Abstract
BACKGROUND—The pathophysiology of enterohaemorrhagic Escherichia coli (EHEC) infection remains unclear. Eicosanoids have been implicated as pathophysiological mediators in other colitides. AIMS—To determine if prostaglandin E2 (PGE2) and leukotriene B4 (LTB4) contribute to mucosal inflammation and dysfunction in EHEC colitis. METHODS—Ten day old rabbits were infected with EHEC. For five days after infection, mucosal synthesis of PGE2 and LTB4 was measured in distal colonic tissue from control and infected animals and 51Cr-EDTA permeability was assessed in vivo. Myeloperoxidase activity was measured and histological inflammation and damage were assessed at five days in control and infected animals and after treatment of infected animals with the LTB4 synthesis inhibitor MK-886. In separate experiments, ion transport was measured in Ussing chambers, before and after in vitro addition of the cyclooxygenase inhibitor indomethacin. RESULTS—LTB4 synthesis was increased from day 2 after infection onwards and PGE2 synthesis was increased on day 3. Mucosal permeability did not increase until day 5 after infection. MK-886 inhibited colonic LTB4 production but did not reduce diarrhoea, inflammation, or mucosal damage. Electrolyte transport was not significantly altered on day 3 after infection. However, both Cl secretion and reduced Na absorption found on day 5 were partially reversed by indomethacin. CONCLUSIONS—Tissue synthesis of PGE2 and LTB4 did not correlate temporally with EHEC induced inflammation or changes in mucosal permeability and ion transport. Cyclooxygenase inhibition partially reversed ion transport abnormalities but lipoxygenase inhibition did not affect mucosal inflammation or histological damage. We conclude that the contribution of eicosanoids to mucosal injury and dysfunction is more complex than previously suggested. Keywords: enterohaemorrhagic; Escherichia coli; electrolyte transport; prostaglandins; leukotrienes; chloride secretion
Full Text
The Full Text of this article is available as a PDF (156.4 KB).
Figure 1 .
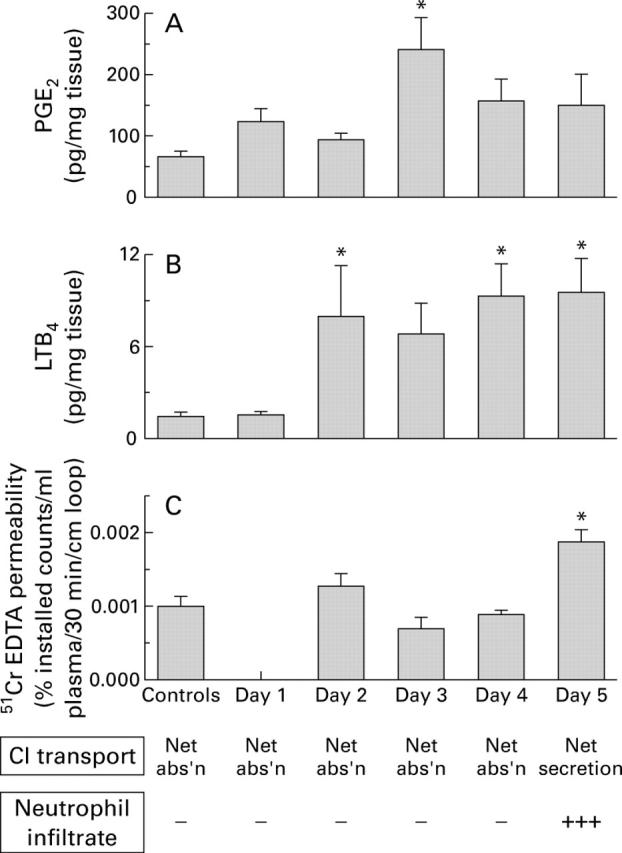
(A) Distal colonic mucosal PGE2 synthetic capacity in uninfected control rabbits and in EHEC infected rabbits 1-5 days after inoculation. Data are mean (SEM), n=12 per group. *p<0.05 compared with control, day 1 infected, and day 2 infected values. (B) Distal colonic mucosal LTB4 synthetic capacity in uninfected control rabbits and in EHEC infected rabbits 1-5 days after inoculation. Data are mean (SEM), n=12 per group. *p<0.05 compared with control and day 1 infected values. (C) Distal colonic mucosal 51Cr-EDTA permeability in uninfected control rabbits and in EHEC infected rabbits 2-5 days after inoculation. Data are mean (SEM), n=4 per group. *p<0. 05 compared with control and other infected values. Lower panels show Cl transport and infiltration of the mucosa by neutrophils. Abs'n, absorption.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argenzio R. A., Lecce J., Powell D. W. Prostanoids inhibit intestinal NaCl absorption in experimental porcine cryptosporidiosis. Gastroenterology. 1993 Feb;104(2):440–447. doi: 10.1016/0016-5085(93)90412-6. [DOI] [PubMed] [Google Scholar]
- Bell C. J., Gall D. G., Wallace J. L. Disruption of colonic electrolyte transport in experimental colitis. Am J Physiol. 1995 Apr;268(4 Pt 1):G622–G630. doi: 10.1152/ajpgi.1995.268.4.G622. [DOI] [PubMed] [Google Scholar]
