Abstract
Effective immunotherapy for human leukemia based on infusions of T lymphocytes requires the identification of effector T cells that target the leukemic stem cell. The transplantation of human acute myeloid leukemia into nonobese diabetic/severe combined immune deficient (SCID) mice has identified a rare leukemic progenitor termed the SCID leukemia-initiating cell, which is present in low frequency in the leukemic population and is essential for establishing leukemic hematopoiesis. Thus, this transplant model may be ideally suited to identify effector T cells with antileukemic activity. We report that CD8+ cytotoxic T lymphocyte (CTL) clones specific for minor histocompatibility antigens inhibit the engraftment of human acute myeloid leukemia cells in nonobese diabetic/SCID mice and demonstrate that this inhibition is mediated by direct CTL recognition of SCID leukemia-initiating cells. These results indicate that CD8+ minor histocompatibility antigen-specific CTL may be mediators of the graft-versus-leukemia effect associated with allogeneic hematopoietic cell transplantation and provide an experimental model to identify and select T cell clones for immunotherapy to prevent or treat relapse after allogeneic hematopoietic cell transplantation.
The efficacy of allogeneic hematopoietic cell transplantation (HCT) as a curative therapy for leukemia depends both on the intensive chemoradiotherapy administered before transplant and a graft-versus-leukemia (GVL) effect mediated by donor T cells. However, a significant fraction of patients with advanced leukemia at the time of HCT will relapse because of persistence of leukemic progenitor cells (1–3). Increasing the intensity of chemoradiotherapy has not improved survival because of increased toxicity (4), and efforts to augment the GVL effect after transplant such as by the administration of unselected donor lymphocytes or the administration of IL-2 were only partially effective and/or complicated by increased graft-versus-host disease (5–10). More effective immunotherapy will require the identification, expansion, and adoptive transfer of donor cells capable of recognizing antigens expressed on residual leukemic progenitors.
Minor histocompatibility (H) antigens are short peptides that are derived from polymorphic regions of cellular proteins and are presented on the cell surface bound to molecules of the MHC (11–14). The adoptive transfer of donor T cells specific for minor H antigens expressed in recipient hematopoietic cells, but not in nonhematopoietic cells such as skin fibroblasts and keratinocytes, has been proposed as one strategy for inducing a GVL effect without causing graft-versus-host disease (15–20). CD8+ cytotoxic T lymphocyte (CTL) clones specific for minor H antigens presented by class I MHC molecules have been demonstrated to lyse a proportion of leukemic cells in vitro (16, 17, 21) and inhibit the growth of clonogenic myeloid leukemic progenitor cells in methylcellulose culture (22–24). However, by transplanting human acute myeloid leukemia (AML) cells into nonobese diabetic/severe combined immune deficient (NOD/SCID) mice, we previously have established that AML is comprised of a hierarchy of cells with vastly different capacities for proliferation, differentiation, and self-renewal. At the top of this hierarchy is a putative AML stem cell that resides exclusively in the subset of CD34+CD38− cells and is required for repopulating NOD/SCID mice with human leukemic hematopoiesis. This cell, termed the SCID leukemia-initiating cell (SL-IC), is present in very low frequency (0.2–100/106 leukemic blasts) in the blood or bone marrow of patients with AML and is distinct from the AML progenitor, termed the AML-colony-forming unit (AML-CFU), which gives rise to AML blast colonies in methylcellulose culture. AML-CFUs are present in at least 1,000-fold higher frequency than SL-IC in blood or bone marrow from AML patients and are found in both the CD34+CD38− and CD34+CD38+ subsets (25, 26). Because of the rarity of the SL-IC in AML populations, in vitro cytotoxicity assays and AML-CFU-inhibition assays cannot be used to address whether SL-ICs express minor H antigens and are targets for CD8+ CTL. In this report, the transplantation of human AML into NOD/SCID mice was used to determine whether CD8+ minor H antigen-specific CTL recognize and eliminate the AML stem cell.
MATERIALS AND METHODS
Collection of AML Cells.
Blood was obtained from five adult patients with refractory or relapsed AML. Disease diagnosis and classification were according to French-American-British criteria (27). Mononuclear cells were isolated by centrifugation on Ficoll-Hypaque and cryopreserved in RPMI-Hepes (GIBCO) with 20% human serum and 10% DMSO for subsequent use. All preparations contained more than 90% malignant cells by morphologic criteria on Wright-Giemsa stained specimens.
