, Baggiolini M, Moser B. Eur J Immunol. 1997;27:1788–1793. doi: 10.1002/eji.1830270729. [DOI] [PubMed] [Google Scholar]
- 6.Aiuti A, Webb I J, Bleul C, Springer T A, Gutierrez-Ramos J C. J Exp Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim C H, Pelus L M, White J R, Broxmeyer H E. Blood. 1998;91:4434–4443. [PubMed] [Google Scholar]
- 8.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S I, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Nature (London) 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 9.Tachibana K, Hirota S, Lizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S I, et al. Nature (London) 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 10.Ma Q, Jones D, Borghesani P R, Segal R A, Nagasawa T, Kishimoto T, Bronson R T, Springer T A. Proc Natl Acad Sci USA. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagasawa T, Kikutani H, Kishimoto T. Proc Natl Acad Sci USA. 1994;91:2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou Y R, Kottmann A H, Kuroda M, Taniuchi I, Littman D R. Nature (London) 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 13.Shirozu M, Nakano T, Inazawa J, Tashiro K, Tada H, Shinohara T, Honjo T. Genomics. 1995;28:495–502. doi: 10.1006/geno.1995.1180. [DOI] [PubMed] [Google Scholar]
- 14.Tashiro K, Tada H, Heilker R, Shirozu M, Nakano T, Honjo T. Science. 1993;261:600–603. doi: 10.1126/science.8342023. [DOI] [PubMed] [Google Scholar]
- 15.Godiska R, Chantry D, Diestsch G N, Gray P W. J Neuroimmunol. 1995;58:167–176. doi: 10.1016/0165-5728(95)00008-p. [DOI] [PubMed] [Google Scholar]
- 16.Tanchou G, Delaunay T, Bodeus M, Roques B P, Darlix J L, Benarous R. J Gen Virol. 1995;76:2457–2466. doi: 10.1099/0022-1317-76-10-2457. [DOI] [PubMed] [Google Scholar]
- 17.Crump M P, Gong J H, Loetscher P, Rajarathnam K, Amara A, Arenzana-Seisdedos F, Virelizier J L, Baggiolini M, Sykes B D, Clark-Lewis I. EMBO J. 1997;16:6996–7007. doi: 10.1093/emboj/16.23.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Rahilly R, Müller F. Carnegie Institute Wash. Publ. No. 637. 1987. [Google Scholar]
- 19.Mondor I, Moulard M, Ugolini S, Klasse P J, Hoxie J, Amara A, Delaunay T, Wyatt R, Sodroski J, Sattentau Q J. Virology. 1998;248:394–405. doi: 10.1006/viro.1998.9282. [DOI] [PubMed] [Google Scholar]
- 20.Gauthier L, Lemmmers B, Guelpa-Fonlupt V, Fougereau M, Schiff C. J Immunol. 1999;162:41–50. [PubMed] [Google Scholar]
- 21.Meffre E, Papavasiliou F, Cohen P, de Bouteiller O, Bell D, Karasuyama H, Schiff C, Banchereau J, Liu Y-J, Nussenzweig M C. J Exp Med. 1998;188:765–772. doi: 10.1084/jem.188.4.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemmers B, Gauthier L, Guelpa-Fonlupt V, Fougereau M, Schiff C. Blood. 1999;93:4336–4346. [PubMed] [Google Scholar]
- 23.Van Eyken P, Sciot R, Callea F, Van Der Steen K, Moerman P, Desmet V J. Hepatology. 1988;8:1586–1595. doi: 10.1002/hep.1840080619. [DOI] [PubMed] [Google Scholar]
- 24.Tarakhovsky A. J Exp Med. 1997;185:981–984. doi: 10.1084/jem.185.6.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solvason N, Kearney J F. J Exp Med. 1992;175:397–404. doi: 10.1084/jem.175.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nunez C, Nishimoto N, Gartland G L, Billips L G, Burrows P D, Kubagawa H, Cooper M D. J Immunol. 1996;156:866–872. [PubMed] [Google Scholar]
- 27.Cuisinier A M, Gauthier L, Boubli L, Fougereau M, Tonnelle C. Eur J Immunol. 1993;23:110–118. doi: 10.1002/eji.1830230118. [DOI] [PubMed] [Google Scholar]
- 28.Kamps W A, Cooper M. J Immunol. 1982;129:526–531. [PubMed] [Google Scholar]
- 29.McCormack W T, Tjoelker L W, Thompson C B. Annu Rev Immunol. 1991;9:219–241. doi: 10.1146/annurev.iy.09.040191.001251. [DOI] [PubMed] [Google Scholar]