Abstract
In response to antigenic stimuli, the multisubunit immune recognition receptors become aggregated and then phosphorylated on their cytoplasmic tyrosines. For the clonotypic receptors of B and T cells and for Fc receptors such as the high-affinity receptor for IgE (FcɛRI), a Src family kinase initiates this phosphorylation. We ask whether aggregation of the initiating kinase itself is required for signal transduction or whether, alternatively, a single associated kinase molecule can phosphorylate the receptors in an aggregate. We formulate the alternative molecular mechanisms mathematically and compare predictions with experimental findings on FcɛRI-bearing cells expressing varying amounts of the transfected Src family kinase Lyn. The data are consistent with the requirement of only a single Lyn molecule per FcɛRI aggregate to initiate signaling and are inconsistent with a mechanism requiring more than one Lyn molecule.
The multisubunit immune recognition receptors (MIRR), unlike the growth factor receptors, have no intrinsic kinase activity. To initiate signaling, MIRR must first associate with a Src kinase (1). In the case of the high-affinity receptor for IgE, FcɛRI, it is the Src kinase Lyn that associates constitutively with the receptor and phosphorylates tyrosines on the β and γ subunits of aggregated receptors (2–4).
There is persuasive experimental evidence for the absolute requirement of aggregation of the receptor (5, 6), but one question that has not been answered is whether aggregation of Lyn kinase itself is required for receptor phosphorylation. A model for the initiation of signaling by another member of the MIRR family, the clonotypic receptor for antigen on B lymphocytes, incorporates the clustering of Lyn (7), but the experimental evidence for the necessity of Lyn aggregation in that system is not conclusive (8). In the case of Syk, the kinase that is activated immediately downstream of the phosphorylation of FcɛRI by Lyn (9–11), there is conflicting evidence regarding the importance of aggregation of the kinase (refs. 12–14; see Discussion). In this study we show that the aggregation of Lyn is not required for Lyn to mediate phosphorylation of aggregated FcɛRI.
In other systems in which aggregation is followed by phosphorylation of receptors, the analogous question has been probed by using altered receptors. For example, aggregation of growth factor receptors, which are intrinsic tyrosine kinases, is followed by transphosphorylation of receptor tyrosines and initiates various signaling pathways. However, the juxtaposition of two functioning kinase domains does not appear to be required for signaling by these receptors. Honegger et al. (15, 16) showed that mutant epidermal growth factor (EGF) receptors having an inactive kinase domain were phosphorylated when they formed heterodimers with wild-type EGF receptors. These experiments, which provided strong evidence for the transphosphorylation mechanism, also demonstrate that a receptor aggregate with only a single functional kinase domain can initiate the phosphorylation that triggers the entire biochemical cascade. Because FcɛRI utilizes an extrinsic kinase that rapidly associates and dissociates from both the unphosphorylated and phosphorylated receptors (17), we cannot use an approach similar to that used with EGF receptors to determine whether the aggregation of Src kinases is required.
Cytokine receptors, like MIRR, undergo ligand-induced receptor aggregation and phosphorylation using constitutively associated extrinsic kinases (in this instance Jak kinases; reviewed in ref. 18). In cases where distinct Jak kinases associate with cytokine subunits, for example the IL-2 receptor system (19, 20), it has been possible to show that heterodimers of Jak kinases are required for optimal signaling. The mutation or absence of one of the Jak kinases decreases or prevents activation of the other (21–23). Because we are analyzing the potential role of homodimers of a Src kinase, the approach used to demonstrate the importance of heterodimerization of Jak kinases in signaling mediated by cytokine receptors is not applicable.
Another tactic, one that does not require altering the functional domains of the Src kinase or the MIRR, is to study the dependence of receptor phosphorylation on the concentration of the initiating Src kinase. For example, if one could titrate the initiating Src kinase into a cell and observe how the concentration of the kinase influences the phosphorylation of the receptors, one should be able to determine how many Src kinases per receptor aggregate are required to initiate phosphorylation. When the Src kinase is limiting, one expects that phosphorylation of the receptor will be proportional to the concentration of the Src kinase if only one kinase molecule per aggregate is required. If two molecules of Lyn are required, phosphorylation should be proportional to the square of the concentration.
This is essentially the approach we take. Because Lyn must be anchored by lipid tethers to the inner leaflet of the plasma membrane, we could not rely on a simple titration, e.g., by incubating permeabilized cells in medium containing variable amounts of Lyn. Instead, we used a clone of Chinese hamster ovary (CHO) cells that we had stably transfected with FcɛRI. This clone has little endogenous Lyn, and we prepared from it a secondary set of stable transfectants expressing a spectrum of amounts of Lyn (24). To confirm our intuition concerning the dependence of phosphorylation on the concentration of Lyn when Lyn is limiting, we present two mathematical models, one requiring only a single Lyn per aggregate and one requiring two Lyns per aggregate to initiate phosphorylation. Comparing predictions of the models to experiment, we can reject the two-Lyn model and show that the single-Lyn model is consistent with experiment.