CD8+ Minor H Antigen-Specific CTL Clones.
CD8+ minor H antigen-specific CTL clones were isolated from recipients of MHC-matched allogeneic HCTs and characterized as described (17). In vitro cytotoxicity assays to detect reactivity with donor and recipient Epstein–Barr virus (EBV)-transformed lymphoblastoid cell lines (LCLs), fibroblasts, and leukemic cells were performed by labeling aliquots of 1–2 × 106 target cells with 50 μCi of 51Cr overnight, washing the target cells three times in RPMI-Hepes, and dispensing at 5 × 103 cells/well into triplicate cultures in 96-well round-bottom plates. CD8+ CTL clones were added at various effector-to-target ratios in a volume of 200 μl, and the assay was incubated for 4 hr. Supernatants were harvested for gamma counting, and the percent of specific lysis was calculated by using the standard formula (17).
Cocultivation of AML Cells with CD8+ CTL Clones.
CD8+ CTL clones were expanded in vitro as described (28). After 11–13 days of expansion, CTL were washed and resuspended in RPMI-Hepes supplemented with 10% human serum, 2 mM l-glutamine, and 1% penicillin/streptomycin (termed CTL medium). Cryopreserved mononuclear cells from AML patients were thawed at 37°C, washed once, resuspended in CTL medium, and counted. The viability determined by trypan blue exclusion was always >90%. Identical aliquots of AML cells (1.5 × 106 to 25 × 106 cells) were incubated in CTL medium supplemented with 25 units/ml recombinant human IL-2 (Chiron) at 37°C with 5% CO2 either alone or in the presence of CD8+ minor H antigen-specific CTL at a T cell/AML ratio of 10:1. After 8–24 hr, the cultures were transported to Toronto by overnight courier for transplantation. Before inoculation into NOD/SCID mice, the cultures were harvested, pelleted, and resuspended in a total volume of 500 μl.
Transplantation of AML Cells into NOD/SCID Mice.
NOD/SCID mice were bred and maintained under defined flora conditions in the animal facilities at the Ontario Cancer Institute and The Hospital for Sick Children, Toronto. The studies were approved by the animal care committees of the Ontario Cancer Institute, the Hospital for Sick Children, and the Fred Hutchinson Cancer Research Center. Eight- to 12-week-old NOD/SCID mice were sublethally irradiated with 375 cGy from a 137Cs source, then injected via the tail vein with the aliquots of AML cells that had been cultured in either medium alone or with CD8+ minor H antigen-specific CTL clones. After inoculation all mice received alternate day i.p. injections of 10 μg human stem cell factor (provided by Amgen Biologicals) and 7 μg PIXY-321 (provided by Doug Williams, Immunex).
Harvest and Phenotypic Analysis of Marrow Cell Suspensions from Transplanted NOD/SCID Mice.
At indicated time points (4–5 or 7–10 weeks after infusion), mice transplanted with AML cells alone or AML cells incubated with CD8+ CTL were euthanized, and bone marrow cells were obtained by flushing the femora, tibiae, and iliac crests with Iscove’s DMEM containing 10% FCS. Nucleated cells were counted and prepared for flow cytometry by incubation at 4°C for 20 min in PBS and 5% FCS with one or more of the following antibodies: peridinin chlorophyll protein-conjugated anti-human CD45 and phycoerythrin (PE)-conjugated anti-human CD33 (both from Becton Dickinson Immunocytometry Systems), FITC-conjugated anti-human CD8 (Coulter), biotinylated anti-HLA A1, biotinylated anti-HLA A3 (both from One Lambda, Canoga Park, CA), or PE-conjugated anti-HLA B7 (Chemicon). Samples stained with biotinylated anti-HLA A1 or A3 then were stained with streptavidin-PE (Jackson Immunoresearch). Isotype-matched FITC- or PE-conjugated antibodies (Becton Dickinson Immunocytometry Systems) were used as controls. After antibody labeling, cells were washed twice with PBS containing 5% FCS. Analysis was performed on a FACscan cytometer by using lysis ii software (Becton Dickinson Immunocytometry Systems). Dead cells were gated out by their staining with 1 mg/ml of propidium iodide (Calbiochem). Each phenotype analysis was generated by analyzing 10,000 viable cells.
