Abstract
BACKGROUND—Antisecretory factor (AF), a 41 kDa cloned and sequenced protein, suppresses intestinal inflammation and hypersecretion in animals. Endogenous AF production can be induced by dietary modifications in several animal species, and this feed has been shown to reduce the incidence of diarrhoeal disease in weaning piglets. The role of AF in intestinal disease in humans is not known. AIMS—To study the effects of hydrothermally processed cereals, optimised for AF induction in animals, added to the diet of patients with longstanding symptoms of inflammatory bowel disease (IBD). PATIENTS—Fifty three patients with IBD (ulcerative colitis and Crohn's disease) were entered into the study, and 50 completed follow up. The experimental group consisted of 16 females (mean age 50 (SEM 5) years) and 10 males (41 (4) years) and the placebo group of 12 women (41 (4) years old) and 12 men (51 (5) years). METHODS—Patients were randomised to receive either hydrothermally processed cereals (active treatment) or the same amount of ordinary cereals (placebo treatment) for four weeks in a double blind study design. Baseline diet and medications remained unchanged. Bowel symptoms, plasma levels of AF, and colonic biopsies were evaluated before and after treatment. RESULTS—The active treatment significantly improved subjective ratings of clinical symptoms and increased plasma AF levels compared with placebo. Plasma lipid levels were unaffected. CONCLUSION—Hydrothermally processed cereals can induce AF production in human IBD. This increase in endogenous AF activity is associated with clinical improvement. Further studies are warranted to clarify the exact role of AF in human intestinal disease. Keywords: antisecretory factor; functional food; ulcerative colitis; Crohn's disease
Full Text
The Full Text of this article is available as a PDF (220.9 KB).
Figure 1 .
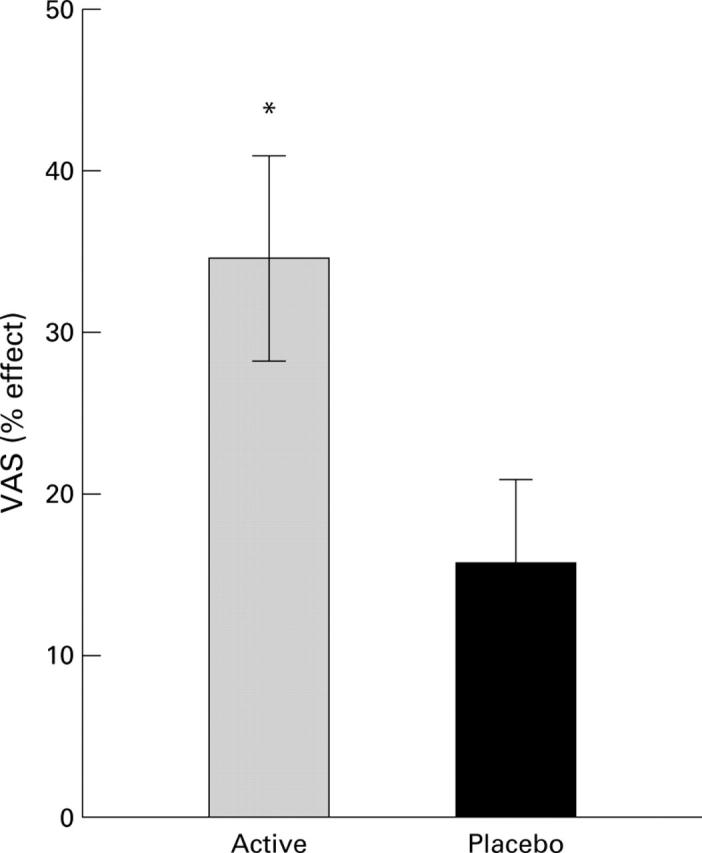
Subjective estimation of the effects of the two diets after four weeks of treatment, expressed as percentage improvement of the condition (VAS). Data are mean (SEM). *Significant difference between the active and placebo groups (p<0.05). VAS, visual analogue scale.
Figure 2 .
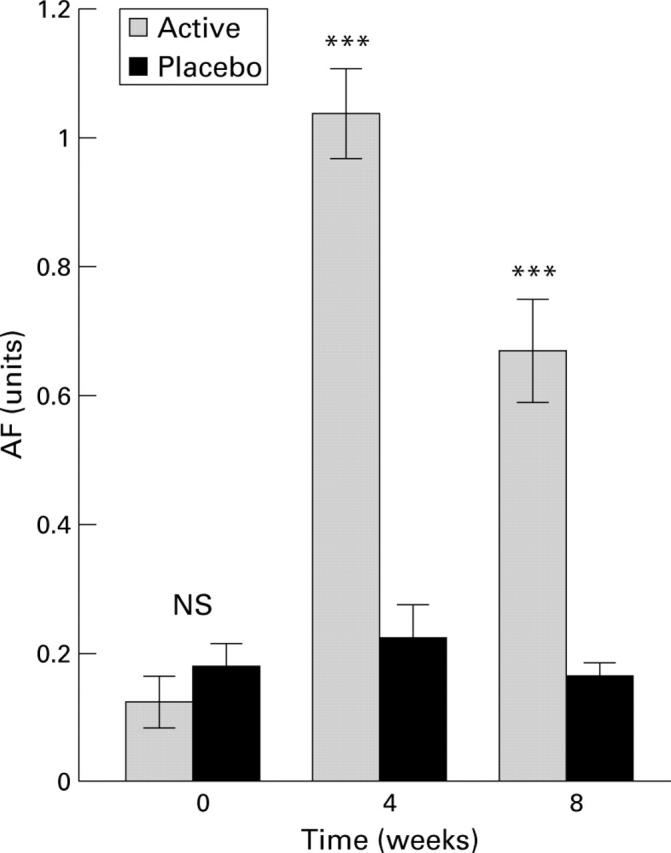
Plasma levels of antisecretory factor (AF) in the two groups of patients at the start of the experiment, after four weeks of active or placebo diet, and after eight weeks (that is, four weeks after termination of the diets). Data are mean (SEM). ***Significant difference between groups (p<0.001) at four and eight weeks. NS, not significant.