MATERIALS AND METHODS
A series of stable double transfectants were generated by electroporating the cDNA for rat Lyn B kinase into a CHO cell line previously stably transfected with the α, β, and γ subunits of rat FcɛRI (24). Clones doubly resistant to G418 and zeocin were characterized for expression of FcɛRI (by incubation with 125I-labeled IgE) and of Lyn (by Western blotting whole cell lysates with polyclonal anti-Lyn (Upstate Biotechnology, Lake Placid, NY)). Phosphotyrosine (PY) on the subunits of FcɛRI was determined by stimulating 5 × 106 transfected cells with covalently crosslinked dimers of IgE, at 37°C. Control cells were incubated with monomeric IgE. FcɛRI were immunoprecipitated with anti-rat IgE, Western blotted with anti-PY directly or indirectly coupled with horseradish peroxidase (PY-20-HRP from Transduction Laboratories, Lexington, KY, or 4G10-biotin from Upstate Biotechnology plus avidin-HRP from Sigma), and detected by using an enhanced chemiluminescence (ECL) system (Amersham). The blots were then stripped and reprobed with a monoclonal antibody (JRK) to the β subunit of rat FcɛRI (25). Autophotographs of the blots were scanned with computing densitometry. The small amount of PY observed on the receptors from unstimulated cells was subtracted from the values for activated receptors. The normalized PY per receptor was calculated by dividing the densitometric value for PY of the β or γ subunit (anti-PY blot) by the value for the JRK blot of the β chain. For each Western blotting antibody, the linear range of detection was determined by scanning a blot from a gel loaded with increasing amounts of CHO lysate (for the blots with anti-Lyn) or with immunoprecipitated FcɛRI (for the blots with anti-PY and anti-β) (see also Results). Appropriate exposures were chosen for scanning that fell within the linear range of antibody staining. An aliquot of tyrosine-phosphorylated human Lyn was included on each gel to normalize for differences in transfer and amount of anti-PY between blots. Additional methodological details are described in ref. 24.
RESULTS
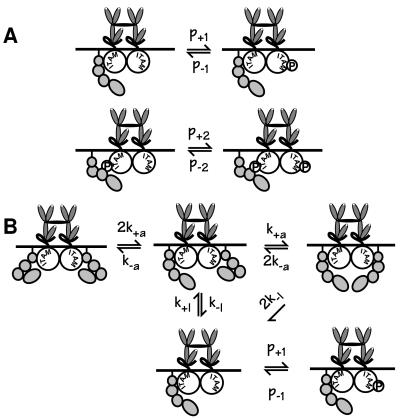
In the experiments we analyze, CHO cells stably transfected with FcɛRI and the short form of Lyn (Lyn-B, see Discussion) were stimulated with covalently linked dimers of IgE. Aggregates then consist of exactly two receptors. We test two alternative models, one in which the activation of Lyn requires both receptors in an aggregate to be associated with Lyn, and one in which a single molecule of Lyn, associated with one of two receptors in an aggregate, is sufficient for phosphorylation to proceed. Fig. 1 sketches the key phosphorylation reactions in the two models. Each model is formulated as a set of coupled chemical rate equations. Quantitative predictions are obtained by solving the systems of equations numerically, using parameters derived from the literature and our earlier experiments (26, 27). Both models include reversible, constitutive association of Lyn with unaggregated FcɛRI, and higher-affinity association of additional Lyn with aggregated, phosphorylated receptors, consistent with data from rat basophilic leukemia (RBL) cells (28). Although the models do not explicitly include membrane domains that may mediate the interaction of Src kinases with MIRR (29–32), the rates of constitutive association and dissociation of Lyn and unaggregated receptors may reflect the reversible association of a fraction of FcɛRI with lipid domains enriched in Lyn. (See the supplemental material on the PNAS web site at www.pnas.org for the details of model A, additional information on model B, and values of the parameters used in simulations.)
Figure 1.