DNA Analysis.
High molecular weight DNA was isolated from murine bone marrow by phenol/chloroform extraction using standard protocols. DNA (1 μg) was digested with EcoRI, separated electrophoretically on agarose gels, blotted onto a nylon membrane (Amersham Pharmacia), and probed with 32P-labeled p17H8, an α-satellite probe specific for sequences on human chromosome 17 (29). The percentage of human cells present in the murine marrow was estimated by comparing the intensity of the characteristic 2.7-kb band in the samples with standard human/mouse DNA mixtures consisting of 0.1%, 1.0%, 10%, and 50% human DNA. Multiple exposures of the autoradiographs were taken to ensure sensitivity down to 0.1% human DNA.
RESULTS
Engraftment of AML Cells in NOD/SCID Mice.
Five individuals with AML who expressed one or more minor H antigens recognized by CD8+ CTL clones were identified for these studies by assessing cytolytic activity of a panel of CD8+ minor H antigen-specific CTL clones against phytohemagglutinin-induced T cell blasts prepared from the peripheral blood of each patient. Peripheral blood mononuclear cells collected from these five individuals were inoculated in incremental cell doses into cohorts of NOD/SCID mice to define the minimum cell dose, termed the engraftment dose, that would result in easily measurable levels of human cells in the bone marrow of all mice 4–8 weeks after inoculation (Table 1). An AML cell dose of 1–15 times the engraftment dose was used in subsequent experiments to evaluate the effects of CD8+ minor H antigen-specific CTL clones on leukemic engraftment.
Table 1.
Characteristics of the five AML patients from whom AML cells were obtained for this study
| Patient UPN | Age/Sex | FAB subtype | HLA-A alleles | HLA-B alleles | Engraftment dose | % human CD45+ cells at engraftment dose, mean ± SEM |
|---|---|---|---|---|---|---|
| 10885 | 49/F | M4 | 24,3 | 44,51 | 1.5 × 106 | 38.1 ± 4.9 |
| 10827 | 44/F | M4 | 3,30 | 53,70 | 4 × 106 | 30.0 ± 5.1 |
| 10138 | 28/M | M1 | 1,3 | 8,14 | 5 × 106 | 29.2 ± 1.9 |
| 11720 | 42/F | M7 | 3 | 7,8 | 5 × 106 | 5.1 ± 2.4 |
| 10911 | 45/M | M4 | 1,2 | 37,35 | 10 × 106 | 10.0 ± 1.0 |
CD8+ Minor H Antigen-Specific CTL Clones Inhibit AML Engraftment.
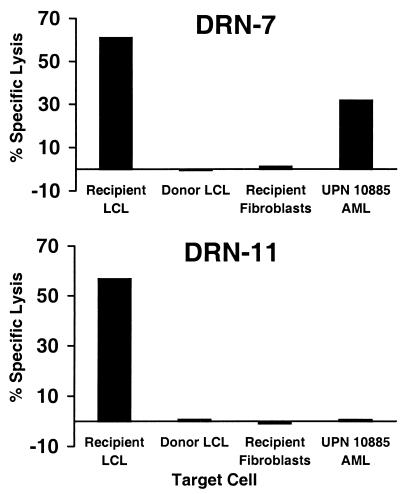
CD8+ CTL clones DRN-7 and DRN-11 are specific for distinct minor H antigens that are presented in association with the class I HLA alleles A3 and B7, respectively (17). The minor H antigens recognized by DRN-7 and DRN-11 are presented by recipient cells of hematopoietic origin such as EBV-transformed B cells and phytohemagglutinin-stimulated T cells, but are not presented by cells derived from nonhematopoietic tissues such as skin fibroblasts, suggesting that they may be suitable targets to induce a selective GVL response (Fig. 1). AML blasts were obtained from a patient (10885) who expressed the HLA A3 restricting allele for clone DRN-7, but not the HLA B7 restricting allele for clone DRN-11, and were tested for recognition by these CTLs. As expected, clone DRN-11 exhibited no lytic activity against 10885 AML cells. However, clone DRN-7 lysed 10885 AML cells, demonstrating that the minor H antigen recognized by DRN-7 was expressed by at least a proportion of the leukemic population (Fig. 1).
Figure 1.