Figure 3 .
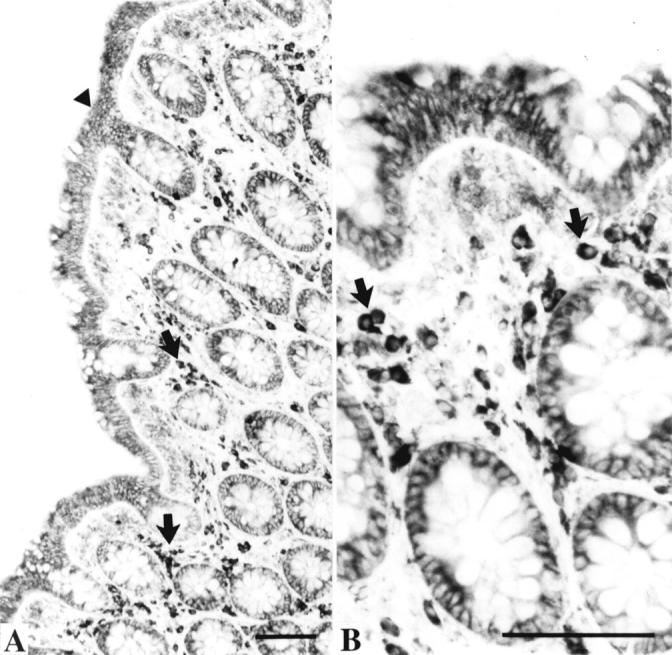
Biopsies from a healthy large intestine processed to demonstrate immunoreactivity of antisecretory factor (AF). Bars=100 µm. (A) Low magnification showing AF immunoreactivity in the surface epithelium (arrowhead), in crypt cells, and in lymphocyte-like cells (arrows) in the lamina propria. (B) Larger magnification showing the AF positive lymphocyte-like cells (arrows).
Figure 4 .
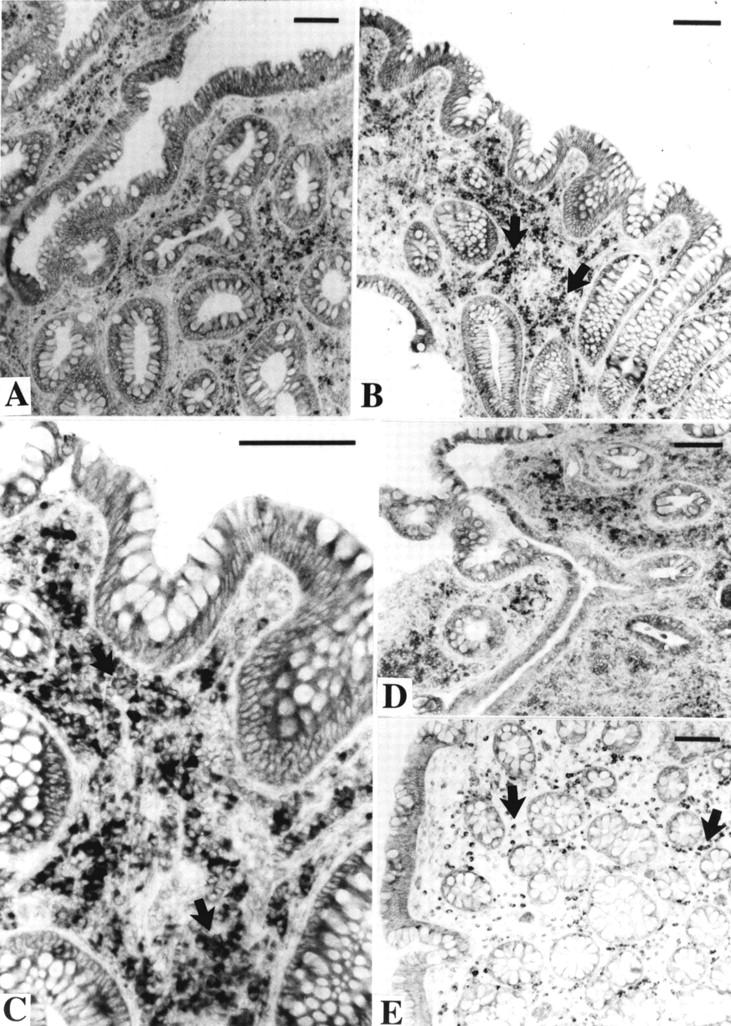
Rectal biopsies from patients with ulcerative colitis treated with the active diet. The sections were incubated with an antiserum against antisecretory factor (AF). Bars=100 µm. (A) Patient No 31 before the diet period. (B) Patient No 31 after the diet period. There appears to be more AF positive cells (arrows) in the lamina propria after the diet period. (C) Larger magnification of (B), showing AF positive cells (arrows) in the lamina propria. (D) Patient No 12 before the diet period. There are signs of an acute inflammatory reaction. Many small inflammatory cells show moderate AF immunoreactivity. (E) The same patient after the diet period. In this biopsy there is no acute inflammation. Large AF positive cells (arrows) are evenly distributed within the entire lamina propria.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fuss I. J., Neurath M., Boirivant M., Klein J. S., de la Motte C., Strong S. A., Fiocchi C., Strober W. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996 Aug 1;157(3):1261–1270. [PubMed] [Google Scholar]
- Göransson L., Martinsson K., Lange S., Lönnroth I. Feed-induced lectins in piglets. Feed-induced lectins and their effect on post-weaning diarrhoea, daily weight gain and mortality. Zentralbl Veterinarmed B. 1993 Sep;40(7):478–484. [PubMed] [Google Scholar]
- Johansson E., Jennische E., Lange S., Lönnroth I. Antisecretory factor suppresses intestinal inflammation and hypersecretion. Gut. 1997 Nov;41(5):642–645. doi: 10.1136/gut.41.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson E., Lönnroth I., Lange S., Jonson I., Jennische E., Lönnroth C. Molecular cloning and expression of a pituitary gland protein modulating intestinal fluid secretion. J Biol Chem. 1995 Sep 1;270(35):20615–20620. doi: 10.1074/jbc.270.35.20615. [DOI] [PubMed] [Google Scholar]