The key phosphorylation steps for model A, in which one Lyn can initiate phosphorylation of aggregated FcɛRI, and model B, in which two Lyns per aggregate are required for phosphorylation to proceed. FcɛRI on mast cells and basophils consists of four chains, α (an IgE-binding chain), β, and two γs. The β and γ chains each contain one immunoreceptor tyrosine-based activation motif (ITAM). Each ITAM contains two essential tyrosines, although within the β chain ITAM there is an additional tyrosine. In modeling the receptor we do not keep track of distinct ITAMS but simply take receptors to be phosphorylated or not phosphorylated. Additional reactions in the models include weak constitutive association of Lyn with unphosphorylated FcɛRI and stronger association of Lyn with phosphorylated receptors. For model A, the rate constants p+1 and p+2 govern, respectively, the phosphorylation of FcɛRI when Lyn is bound constitutively and when Lyn is bound to a phosphorylated ITAM. Although the model does not include phosphatases explicitly, their effects are included implicitly in the dephosphorylation rates p−1 and p−2. This formulation assumes that the availability of phosphatases remains essentially constant for the duration of the experiments under consideration. In model B, phosphorylation of the receptors explicitly requires juxtaposition of two Lyns. Possibly this would be required to induce a change in the specific activity of Lyn, as might be evidenced by enhanced activity toward an exogenous test substrate. Alternatively, it might be required to enhance the susceptibility of the receptor to modification, through some other mechanism. In the figure, we depict this phenomenon as a change in the configuration of Lyn, without thereby implying a particular mechanism. The forward and reverse rate constants that govern this transition are denoted by k+a and k−a. In both models, we have made the simplifying assumption that a receptor cannot be in the phosphorylated state and still have Lyn bound constitutively. The difference we will demonstrate between predictions of the two models does not depend on this assumption. (For additional detail on the equations, states, and parameters of the models, see the supplemental material on the PNAS web site at www.pnas.org.)
Because our analysis relies on the quantitative validity of our results, we took special care to assess the credibility of our procedures. The number of FcɛRI molecules per cell was regularly monitored for each clone, using monomeric IgE of carefully determined specific activity (33). Quadruplicate estimates of specific binding had a standard deviation within 4% of the mean, and it was gratifying that clones studied over a period of 2 years, with different preparations of labeled IgE, yielded consistent values (Table 1).
Table 1.
Characteristics of clones examined
| Clone | First assessment*
|
Second assessment†
|
||
|---|---|---|---|---|
| FcɛRI/cell (×10−5) | Lyn/FcɛRI (relative) | FcɛRI/cell (×10−5) | Lyn/FcɛRI (relative) | |
| A11 | 1.3 | 1.00 | 1.4 | 1.00 |
| D1 | 1.3 | 0.52 | 1.3 | 0.73 |
| A6 | 1.0 | 0.15 | 1.2 | 0.13 |
| A9 | 0.7 | 0.14 | 0.7 | 0.088 |
| D7 | 1.3 | 0.048 | 1.4 | 0.24 |
| D8 | 1.3 | 0.014 | 1.5 | 0.038 |
| B12 | 1.7 | 0.038 | 1.4 | 0.064 |
| RBL | ND | ND | 3.7 | 0.34 |
These results have been published in ref. 24, but the values in the third column of this table, which are based on duplicate determinations of FcɛRI and two to five determinations of Lyn, are normalized on the basis of the relative ratio of Lyn/FcɛRI for the highest expressing clone A11 (in boldface). ND, not determined.
These new analyses involved quadruplicate determinations of FcɛRI and four to eight determinations of Lyn.
Determining the amount of expressed Lyn and the phosphorylation of receptor tyrosines involved using three antibodies: anti-Lyn, anti-PY, and anti-β for Western blotting (Materials and Methods). This technique requires special care if it is to be used quantitatively. The following procedures were used to promote the accuracy of our measurements. (i) To the maximum extent possible, the samples to be compared were run on the same gel, and when replicates on different gels were to be compared, a fixed amount of a standard (e.g., recombinant phosphorylated Lyn) was run in one of the lanes to be able to correct for differences between gels and transfer to the nitrocellulose sheets used for blotting. (ii) We assessed the range over which the densitometric readings of the autophotographs of the blots were linear and found that within the range 300 to 3,500, linearity was satisfactory for each of the antibody reagents (Table 2). We have found that care must be taken to load equal amounts of total protein and equal volumes in the gel wells, to obtain a linear response. (iii) The instability of the solubilized receptor at the detergent concentrations used for the immunoprecipitation of the solubilized receptors can lead to (generally small) variations in the recovery of the β and γ chains. However, when it occurs, dissociations of β and γ are comparable (34), and we have found that correcting for the recovery of the β subunits is sufficient to adjust for the yield of both chains. These corrections were made with the same gels used for the quantitation of PY, by stripping away the anti-PY before reblotting with anti-β. (iv) With RBL cells, under the conditions generally used for stimulation, the ratio of phosphorylation of tyrosines on the β and γ chains is invariant and is approximately 1:2 (35). For the current studies we independently assessed the dependence of phosphorylation of the β and γ chains on the concentration of Lyn (Tables 2 and 3). (v) We also examined different times of stimulation and replicates to determine the extent of variability (Table 3). Fig. 2 illustrates how our experiments were performed.