Cytolytic activity of CD8+ minor H antigen-specific CTL clones DRN-7 and DRN-11. Clones DRN-7 and DRN-11 were isolated from an HLA A3+, HLA B7+ allogeneic HCT recipient (17). The CTLs were tested in a 4-hr 51Cr release assay against EBV-LCL derived from pretransplant recipient lymphocytes, EBV-LCL derived from the stem cell donor, dermal fibroblasts derived from the recipient, and AML cells from patient 10885 who was HLA A3+ but also HLA B7−. Data shown are for an effector-to-target ratio of 10:1. UPN, unique patient number.
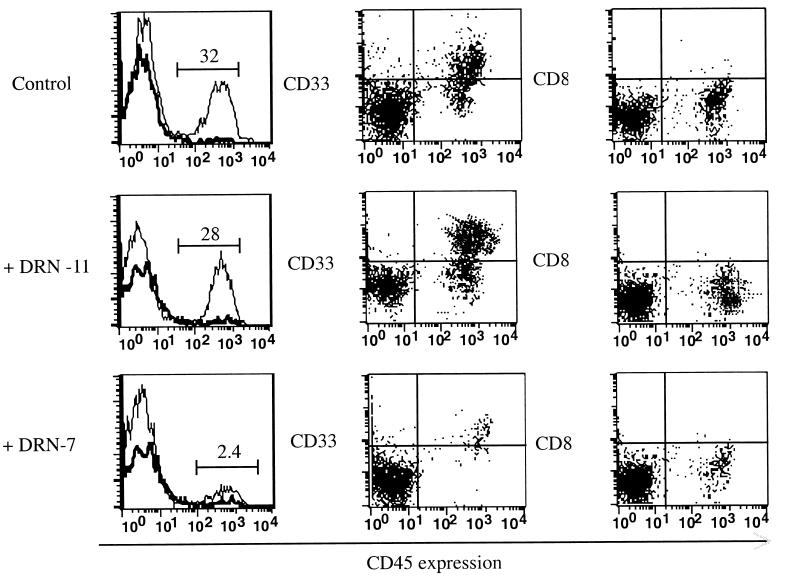
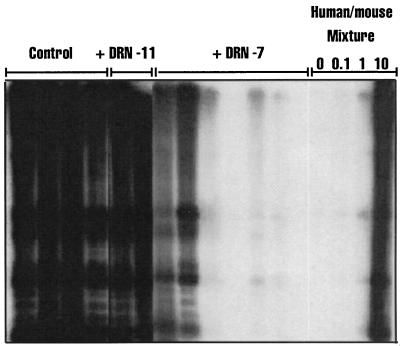
To determine whether the minor H antigen recognized by DRN-7 may be expressed by the rare SL-IC, AML cells from patient 10885 were cultured for 24 hr in vitro in medium alone, with CTL clone DRN-7, or with CTL clone DRN-11 as a control for potential nonspecific effects of T cells, at a T cell/AML ratio of 10:1. The mixtures then were inoculated into cohorts of sublethally irradiated NOD/SCID mice. Our prior studies revealed extensive leukemic cell engraftment by 30 days (25, 26). To provide double the standard observation period in these experiments, we sacrificed a cohort of mice from each treatment group at 29–32 days and at 65–70 days after inoculation. Human cell engraftment was evaluated by flow cytometry of bone marrow mononuclear cells by using mAbs specific for human CD45, CD33, and CD8, and by Southern blot using a human chromosome 17-specific α-satellite probe (29). Flow cytometry of bone marrow mononuclear cells obtained 65–70 days after transplantation of control mice with 10885 AML cells cultured in medium alone or with clone DRN-11 showed 27–36% human cells. These cells were uniformly positive for the CD33 antigen expressed on the leukemic blasts of patient 10885 and negative for the CD8 antigen expressed by T cells (Fig. 2). In contrast, the bone marrow of all mice transplanted with AML cells cultured with CD8+ CTL clone DRN-7 revealed ≤3% human cells (range, <0.5 to 3.0%) (Fig. 2). Flow cytometric data from all mice sacrificed at 29–32 days and at 65–70 days postinoculation showed that the coculture with CTL clone DRN-7 inhibited the engraftment of 10885 cells by 95% when compared with control mice receiving AML cells alone or AML cells cultured with CTL clone DRN-11 (Fig. 3). Southern blot analysis of DNA from the bone marrow confirmed the selective inhibition of AML engraftment in the cohorts of mice that received leukemic cells cocultured with DRN-7 (Fig. 4). In two mice injected with AML cells that had been cultured with clone DRN-7, the amount of human DNA was below the detection limit of 0.1%, demonstrating that leukemic cells were completely eliminated from the inocula (Fig. 4). The few residual human cells detected in the marrow of other DRN-7-treated mice were leukemic in origin because they stained positive for CD33 and not CD8 (Fig. 2).