- Lange S., Jennische E., Johansson E., Lönnroth I. The antisecretory factor: synthesis and intracellular localisation in porcine tissues. Cell Tissue Res. 1999 Jun;296(3):607–617. doi: 10.1007/s004410051322. [DOI] [PubMed] [Google Scholar]
- Lange S., Lönnroth I., Skadhauge E. Effects of the antisecretory factor in pigs. Pflugers Arch. 1987 Jul;409(3):328–332. doi: 10.1007/BF00583485. [DOI] [PubMed] [Google Scholar]
- Lennard-Jones J. E. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2–19. doi: 10.3109/00365528909091339. [DOI] [PubMed] [Google Scholar]
- Lönnroth I., Lange S. Intake of monosaccharides or amino acids induces pituitary gland synthesis of proteins regulating intestinal fluid transport. Biochim Biophys Acta. 1987 Aug 13;925(2):117–123. doi: 10.1016/0304-4165(87)90100-0. [DOI] [PubMed] [Google Scholar]
- Lönnroth I., Lange S., Skadhauge E. The antisecretory factors: inducible proteins which modulate secretion in the small intestine. Comp Biochem Physiol A Comp Physiol. 1988;90(4):611–617. doi: 10.1016/0300-9629(88)90675-5. [DOI] [PubMed] [Google Scholar]
- MacDermott R. P., Stenson W. F. Alterations of the immune system in ulcerative colitis and Crohn's disease. Adv Immunol. 1988;42:285–328. doi: 10.1016/s0065-2776(08)60848-2. [DOI] [PubMed] [Google Scholar]
- Miyamoto H., Tanaka T., Kitamoto N., Fukuda Y., Shimoyama T. Detection of immunoreactive antigen, with a monoclonal antibody to measles virus, in tissue from a patient with Crohn's disease. J Gastroenterol. 1995 Feb;30(1):28–33. doi: 10.1007/BF01211371. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K. Inflammatory bowel disease (1) N Engl J Med. 1991 Sep 26;325(13):928–937. doi: 10.1056/NEJM199109263251306. [DOI] [PubMed] [Google Scholar]
- Powrie F. T cells in inflammatory bowel disease: protective and pathogenic roles. Immunity. 1995 Aug;3(2):171–174. doi: 10.1016/1074-7613(95)90086-1. [DOI] [PubMed] [Google Scholar]
- Thompson N. P., Montgomery S. M., Pounder R. E., Wakefield A. J. Is measles vaccination a risk factor for inflammatory bowel disease? Lancet. 1995 Apr 29;345(8957):1071–1074. doi: 10.1016/s0140-6736(95)90816-1. [DOI] [PubMed] [Google Scholar]
- Wakefield A. J., Sim R., Akbar A. N., Pounder R. E., Dhillon A. P. In situ immune responses in Crohn's disease: a comparison with acute and persistent measles virus infection. J Med Virol. 1997 Feb;51(2):90–100. doi: 10.1002/(sici)1096-9071(199702)51:2<90::aid-jmv2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]