Table 2.
Linearity of Western blotting data
| Antibody | Specimen | Cell equiv. (relative) | Densitometric value* |
|---|---|---|---|
| Anti-Lyn | D1 lysate | 1.0 | 1,800 |
| 0.5 | 933 ± 44 | ||
| 0.25 | 415 ± 36 | ||
| β γ | |||
| Anti-PY | Anti-IgE ppt. | 1.0 | 1,283 ± 16 2,979 ± 49 |
| (4G10) | (FcɛRI) | 0.5 | 588 ± 23 1,391 ± 86 |
| 0.25 | 286 ± 20 730 ± 70 |
Table 3.
Variation with stimulation time and reproducibility of replicates
| Assay | tstim, min | Clone | PY*
|
D1/D7†
|
||
|---|---|---|---|---|---|---|
| β | γ2 | β | γ2 | |||
| 1 | 7.5 | D1 | 0.294 | 0.770 | ||
| 2.91 | 2.17 | |||||
| D7 | 0.101 | 0.354 | ||||
| 15 | D1 | 0.527 | 1.317 | |||
| 3.11 | 2.95 | |||||
| D7 | 0.169 | 0.446 | ||||
| 30 | D1 | 2.060 | 4.368 | |||
| 3.51 | 2.81 | |||||
| D7 | 0.586 | 1.550 | ||||
| 2 | 15 | D1 | 0.371 | 0.724 | ||
| 6.74 | 4.02 | |||||
| D7 | 0.055 | 0.180 | ||||
| 15 | D1 | 0.267 | 0.606 | |||
| 2.64 | 3.42 | |||||
| D7 | 0.101 | 0.177 | ||||
| 15 | D1 | 0.414 | 0.391 | |||
| 7.39 | 7.24 | |||||
| D7 | 0.056 | 0.054 | ||||
| Mean ± SEM 4.3 ± 0.83.7 ± 0.6 | ||||||
The values shown are the corrected densitometry readings obtained from the Western blots using anti-PY, divided by the readings similarly obtained with the anti-β (JRK).
As seen in Table 1, the ratio Lynrel/FcɛRI in D1 is currently 3 times greater than in D7.
Figure 2.
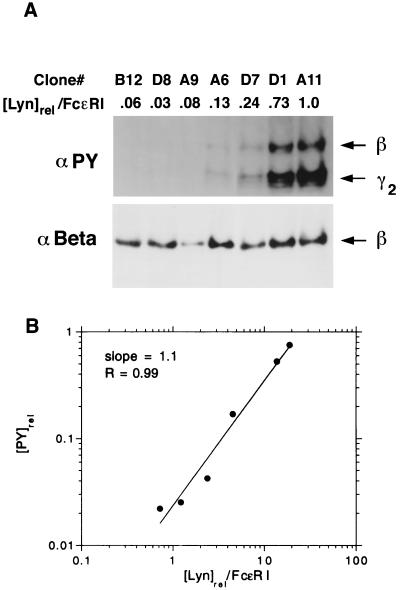
Relative phosphorylation of FcɛRI after stimulation, as a function of expressed Lyn. This figure is illustrative of the analyses that form the basis of this study. Each clone was reacted with 0.5 μg/ml dimeric IgE for 15 min before solubilization of the cells, reaction of the centrifuged lysate with anti-IgE, and analysis of the immunoprecipitates on gels. (A) Line 2 reports the relative amount of Lyn per FcɛRI for each of the seven clones. (Upper) Western blot with anti-PY. (Lower) Western blot with anti-β after stripping of the gel shown in Upper. For reasons that have not been investigated, the immunoprecipitates from clone A9 regularly showed an abnormally low yield of β chains relative to recovery of radiolabeled IgE, suggesting that the receptors were unusually unstable. (B) log–log plot of the phosphorylation of the β chain ([PY]rel) vs. the relative expression of Lyn per receptor ([Lyn]rel/FcɛRI), for the experiment shown in A. The line that gives the best fit to the data is shown. Its slope is 1.1. The correlation coefficient is 0.99. The data from clone A9 were not included because the small yield of β chains (see above) precluded accurate quantification. In 26 similar plots, reflecting measurements of tyrosine phosphorylation on the β and γ chains at distinct doses of ligand and distinct times, the mean slope is 0.96 ± 0.05.
Fig. 2B shows one of 26 log–log plots of the level of tyrosine phosphorylation as a function of the ratio of cellular Lyn to FcɛRI. Each data point represents a different transfectant. The line shown, which gives the best fit to the data, has slope 1.1. The 26 data sets and corresponding slopes are from separate measurements of the phosphorylation of tyrosines on FcɛRIβ and FcɛRIγ in experiments at three doses of ligand (0.3, 0.5, and 1.0 μg/ml of dimeric IgE) and three times of exposure (7.5, 15, and 30 min). Eight additional data sets, from two experiments, had densitometric readings outside the linear range and are not included in the results and statistical analysis we report. The remaining 26 log–log plots are fit well by straight lines; in 20 of 26 cases, the correlation coefficient is above 0.9. The mean slope is 0.96 and the standard error is 0.05.
We analyzed the results statistically in several ways. The distribution of slopes was consistent with a normal distribution. We tested two hypotheses concerning the mean slope of the log–log plots. The slope gives the exponent in a power law relating phosphorylation to the expression of Lyn, so we are interested in knowing if the results are consistent with a slope of 1 (expected when one Lyn is sufficient to initiate receptor phosphorylation) or a slope of 2 (expected if two Lyns are required). The slope of 0.96 ± 0.05 was consistent with a slope of 1 (P = 0.81) and inconsistent with a slope of 2 (P < 10−100). We also performed analyses of variance (ANOVA tests) to see if the slopes differed significantly for the two receptor subunits, for the three doses of ligand, or for the three times of stimulation. We found no statistically significant differences.
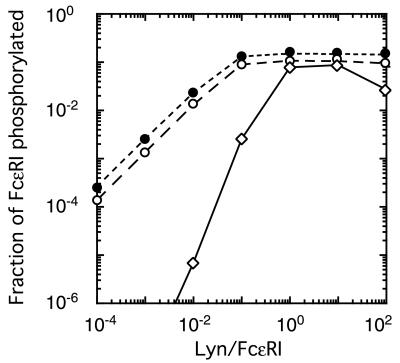
Fig. 3 shows the results of simulations, based on the alternative models. In the linear range of the log–log plot (i.e., when Lyn is not saturating), the model in which Lyn must dimerize to induce activation, model B, gives a slope of 2.1. For the model in which only one Lyn is required for phosphorylation of aggregated receptors, model A, the slope is 1.0. Model A is consistent with the experimental observations, whereas model B, requiring two paired Lyns for activation, is inconsistent with the data.
Figure 3.
Results of simulations of the experiments, based on alternative models. Markers indicate the fraction of receptors phosphorylated, as predicted by models A (○, Fig. 1A), B (⋄, Fig. 1B), and an analogue of model A in which receptors are aggregated by trimers of IgE (●), for the experiments in which cells were exposed to 0.3 μg/ml dimeric IgE for 15 min. For each of the three curves, we determined the line that gave the best fit to predicted values of the fraction of FcɛRI phosphorylated, for Lyn/FcɛRI in the range 10−5 to 10−2 (some of the values used lie outside the range shown in this plot). The line giving the best fit to the simulated data, in this linear range, has a slope of 1.0 for model A, in which one Lyn can initiate phosphorylation, and 2.1 for model B, in which two juxtaposed Lyn molecules are required. In the model where aggregation is induced by trimers of IgE but only one Lyn is needed for phosphorylation of receptor tyrosines, the predicted slope is 1.0. Predictions of the slopes are robust. Large changes in the rates of phosphorylation and dephosphorylation, the rates of association and dissociation of the receptor with Lyn, and the times at which phosphorylation is measured result in almost no change in the predicted slopes. Even when we chose the rate of dephosphorylation to be slow compared with the rate of dissociation of Lyn, so that by 15 min a significant fraction of the receptor aggregates that are phosphorylated are not associated with Lyn, the slopes for the two models remain essentially equal to 1 and 2.
We also simulated experiments in which FcɛRI is aggregated into trimers by covalently crosslinked trimers of IgE. The simulations were based on a model in which a single Lyn, associated with one receptor in a trimer, can mediate phosphorylation of tyrosines on opposing receptors. As shown in Fig. 3, the slope of the log–log plot is 1, as it was in the corresponding dimer model.
Predictions of all three models do not depend sensitively on the rate constants governing processes that occur on a faster time scale than that of the ligand binding from solution. For example, the value we took for the forward rate of receptor crosslinking is the calculated diffusion limit, but the predicted levels of phosphorylation are essentially unchanged if the value is up to three orders of magnitude lower. The rates of association and dissociation of Lyn with both the phosphorylated and the nonphosphorylated receptor, rates of phosphorylation and dephosphorylation, and rates of activation and inactivation of Lyn in model B, are other parameters that can change by at least an order of magnitude without changing quantitative predictions significantly.