Figure 2.
Flow cytometric analysis for human CD45+, CD33+, and CD8+ cells in the marrow of transplanted mice. Bone marrow was obtained 70 days after transplant from mice receiving 1.5 × 106 10885 AML that had been cultured in either medium alone (control; Top), with clone DRN-11 (Middle), or with clone DRN-7 (Bottom), at a T cell/AML cell ratio of 10:1. Cells were analyzed by flow cytometry to detect those expressing human CD45 (Left); the dark solid line shows staining with an isotype control mAb and the light solid line, staining with the anti-human CD45 mAb. (Center) Dual staining for CD33 and CD45. (Right) Dual staining for CD8 and CD45.
Figure 3.
CD8+ minor H antigen-specific CTL clone DRN-7 specifically inhibits engraftment of 10885 AML cells in NOD/SCID mice. Mean (±SE) engraftment of human cells in mice transplanted with 10885 AML cells cultured with either the control clone DRN-11 (open columns) or with clone DRN-7 (filled columns), at 29–32 days (1 month) or 65–70 days (2 months). The data are expressed as a percentage of the mean engraftment in control mice who received the same dose of 10885 AML cells cultured in medium alone. The numbers of control, DRN-11-treated, and DRN-7-treated mice analyzed at 1 month were 5, 3, and 5, respectively, and at 2 months, 6, 2, and 6, respectively. P values refer to the comparison between the engraftment in DRN-7-treated mice and that in control mice, as computed by the Mann–Whitney u test.
Figure 4.
Southern blot analysis. DNA was extracted from the bone marrow of mice transplanted with 10885 AML cells, digested with EcoRI, and analyzed by Southern blot using a probe (p17H8) specific for sequences on human chromosome 17 (29). Each lane contains DNA of a single mouse. The annotation above each lane indicates whether the mouse received AML cells cultured in medium alone (Control), with CTL clone DRN-11, or with CTL clone DRN-7. The four lanes on the right contain standard human/mouse DNA mixtures consisting of 0%, 0.1%, 1.0%, and 10% human DNA.
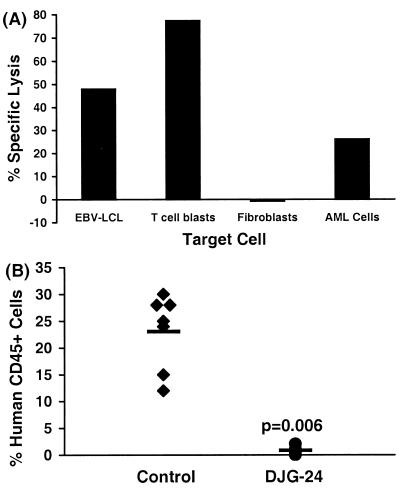
Further evidence that AML stem cells can be eliminated by minor H antigen-specific CTL clones was provided by independent experiments in which different combinations of CTL clones and AML samples were used. The DRN-7 CTL clone also inhibited the engraftment of an AML sample obtained from patient 10827 who expressed both HLA A3 and the minor H antigen recognized by the DRN-7 CTL, but had no effect on the engraftment of AML cells obtained from patient 10911, against which the clone demonstrated no lytic activity in vitro (data not shown). Engraftment of AML cells from patient 10827 also was inhibited by CD8+ CTL clone DJG-24, which was derived from this patient’s MHC-identical HCT donor. DJG-24 recognizes a minor H antigen presented by HLA B53 and expressed in EBV-transformed B cells, phytohemagglutinin-stimulated T cells, and AML blasts but not in fibroblasts (Fig. 5A). AML cells from patient 10827 were cultured in medium alone or with DJG-24 CTL and inoculated into cohorts of NOD/SCID mice. At 8 weeks, mice inoculated with AML cells alone showed a mean of 23.1% human cell engraftment whereas mice inoculated with AML cells cultured with DJG-24 exhibited a mean of 0.9% human cell engraftment (Fig. 5B).
Figure 5.
CD8+ minor H antigen-specific CTL clone DJG-24 specifically inhibits engraftment of 10827 AML cells in NOD/SCID mice. (A) Cytolytic activity of donor CD8+ CTL clone DJG-24, against EBV-LCL, phytohemagglutinin-stimulated T cells, fibroblasts, and AML cells from the HCT recipient. Data shown are for an effector-to-target ratio of 10:1. (B) Flow cytometric analysis of human CD45+ cell engraftment 8 weeks after transplantation in cohorts of mice that received 5 × 106 10827 AML cells that had been cultured for 24 hr in medium alone (Control) or with DJG-24 CTL at a T cell/AML cell ratio of 10:1. Each symbol indicates the engraftment measured in an individual mouse. The mean levels of engraftment are indicated by the horizontal bars. The P value refers to the comparison of engraftment in the DJG-24 group and control mice, as computed by the Mann–Whitney u test.
CD8+ CTL Inhibit AML Engraftment by Direct Recognition of SL-IC.
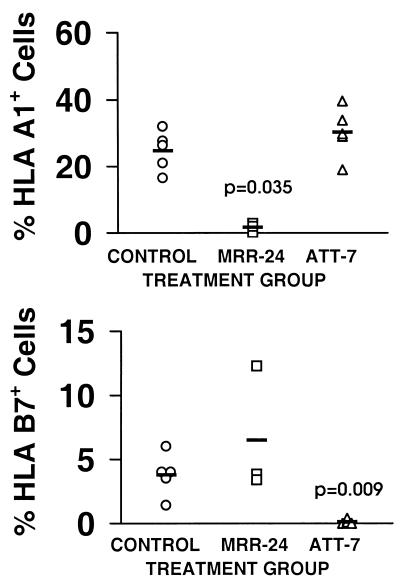
The inhibition of leukemic engraftment by CD8+ minor H antigen-specific CTL could be mediated by direct T cell elimination of SL-IC, or by indirect effects of cytokines released by T cells activated by recognition of more differentiated cells in the leukemic population. To distinguish between these two potential mechanisms, 5 × 106 AML cells from a male patient (10138) were mixed with 5 × 106 AML cells from a female patient (11720), and the mixture then was cultured for 24 hr in either medium alone, with CTL clone MRR-24 that recognizes a novel H-Y antigen encoded by the UTY gene (E.H.W. and M. Gavin, unpublished work) and expressed by 10138 but not 11720 AML cells, or with CTL clone ATT-7, which recognizes a distinct minor H antigen presented by 11720 but not 10138 AML cells. Cohorts of mice were inoculated with each of these mixtures and evaluated for engraftment of each leukemia 7–10 weeks later by using flow cytometry to detect HLA A1+ 10138 AML cells and HLA B7+ 11720 AML cells.
Control mice transplanted with the mixture of AML cells incubated in medium alone showed engraftment of both the HLA A1+ 10138 AML cells (mean 24.7%) and the HLA B7+ 11720 AML cells (mean 3.8%; Fig. 6). However, mice transplanted with the mixture of AML cells that had been cultured with CTL clone MRR-24 exhibited barely detectable engraftment of the 10138 AML but had equivalent or greater engraftment than control mice of the 11720 AML (Fig. 6). Conversely, mice transplanted with the mixture of AML cells that had been cultured with CTL clone ATT-7 exhibited robust engraftment of the 10138 AML and minimal engraftment of the 11720 AML (Fig. 6). These results demonstrate that minor H antigen-specific CTL clones selectively inhibit the engraftment of AML cells expressing the relevant minor H antigen and are consistent with direct recognition of SL-IC by CTL rather than indirect effects on SL-IC activity mediated by cytokines produced by CTL.
Figure 6.
CD8+ minor H antigen-specific CTL clones inhibit AML engraftment in NOD/SCID mice by direct recognition of SL-ICs. Cohorts of mice were transplanted with a mixture of AML cells from patient 10138 (HLA A1+, A3+, B8+, B14+) and patient 11720 (HLA A3+, B7+, B8+) that had been cultured in either medium alone (○; n = 5), with clone MRR-24 (□; n = 3), or with clone ATT-7 (▵; n = 5). Bone marrow was obtained 10 weeks later and examined by flow cytometry using an HLA A1-specific mAb to detect 10138 AML cells (Upper) and an HLA B7-specific mAb to detect 11720 AML cells (Lower). The mean levels of engraftment in each treatment group are indicated by the horizontal bars. P values refer to the comparison between the engraftment in the indicated treatment group and that in control mice, as computed by the Mann–Whitney u test.
DISCUSSION
The eradication of leukemia in patients receiving allogeneic HCT is in part the result of an immune-mediated GVL effect (30–35). The nature and specificity of the effector cells mediating GVL activity have been the subject of intense speculation. It has been suggested that recipient minor H antigens recognized by donor T cells are targets of the GVL reaction (30), but experimental studies have yet to provide direct evidence for recognition of leukemic stem cells by donor T cells. Donor T cell clones reactive with recipient minor H antigens have been derived from recipients of allogeneic HCT and have been shown to lyse 51Cr-labeled AML cells in vitro and inhibit the formation of leukemic colonies in methylcellulose (22–24). However, the predictive value of 51Cr release assays in assessing recognition of leukemic progenitors is not known and the AML-colony-forming unit assay does not detect the most primitive leukemic stem cell (25, 26).
Engraftment of AML cells into NOD/SCID mice has defined a putative leukemic stem cell, the SL-IC, with the potential for both differentiation and self-renewal (25, 26). By using this model to assess recognition of SL-IC by CD8+ minor H antigen-specific CTL, we demonstrate that the rare SL-IC expresses minor H antigens and is a target for CD8+ CTL. The inhibitory effect of CTL on leukemic engraftment is specific for leukemic cells derived from patients expressing the relevant minor H antigen and class I MHC restricting allele, thus supporting direct recognition of leukemic stem cells by CTL rather than inhibition of stem cell proliferation by diffusable factors as the mechanism for the antileukemic activity. Recognition by minor H antigen-specific CTL in the 51Cr release assay was predictive of activity against the SL-IC for all five AML samples studied here. Analysis of additional AML samples and CD8+ CTL specific for other minor H antigens in the NOD/SCID model will permit comprehensive definition of the predictive value of the 51Cr release assay.
A low level of AML engraftment was observed in some mice that received AML cells and specific CTL and it is possible these leukemic cells would have expanded further with longer observation. The failure to eradicate every stem cell in some experiments suggests a longer duration of in vitro coculture or the in vivo administration of CD8+ CTL may be required. However, it will be essential to address issues related to the persistence, function, and migration of human CTL in NOD/SCID mice to develop this model for in vivo therapy (36, 37).
The demonstration that AML stem cells express minor H antigens and are targets for CD8+ T cells indicates that minor H-specific CTL participate in the GVL effect associated with allogeneic HCT and suggests that the adoptive transfer of CD8+ CTL could augment GVL activity. Many minor H antigens are preferentially expressed in hematopoietic cells but not in nonhematopoietic cells derived from tissues that are targets of graft-versus-host disease (GVHD) (15–17). Thus, T cell therapy potentially could be designed to enhance the GVL effect without aggravating GVHD, as has been done in murine models (38). Considerable effort now is being devoted to identifying the genes encoding minor H antigens to permit comprehensive analysis of their tissue expression (19). The NOD/SCID engraftment assay described in this report should assist in identifying those minor H antigens that are expressed on leukemic stem cells, selecting clones for adoptive immunotherapy to prevent or treat leukemic relapse after allogeneic HCT, and analyzing potential mechanisms by which leukemic cells may evade elimination.
Acknowledgments
This work was supported by grants to J.E.D. from the Medical Research Council of Canada, the National Cancer Institute of Canada, with funds from the Canadian Cancer Society, the Canadian Genetic Diseases Network of the National Centers of Excellence, a Medical Research Council Scientist Award to J.E.D., a grant to S.R.R. from the National Institutes of Health (CA 18029), and postdoctoral fellowships from the Human Frontier Science Organization Program (D.B.), the American Medical Association (E.H.W.), and the Lady Tata Memorial Trust (E.H.W.).
ABBREVIATIONS
- HCT
hematopoietic cell transplantation
- GVL
graft versus leukemia
- H
histocompatibility
- CTL
cytotoxic T lymphocyte
- AML
acute myeloid leukemia
- NOD/SCID
nonobese diabetic/severe combined immune deficient
- SL-IC
SCID leukemia-initiating cell
- EBV
Epstein–Barr virus
- LCL
lymphoblastoid cell line
References
- 1.Thomas E D, Storb R, Clift R A, Fefer A, Johnson L, Neiman P E, Lerner K G, Glucksberg H, Buckner C D. N Engl J Med. 1975;292:832–843. doi: 10.1056/NEJM197504172921605. [DOI] [PubMed] [Google Scholar]
- 2.Thomas E D, Storb R, Clift R A, Fefer A, Johnson L, Neiman P E, Lerner K G, Glucksberg H, Buckner C D. N Engl J Med. 1975;292:895–902. doi: 10.1056/NEJM197504242921706. [DOI] [PubMed] [Google Scholar]
- 3.Appelbaum F R. Semin Oncol. 1997;24:114–123. [PubMed] [Google Scholar]
- 4.Clift R A, Buckner C D, Appelbaum F R, Sullivan K M, Storb R, Thomas E D. Blood. 1998;92:1455–1456. [PubMed] [Google Scholar]
- 5.Sullivan K M, Storb R, Buckner C D, Fefer A, Fisher L, Weiden P L, Witherspoon R P, Appelbaum F R, Banaji M, Hansen J, et al. N Engl J Med. 1989;320:828–834. doi: 10.1056/NEJM198903303201303. [DOI] [PubMed] [Google Scholar]
- 6.Kolb H J, Mittermuller J, Clemm C, Holler E, Ledderose G, Brehm G, Heim M, Wilmanns W. Blood. 1990;76:2462–2465. [PubMed] [Google Scholar]
- 7.Kolb H J, Schattenberg A, Goldman J M, Hertenstein B, Jacobsen N, Arcese W, Ljungman P, Ferrant A, Verdonck L, Niederwieser D, et al. Blood. 1995;86:2041–2050. [PubMed] [Google Scholar]
- 8.Slavin S, Naparstek E, Nagler A, Ackerstein A, Samuel S, Kapelushnik J, Brautbar C, Or R. Blood. 1996;87:2195–2204. [PubMed] [Google Scholar]
- 9.Robinson N, Sanders J E, Benyunes M C, Beach K, Lindgren C, Thompson J A, Appelbaum F R, Fefer A. Blood. 1996;87:1249–1254. [PubMed] [Google Scholar]
- 10.Collins R H, Jr, Shpilberg O, Drobyski W R, Porter D L, Giralt S, Champlin R, Goodman S A, Wolff S N, Hu W, Verfaillie C, et al. J Clin Oncol. 1997;15:433–444. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]
- 11.den Haan J M, Sherman N E, Blokland E, Huczko E, Koning F, Drijfhout J W, Skipper J, Shabanowitz J, Hunt D F, Engelhard V H, et al. Science. 1995;268:1476–1480. doi: 10.1126/science.7539551. [DOI] [PubMed] [Google Scholar]
- 12.Wang W, Meadows L R, den Haan J M, Sherman N E, Chen Y, Blokland E, Shabanowitz J, Agulnik A I, Hendrickson R C, Bishop C E, et al. Science. 1995;269:1588–1590. doi: 10.1126/science.7667640. [DOI] [PubMed] [Google Scholar]
- 13.den Haan J M M, Meadows L M, Wang W, Pool J, Blokland E, Bishop T L, Reinhardus C, Shabanowitz J, Offringa R, Hunt D F, et al. Science. 1998;279:1054–1057. doi: 10.1126/science.279.5353.1054. [DOI] [PubMed] [Google Scholar]
- 14.Dolstra H, Fredrix H, Maas F, Coulie P G, Brasseur F, Mensink E, Adema G J, de Witte T M, Figdor C G, van de Wiel-van Kemenade E. J Exp Med. 1999;189:301–308. doi: 10.1084/jem.189.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Bueger M, Bakker A, Van Rood J J, Van der Woude F, Goulmy E. J Immunol. 1992;149:1788–1794. [PubMed] [Google Scholar]
- 16.Dolstra H, Fredrix H, Preijers F, Goulmy E, Figdor C G, de Witte T M, van de Wiel-van Kemenade E. J Immunol. 1997;158:560–565. [PubMed] [Google Scholar]
- 17.Warren E H, