Abstract
BACKGROUND—Faecal concentrations of the protein calprotectin have been found to be elevated in patients with colorectal neoplasia, suggesting that it might be used as a screening tool for colorectal cancer as well as adenomas. AIMS—To measure the sensitivity and specificity of faecal calprotectin for the detection of adenomas in high risk individuals undergoing colonoscopy. Also, to investigate between and within stool variability of calprotectin concentrations. SUBJECTS—A total of 814 patients planned for colonoscopy were included for the following indications: positive faecal occult blood test, 25; neoplasia surveillance, 605; newly detected polyp, 130; and family risk, 54. METHODS—Two faecal samples from each of two stools were analysed using the PhiCal ELISA test device (Nycomed Pharma AS). RESULTS—Adenoma patients had significantly higher calprotectin levels than normal subjects (median 9.1 (95% confidence interval 7.5-10.1) v 6.6 (5.6-7.4)mg/l). There was no significant decrease in calprotectin levels after polypectomy. Levels in cancer patients were significantly higher than those in all other subgroups (median 17.6 mg/l (11.5-31.0)). With a cut off limit of 10 mg/l, the sensitivity for cancer was 74% and for adenoma 43%. Corresponding specificity values were 64% for no cancer and 67% for no neoplasia (cancer+adenoma). Specificity varied from 71% for one stool sample to 63% for four samples. Stool variability was small, suggesting that two spots from one stool were as discriminative as two spots from each of two stools. CONCLUSIONS—The sensitivity and specificity of faecal calprotectin levels as a marker for colorectal adenoma and carcinoma justifies its use in high risk groups, but specificity is too low for screening of average risk persons. Lack of a decrease in levels after polypectomy may be due to a more widespread leucocyte migration into the intestinal lumen than that at the polyp site, and needs further investigation. Keywords: calprotectin; colonoscopy; colorectal cancer; polyps; screening; tumour markers
Full Text
The Full Text of this article is available as a PDF (142.5 KB).
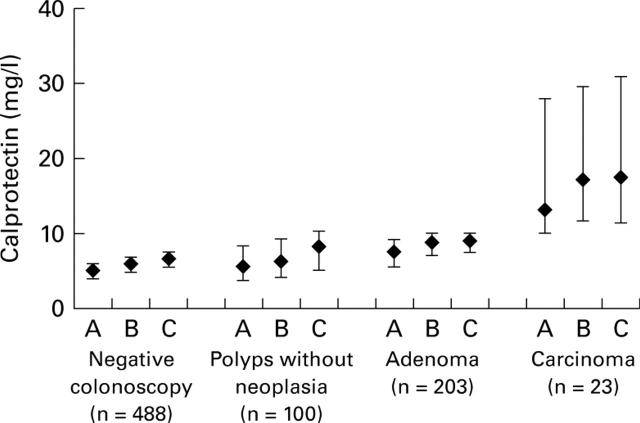
Figure 1 .
Median (95% confidence intervals) faecal calprotectin levels in the four groups using the three sampling methods: A, one spot; B, maximum of two spots in the first stool; C, maximum of one spot from two stools.
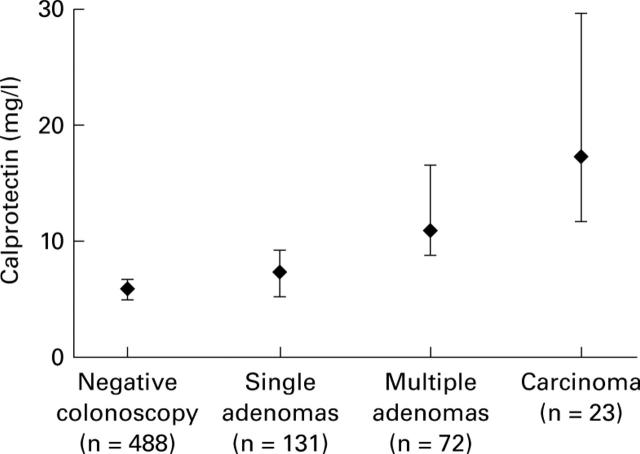
Figure 2 .
Faecal calprotectin levels by number of adenomas.
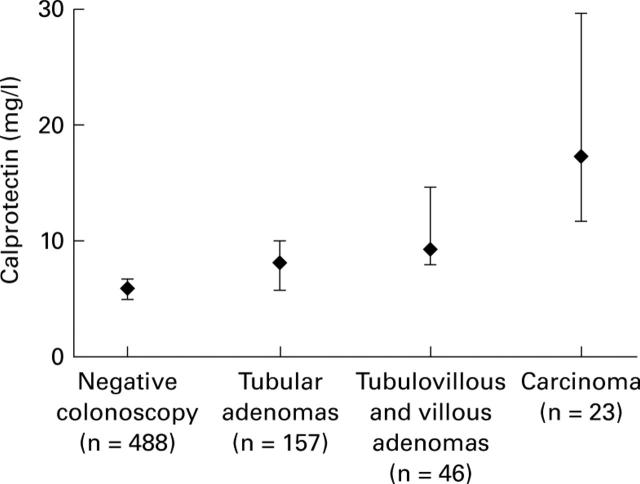
Figure 3 .
Faecal calprotectin levels by histology.
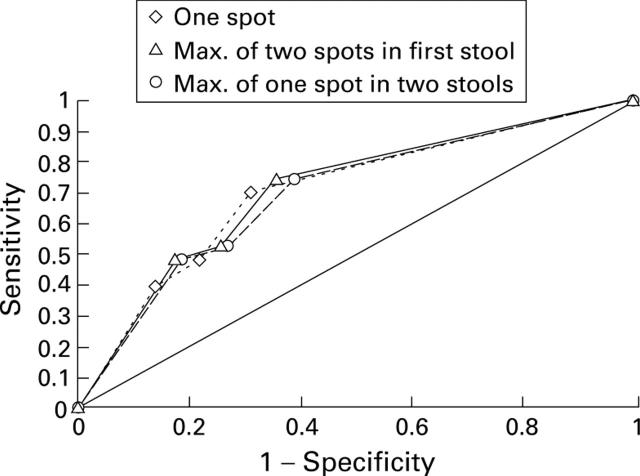
Figure 4 .
Sensitivity and specificity of the three sampling methods for asymptomatic colorectal cancer.
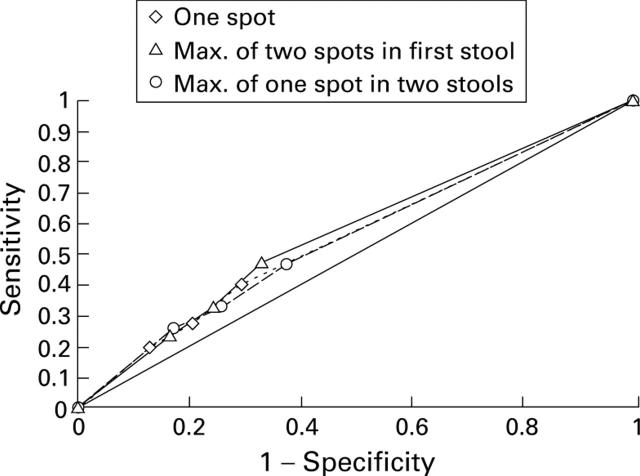
Figure 5 .
Sensitivity and specificity of the three sampling methods for asymptomatic colorectal neoplasia.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J. E. Review article: faecal occult blood testing for colorectal cancer. Aliment Pharmacol Ther. 1998 Jan;12(1):1–10. doi: 10.1046/j.1365-2036.1998.00231.x. [DOI] [PubMed] [Google Scholar]
- Allison J. E., Tekawa I. S., Ransom L. J., Adrain A. L. A comparison of fecal occult-blood tests for colorectal-cancer screening. N Engl J Med. 1996 Jan 18;334(3):155–159. doi: 10.1056/NEJM199601183340304. [DOI] [PubMed] [Google Scholar]
- Dutta S. K., Nair P. P. Noninvasive detection of colorectal cancer by molecular tools: coming of age. Gastroenterology. 1998 Jun;114(6):1333–1335. doi: 10.1016/s0016-5085(98)70442-1. [DOI] [PubMed] [Google Scholar]
- Gilbert J. A., Ahlquist D. A., Mahoney D. W., Zinsmeister A. R., Rubin J., Ellefson R. D. Fecal marker variability in colorectal cancer: calprotectin versus hemoglobin. Scand J Gastroenterol. 1996 Oct;31(10):1001–1005. doi: 10.3109/00365529609003120. [DOI] [PubMed] [Google Scholar]
- Jahn H., Joergensen O. D., Kronborg O., Fenger C. Can Hemoccult-II replace colonoscopy in surveillance after radical surgery for colorectal cancer and after polypectomy? Dis Colon Rectum. 1992 Mar;35(3):253–256. doi: 10.1007/BF02051018. [DOI] [PubMed] [Google Scholar]
- Johne B., Fagerhol M. K., Lyberg T., Prydz H., Brandtzaeg P., Naess-Andresen C. F., Dale I. Functional and clinical aspects of the myelomonocyte protein calprotectin. Mol Pathol. 1997 Jun;50(3):113–123. doi: 10.1136/mp.50.3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen O. D., Kronborg O., Fenger C. The Funen Adenoma Follow-Up Study. Characteristics of patients and initial adenomas in relation to severe dysplasia. Scand J Gastroenterol. 1993 Mar;28(3):239–243. doi: 10.3109/00365529309096079. [DOI] [PubMed] [Google Scholar]
- Jørgensen O. D., Kronborg O., Fenger C. The Funen Adenoma Follow-up Study. Incidence and death from colorectal carcinoma in an adenoma surveillance program. Scand J Gastroenterol. 1993 Oct;28(10):869–874. doi: 10.3109/00365529309103127. [DOI] [PubMed] [Google Scholar]
- Kewenter J., Björk S., Haglind E., Smith L., Svanvik J., Ahrén C. Screening and rescreening for colorectal cancer. A controlled trial of fecal occult blood testing in 27,700 subjects. Cancer. 1988 Aug 1;62(3):645–651. doi: 10.1002/1097-0142(19880801)62:3<645::aid-cncr2820620333>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Kristinsson J., Røseth A., Fagerhol M. K., Aadland E., Schjønsby H., Børmer O. P., Raknerud N., Nygaard K. Fecal calprotectin concentration in patients with colorectal carcinoma. Dis Colon Rectum. 1998 Mar;41(3):316–321. doi: 10.1007/BF02237485. [DOI] [PubMed] [Google Scholar]
- O'Brien M. J., O'Keane J. C., Zauber A., Gottlieb L. S., Winawer S. J. Precursors of colorectal carcinoma. Biopsy and biologic markers. Cancer. 1992 Sep 1;70(5 Suppl):1317–1327. doi: 10.1002/1097-0142(19920901)70:3+<1317::aid-cncr2820701519>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Robinson M. H., Kronborg O., Williams C. B., Bostock K., Rooney P. S., Hunt L. M., Hardcastle J. D. Faecal occult blood testing and colonoscopy in the surveillance of subjects at high risk of colorectal neoplasia. Br J Surg. 1995 Mar;82(3):318–320. doi: 10.1002/bjs.1800820310. [DOI] [PubMed] [Google Scholar]
- Robinson M. H., Marks C. G., Farrands P. A., Bostock K., Hardcastle J. D. Screening for colorectal cancer with an immunological faecal occult blood test: 2-year follow-up. Br J Surg. 1996 Apr;83(4):500–501. doi: 10.1002/bjs.1800830420. [DOI] [PubMed] [Google Scholar]
- Røseth A. G., Fagerhol M. K., Aadland E., Schjønsby H. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol. 1992 Sep;27(9):793–798. doi: 10.3109/00365529209011186. [DOI] [PubMed] [Google Scholar]
- Røseth A. G., Kristinsson J., Fagerhol M. K., Schjønsby H., Aadland E., Nygaard K., Roald B. Faecal calprotectin: a novel test for the diagnosis of colorectal cancer? Scand J Gastroenterol. 1993 Dec;28(12):1073–1076. doi: 10.3109/00365529309098312. [DOI] [PubMed] [Google Scholar]
- St John D. J., Young G. P., Alexeyeff M. A., Deacon M. C., Cuthbertson A. M., Macrae F. A., Penfold J. C. Evaluation of new occult blood tests for detection of colorectal neoplasia. Gastroenterology. 1993 Jun;104(6):1661–1668. doi: 10.1016/0016-5085(93)90643-q. [DOI] [PubMed] [Google Scholar]
- Swidsinski A., Khilkin M., Kerjaschki D., Schreiber S., Ortner M., Weber J., Lochs H. Association between intraepithelial Escherichia coli and colorectal cancer. Gastroenterology. 1998 Aug;115(2):281–286. doi: 10.1016/s0016-5085(98)70194-5. [DOI] [PubMed] [Google Scholar]
- Yamao T., Matsumura Y., Shimada Y., Moriya Y., Sugihara K., Akasu T., Fujita S., Kakizoe T. Abnormal expression of CD44 variants in the exfoliated cells in the feces of patients with colorectal cancer. Gastroenterology. 1998 Jun;114(6):1196–1205. doi: 10.1016/s0016-5085(98)70425-1. [DOI] [PubMed] [Google Scholar]