- Bern M. J., Sturbaum C. W., Karayalcin S. S., Berschneider H. M., Wachsman J. T., Powell D. W. Immune system control of rat and rabbit colonic electrolyte transport. Role of prostaglandins and enteric nervous system. J Clin Invest. 1989 Jun;83(6):1810–1820. doi: 10.1172/JCI114086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter A. O., Borczyk A. A., Carlson J. A., Harvey B., Hockin J. C., Karmali M. A., Krishnan C., Korn D. A., Lior H. A severe outbreak of Escherichia coli O157:H7--associated hemorrhagic colitis in a nursing home. N Engl J Med. 1987 Dec 10;317(24):1496–1500. doi: 10.1056/NEJM198712103172403. [DOI] [PubMed] [Google Scholar]
- Elliott E., Li Z., Bell C., Stiel D., Buret A., Wallace J., Brzuszczak I., O'Loughlin E. Modulation of host response to Escherichia coli o157:H7 infection by anti-CD18 antibody in rabbits. Gastroenterology. 1994 Jun;106(6):1554–1561. doi: 10.1016/0016-5085(94)90410-3. [DOI] [PubMed] [Google Scholar]
- Ford-Hutchinson A. W., Bray M. A., Doig M. V., Shipley M. E., Smith M. J. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980 Jul 17;286(5770):264–265. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- Giannella R. A., Rout W. R., Formal S. B. Effect of indomethacin on intestinal water transport in salmonella-infected rhesus monkeys. Infect Immun. 1977 Jul;17(1):136–139. doi: 10.1128/iai.17.1.136-139.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillard J., Ford-Hutchinson A. W., Chan C., Charleson S., Denis D., Foster A., Fortin R., Leger S., McFarlane C. S., Morton H. L-663,536 (MK-886) (3-[1-(4-chlorobenzyl)-3-t-butyl-thio-5-isopropylindol-2-yl]-2,2 - dimethylpropanoic acid), a novel, orally active leukotriene biosynthesis inhibitor. Can J Physiol Pharmacol. 1989 May;67(5):456–464. doi: 10.1139/y89-073. [DOI] [PubMed] [Google Scholar]
- Goldhill J. M., Burakoff R., Donovan V., Rose K., Percy W. H. Defective modulation of colonic secretomotor neurons in a rabbit model of colitis. Am J Physiol. 1993 Apr;264(4 Pt 1):G671–G677. doi: 10.1152/ajpgi.1993.264.4.G671. [DOI] [PubMed] [Google Scholar]
- Hanglow A. C., Bienenstock J., Perdue M. H. Effects of platelet-activating factor on ion transport in isolated rat jejunum. Am J Physiol. 1989 Nov;257(5 Pt 1):G845–G850. doi: 10.1152/ajpgi.1989.257.5.G845. [DOI] [PubMed] [Google Scholar]
- Harris D. W., Smith P. R., Swan C. H. Determination of prostaglandin synthetase activity in rectal biopsy material and its significance in colonic disease. Gut. 1978 Oct;19(10):875–877. doi: 10.1136/gut.19.10.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashimura M., Okuno M., Himeno S., Kuroshima T., Kawamoto H., Shinomura Y., Tarui S. Prostaglandin D2 diminishes transmucosal potential difference in rat colonic mucosa in vitro in contrast to the increasing effect of prostaglandin E1. Prostaglandins. 1988 Apr;35(4):583–595. doi: 10.1016/0090-6980(88)90033-0. [DOI] [PubMed] [Google Scholar]
- Keenan C. M., Rangachari P. K. Eicosanoid interactions in the canine proximal colon. Am J Physiol. 1989 Apr;256(4 Pt 1):G673–G679. doi: 10.1152/ajpgi.1989.256.4.G673. [DOI] [PubMed] [Google Scholar]
- Krawisz J. E., Sharon P., Stenson W. F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984 Dec;87(6):1344–1350. [PubMed] [Google Scholar]
- Kubes P., Hunter J., Granger D. N. Ischemia/reperfusion-induced feline intestinal dysfunction: importance of granulocyte recruitment. Gastroenterology. 1992 Sep;103(3):807–812. doi: 10.1016/0016-5085(92)90010-v. [DOI] [PubMed] [Google Scholar]
- Li Z., Bell C., Buret A., Robins-Browne R., Stiel D., O'Loughlin E. The effect of enterohemorrhagic Escherichia coli O157:H7 on intestinal structure and solute transport in rabbits. Gastroenterology. 1993 Feb;104(2):467–474. doi: 10.1016/0016-5085(93)90415-9. [DOI] [PubMed] [Google Scholar]
- Li Z., Elliott E., Payne J., Isaacs J., Gunning P., O'loughlin E. V. Shiga toxin-producing Escherichia coli can impair T84 cell structure and function without inducing attaching/effacing lesions. Infect Immun. 1999 Nov;67(11):5938–5945. doi: 10.1128/iai.67.11.5938-5945.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNaughton W. K., Leach K. E., Prud'homme-Lalonde L., Ho W., Sharkey K. A. Ionizing radiation reduces neurally evoked electrolyte transport in rat ileum through a mast cell-dependent mechanism. Gastroenterology. 1994 Feb;106(2):324–335. doi: 10.1016/0016-5085(94)90589-4. [DOI] [PubMed] [Google Scholar]
- Madara J. L., Patapoff T. W., Gillece-Castro B., Colgan S. P., Parkos C. A., Delp C., Mrsny R. J. 5'-adenosine monophosphate is the neutrophil-derived paracrine factor that elicits chloride secretion from T84 intestinal epithelial cell monolayers. J Clin Invest. 1993 May;91(5):2320–2325. doi: 10.1172/JCI116462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen K. L., Tavernini M. M., Mosmann T. R., Fedorak R. N. Interleukin 10 modulates ion transport in rat small intestine. Gastroenterology. 1996 Oct;111(4):936–944. doi: 10.1016/s0016-5085(96)70061-6. [DOI] [PubMed] [Google Scholar]
- March S. B., Ratnam S. Latex agglutination test for detection of Escherichia coli serotype O157. J Clin Microbiol. 1989 Jul;27(7):1675–1677. doi: 10.1128/jcm.27.7.1675-1677.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March S. B., Ratnam S. Sorbitol-MacConkey medium for detection of Escherichia coli O157:H7 associated with hemorrhagic colitis. J Clin Microbiol. 1986 May;23(5):869–872. doi: 10.1128/jcm.23.5.869-872.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton-Thompson G. J., Cummings J. H., Newman A., Billings J. A., Misiewicz J. J. Colonic and small intestinal response to intravenous prostaglandin F2 alpha and E2 in man. Gut. 1975 Jan;16(1):42–46. doi: 10.1136/gut.16.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mion F., Cuber J. C., Minaire Y., Chayvialle J. A. Short term effects of indomethacin on rat small intestinal permeability. Role of eicosanoids and platelet activating factor. Gut. 1994 Apr;35(4):490–495. doi: 10.1136/gut.35.4.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musch M. W., Miller R. J., Field M., Siegel M. I. Stimulation of colonic secretion by lipoxygenase metabolites of arachidonic acid. Science. 1982 Sep 24;217(4566):1255–1256. doi: 10.1126/science.6810465. [DOI] [PubMed] [Google Scholar]
- Norris A. A., Lewis A. J., Zeitlin I. J. Changes in colonic tissue levels of inflammatory mediators in a guinea-pig model of immune colitis. Agents Actions. 1982 Apr;12(1-2):243–246. doi: 10.1007/BF01965154. [DOI] [PubMed] [Google Scholar]
- O'Loughlin E. V., Hunt D. M., Kreutzmann D. Postnatal development of colonic electrolyte transport in rabbits. Am J Physiol. 1990 Mar;258(3 Pt 1):G447–G453. doi: 10.1152/ajpgi.1990.258.3.G447. [DOI] [PubMed] [Google Scholar]
- Pai C. H., Gordon R., Sims H. V., Bryan L. E. Sporadic cases of hemorrhagic colitis associated with Escherichia coli O157:H7. Clinical, epidemiologic, and bacteriologic features. Ann Intern Med. 1984 Dec;101(6):738–742. doi: 10.7326/0003-4819-101-6-738. [DOI] [PubMed] [Google Scholar]
- Peskar B. M., Lange K., Hoppe U., Peskar B. A. Ethanol stimulates formation of leukotriene C4 in rat gastric mucosa. Prostaglandins. 1986 Feb;31(2):283–293. doi: 10.1016/0090-6980(86)90054-7. [DOI] [PubMed] [Google Scholar]
- Powell D. W. New paradigms for the pathophysiology of infectious diarrhea. Gastroenterology. 1994 Jun;106(6):1705–1707. doi: 10.1016/0016-5085(94)90430-8. [DOI] [PubMed] [Google Scholar]
- Rath H. C., Herfarth H. H., Ikeda J. S., Grenther W. B., Hamm T. E., Jr, Balish E., Taurog J. D., Hammer R. E., Wilson K. H., Sartor R. B. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest. 1996 Aug 15;98(4):945–953. doi: 10.1172/JCI118878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley L. W., Remis R. S., Helgerson S. D., McGee H. B., Wells J. G., Davis B. R., Hebert R. J., Olcott E. S., Johnson L. M., Hargrett N. T. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983 Mar 24;308(12):681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- Roberts W. G., Simon T. J., Berlin R. G., Haggitt R. C., Snyder E. S., Stenson W. F., Hanauer S. B., Reagan J. E., Cagliola A., Tanaka W. K. Leukotrienes in ulcerative colitis: results of a multicenter trial of a leukotriene biosynthesis inhibitor, MK-591. Gastroenterology. 1997 Mar;112(3):725–732. doi: 10.1053/gast.1997.v112.pm9041233. [DOI] [PubMed] [Google Scholar]
- Sharon P., Stenson W. F. Enhanced synthesis of leukotriene B4 by colonic mucosa in inflammatory bowel disease. Gastroenterology. 1984 Mar;86(3):453–460. [PubMed] [Google Scholar]
- Sharon P., Stenson W. F. Metabolism of arachidonic acid in acetic acid colitis in rats. Similarity to human inflammatory bowel disease. Gastroenterology. 1985 Jan;88(1 Pt 1):55–63. doi: 10.1016/s0016-5085(85)80132-3. [DOI] [PubMed] [Google Scholar]
- Smith H. R., Rowe B., Gross R. J., Fry N. K., Scotland S. M. Haemorrhagic colitis and Vero-cytotoxin-producing Escherichia coli in England and Wales. Lancet. 1987 May 9;1(8541):1062–1065. doi: 10.1016/s0140-6736(87)90485-5. [DOI] [PubMed] [Google Scholar]
- Triadafilopoulos G., Pothoulakis C., Weiss R., Giampaolo C., Lamont J. T. Comparative study of Clostridium difficile toxin A and cholera toxin in rabbit ileum. Gastroenterology. 1989 Nov;97(5):1186–1192. doi: 10.1016/0016-5085(89)91689-2. [DOI] [PubMed] [Google Scholar]
- Tzipori S., Karch H., Wachsmuth K. I., Robins-Browne R. M., O'Brien A. D., Lior H., Cohen M. L., Smithers J., Levine M. M. Role of a 60-megadalton plasmid and Shiga-like toxins in the pathogenesis of infection caused by enterohemorrhagic Escherichia coli O157:H7 in gnotobiotic piglets. Infect Immun. 1987 Dec;55(12):3117–3125. doi: 10.1128/iai.55.12.3117-3125.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J. L., Keenan C. M. An orally active inhibitor of leukotriene synthesis accelerates healing in a rat model of colitis. Am J Physiol. 1990 Apr;258(4 Pt 1):G527–G534. doi: 10.1152/ajpgi.1990.258.4.G527. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., MacNaughton W. K., Morris G. P., Beck P. L. Inhibition of leukotriene synthesis markedly accelerates healing in a rat model of inflammatory bowel disease. Gastroenterology. 1989 Jan;96(1):29–36. doi: 10.1016/0016-5085(89)90760-9. [DOI] [PubMed] [Google Scholar]
- Zipser R. D., Nast C. C., Lee M., Kao H. W., Duke R. In vivo production of leukotriene B4 and leukotriene C4 in rabbit colitis. Relationship to inflammation. Gastroenterology. 1987 Jan;92(1):33–39. doi: 10.1016/0016-5085(87)90836-5. [DOI] [PubMed] [Google Scholar]