DISCUSSION
Aggregation of B cell receptors and various Fc receptors is essential for signal transduction mediated by these receptors, and there is increasing evidence for the importance of aggregation of the T cell receptor in T cell activation (5, 6, 36). Receptor aggregation has the effect of juxtaposing enzymes and substrates whose interactions initiate a signaling cascade, in turn creating new sites for interactions with additional effector molecules. In this study we asked whether the enzyme Lyn, whose action leads to the first known covalent modification in the FcɛRI-initiated cascade, also had to be aggregated.
Because it is experimentally impractical to follow the approaches used to investigate the same question with respect to the receptors for growth factors and cytokines (Introduction), we applied a quantitative method. We took advantage of the availability of CHO cells we had previously transfected with FcɛRI. These cells have minimal amounts of endogenous Lyn (24) and no detectable amounts of the other Src family kinases c-Yes, Fyn, and Src (B.M.V., unpublished observations). The cells were then stably transfected with rat Lyn kinase, and a series of clones that continued to express approximately similar amounts of receptors and a spectrum of amounts of Lyn were identified and maintained (Table 1). We used only the short form of Lyn (Lyn-B) which is missing 21 amino acids corresponding to residues 24–44 in the longer form A. Yamanashi et al. (37) observed that the long, but not the short, form of Lyn was down-regulated in concert with IgM when IgM was aggregated. This observation suggests a differential interaction of the two forms with IgM, at least on the WEHI-231 B cells they examined. On RBL cells we have never observed any differences between the two forms of Lyn, either in the interaction of the wild types with unaggregated or aggregated FcɛRI (28) or in the ability of their corresponding inactive constructs to compete with the wild-type kinase for interaction with the receptors (24).
The results of our analyses clearly demonstrate that juxtaposition of two or more molecules of Lyn is not required to initiate phosphorylation of the tyrosines on the receptor. The conclusion is based on dose–response data showing the dependence of receptor phosphorylation on the amount of cellular Lyn, after ligand-induced aggregation of FcɛRI. The data were consistent with a model in which a single Lyn molecule, associated with a receptor in an aggregate, can phosphorylate other receptors in the aggregate. The data provided strong evidence against an analogous model in which two Lyn molecules are required to initiate phosphorylation of receptors in an aggregate.
The same question we have asked about Lyn can also be posed for Syk, the next kinase implicated in signal transduction mediated by FcɛRI (9–11). Rivera and Brugge (12) showed, by crosslinking chimeric constructs transfected into RBL cells, that aggregation of Syk is sufficient to trigger the spectrum of responses normally induced by the crosslinking of FcɛRI, except for phosphorylation of tyrosines on the receptor itself and on endogenous Syk. On the other hand, evidence that isolated phosphorylated γ chain ITAMs can themselves activate Syk suggests that clustering of Syk is not a requirement for Syk activation (13, 14).
Our new results support a straightforward explanation of these nominally contradictory observations. Membrane-bound phosphorylated Syk is required to generate downstream signaling events. Normally, Lyn creates membrane-localized high-affinity binding sites for Syk by phosphorylating receptor tyrosines in the appropriate ITAMs (38). In addition, Lyn phosphorylates specific tyrosines on Syk, and its presence enhances Syk-mediated phosphorylation of other Syk tyrosines (39). That is, phosphorylation is promoted by heteroaggregates of Lyn and Syk, which play a role analogous to that played by heterodimers of Jak kinases in mediating cytokine signaling. On the other hand, when Syk is expressed as a transmembrane chimera, it is brought to the plasma membrane artificially but not juxtaposed to Lyn. Then the normal function of Lyn in directly and indirectly stimulating phosphorylation of Syk can be mimicked only by artificially generating Syk⋅Syk homodimers that can transphosphorylate themselves.
In the model that we have demonstrated to be consistent with the experiments presented here and with other experiments (17, 26), aggregation physically promotes the phosphorylation of FcɛRI by bringing Lyn together with its substrate, the tyrosines in the ITAMs of β and γ. The simultaneous action of phosphatases on both unaggregated and aggregated receptors promotes continuous cycles of phosphorylation and dephosphorylation (40, 41). Receptor aggregation does not cause measurable changes in the rate of dephosphorylation (41). Thus the role of aggregation is to enhance phosphorylation of the ITAMs by raising the effective substrate concentration for Lyn, rather than to decrease the rate of dephosphorylation.
In the IgE/mast cell system, the phosphorylation of Lyn itself has not yet been systematically explored. As for other Src family kinases, the specific activity of Lyn may be influenced by its own phosphorylation. It has been suggested that the phosphatase CD45 dephosphorylates a regulatory (inhibitory) tyrosine on Lyn (42). In CD45-positive and -negative transfectants expressing the B cell receptor, Lyn activation was modestly elevated, less than 2-fold, in the CD45+ cells compared with the CD45− cells, and Lyn failed to be recruited to aggregated receptors in the CD45− cells (43).
For the subject we analyzed in this paper, the number of molecules of Lyn that are required in an aggregate for phosphorylation of FcɛRI to proceed, a mechanistic model including the details of activation, if any, is not required. The role of the model was simply to check how tyrosine phosphorylation of the receptor is related to the concentration of Lyn, when Lyn is limiting. If phosphatases or other kinases are required for activation of Lyn, or to modulate activation, the conclusion does not change. What the results do exclude is a model that requires a mutual transactivation of Lyn, at least in the case of FcɛRI. However, the overall similarity in the structure of the MIRR, and in the early biochemical events they initiate, make it reasonable to propose that our findings regarding signaling through FcɛRI, mediated by the Src kinase Lyn, may hold more generally among MIRR.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health Grant GM35556 and National Science Foundation Grants MCB9723897 and HRD9627118.
ABBREVIATIONS
- MIRR
multisubunit immune recognition receptors
- FcɛRI
high-affinity receptor for IgE
- PY
phosphotyrosine
- ITAM
immunoreceptor tyrosine-based activation motif
References
- 1.Thomas S M, Brugge J S. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 2.Paolini R, Jouvin M-H, Kinet J-P. Nature (London) 1991;353:855–858. doi: 10.1038/353855a0. [DOI] [PubMed] [Google Scholar]
- 3.Eiseman E, Bolen J B. Nature (London) 1992;355:78–80. doi: 10.1038/355078a0. [DOI] [PubMed] [Google Scholar]
- 4.Benhamou M. In: IgE Receptor (FcɛRI) Function in Mast Cells and Basophils, Hamaway M M, editor. Austin, TX: Landes; 1997. pp. 33–54. [Google Scholar]
- 5.Metzger H, Alcarez G, Hohman R, Kinet J-P, Pribluda V, Quarto R. Annu Rev Immunol. 1986;4:419–470. doi: 10.1146/annurev.iy.04.040186.002223. [DOI] [PubMed] [Google Scholar]
- 6.Klemm J D, Schreiber S L, Crabtree G R. Annu Rev Immunol. 1998;16:569–592. doi: 10.1146/annurev.immunol.16.1.569. [DOI] [PubMed] [Google Scholar]
- 7.Cambier J C. J Immunol. 1995;155:3281–3285. [PubMed] [Google Scholar]
- 8.Johnson S A, Pleiman C M, Pao L, Schneringer J, Hoppen K, Cambier J C. J Immunol. 1995;155:4596–4603. [PubMed] [Google Scholar]
- 9.Benhamou M, Gutkind J S, Robbins K C, Siraganian R P. Proc Natl Acad Sci USA. 1990;87:5327–5330. doi: 10.1073/pnas.87.14.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benhamou M, Ryba N J P, Kihara H, Siraganian R P. J Biol Chem. 1993;268:23318–23324. [PubMed] [Google Scholar]
- 11.Hutchcroft J E, Geahlen R L, Deanin G G, Oliver J M. Proc Natl Acad Sci USA. 1992;89:9107–9111. doi: 10.1073/pnas.89.19.9107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rivera V M, Brugge J S. Mol Cell Biol. 1995;15:1582–1590. doi: 10.1128/mcb.15.3.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiue L, Zoller M J, Brugge J S. J Biol Chem. 1995;270:10498–10502. doi: 10.1074/jbc.270.18.10498. [DOI] [PubMed] [Google Scholar]
- 14.Kimura T, Sakamoto H, Appella E, Siraganian R P. Mol Cell Biol. 1996;16:1471–1478. doi: 10.1128/mcb.16.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honegger A M, Kris R M, Ullrich A, Schlessinger J. Proc Natl Acad Sci USA. 1989;86:925–929. doi: 10.1073/pnas.86.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honegger A M, Schmidt A, Ullrich A, Schlessinger J. Mol Cell Biol. 1990;10:4035–4044. doi: 10.1128/mcb.10.8.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torigoe C, Goldstein B, Wofsy C, Metzger H. Proc Natl Acad Sci USA. 1997;159:5984–5992. [PubMed] [Google Scholar]
- 18.Leonard W J, O’Shea J J. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 19.Russell S M, Johnston J A, Noguchi M, Kawamura M, Bacon C M, Friedmann M, Berg M, McVicar D W, Witthuhn B A, Silvennoinen O, et al. Science. 1994;266:1042–1045. doi: 10.1126/science.7973658. [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki T, Kawahara A, Fujii H, Nakagawa Y, Minami Y, Liu Z-J, Oishi I, Silvennoinen O, Witthuhn B A, Ihle J N, Taniguchi T. Science. 1994;266:1045–1047. doi: 10.1126/science.7973659. [DOI] [PubMed] [Google Scholar]
- 21.Müller M, Briscoe J, Laxton C, Guschin D, Ziemiecki A, Silvennoinen O, Harpur A G, Barbieri G, Witthuhn B A, Schindler C, et al. Nature (London) 1993;366:129–135. doi: 10.1038/366129a0. [DOI] [PubMed] [Google Scholar]
- 22.Briscoe J, Rogers N C, Witthuhn B A, Watling D, Harpur A G, Wilks A F, Stark G R, Ihle J N, Kerr I M. EMBO J. 1996;15:799–809. [PMC free article] [PubMed] [Google Scholar]
- 23.Oakes S A, Candotti F, Johnston J A, Chen Y-Q, Ryan J J, Taylor N, Liu X, Hennighausen L, Notarangelo L D, Paul W E, et al. Immunity. 1996;5:605–615. doi: 10.1016/s1074-7613(00)80274-5. [DOI] [PubMed] [Google Scholar]
- 24.Vonakis B M, Chen H, Haleem-Smith H, Metzger H. J Biol Chem. 1997;272:24072–24080. doi: 10.1074/jbc.272.38.24072. [DOI] [PubMed] [Google Scholar]
- 25.Rivera J, Kinet J-P, Kim J, Pucillo C, Metzger H. Mol Immunol. 1988;25:647–661. doi: 10.1016/0161-5890(88)90100-9. [DOI] [PubMed] [Google Scholar]
- 26.Wofsy C, Torigoe C, Kent U, Metzger H, Goldstein B. J Immunology. 1997;94:77–80. [PubMed] [Google Scholar]
- 27.Wofsy C, Kent U K, Mao S-Y, Metzger H, Goldstein B. J Biol Chem. 1995;270:20264–20272. doi: 10.1074/jbc.270.35.20264. [DOI] [PubMed] [Google Scholar]
- 28.Yamashita Y, Mao S-Y, Metzger H. Proc Natl Acad Sci USA. 1994;91:11251–11255. doi: 10.1073/pnas.91.23.11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Field K A, Holowka D, Baird B. Proc Natl Acad Sci USA. 1995;92:9201–9205. doi: 10.1073/pnas.92.20.9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Field K A, Holowka D, Baird B. J Biol Chem. 1997;272:4276–4280. doi: 10.1074/jbc.272.7.4276. [DOI] [PubMed] [Google Scholar]
- 31.Stauffer T P, Meyer T. J Cell Biol. 1997;139:1447–1454. doi: 10.1083/jcb.139.6.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xavier R, Brennan T, Li Q, McCormack C, Seed B. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- 33.Kulczycki A, Jr, Metzger H. J Exp Med. 1974;140:1676–1695. doi: 10.1084/jem.140.6.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kinet J-P, Alcaraz G, Leonard A, Wank S, Metzger H. Biochemistry. 1985;24:4117–4124. doi: 10.1021/bi00336a046. [DOI] [PubMed] [Google Scholar]
- 35.Pribluda V S, Pribluda C, Metzger H. J Biol Chem. 1997;272:11185–11192. doi: 10.1074/jbc.272.17.11185. [DOI] [PubMed] [Google Scholar]
- 36.Davis M M, Boniface J J, Reich Z, Lyons D, Hampl J, Arden B, Chien Y. Annu Rev Immunol. 1998;16:523–544. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 37.Yamanashi Y, Miyasaka M, Takeuchi M, Ilic D, Mizuguchi J, Yamamoto T. Cell Regul. 1991;2:979–987. doi: 10.1091/mbc.2.12.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jouvin M-H E, Adamczewski M, Numerof R, Letourneur O, Valle A, Kinet J-P. J Biol Chem. 1994;269:5918–5925. [PubMed] [Google Scholar]
- 39.Keshvara L M, Isaacson C C, Yankee T M, Sarac R, Harrison M L, Geahlen R L. J Immunol. 1998;161:5276–5283. [PubMed] [Google Scholar]
- 40.Kent U M, Mao S-Y, Wofsy C, Goldstein B, Ross S, Metzger H. Proc Natl Acad Sci USA. 1994;91:3087–3091. doi: 10.1073/pnas.91.8.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mao S-Y, Metzger H. J Biol Chem. 1997;272:14067–14073. doi: 10.1074/jbc.272.22.14067. [DOI] [PubMed] [Google Scholar]
- 42.Adamczewski M, Numerof R P, Koretzky G A, Kinet J-P. J Immunol. 1995;154:3047–3055. [PubMed] [Google Scholar]
- 43.Pao L I, Cambier J C. J Immunol. 1997;158:2663–2669. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.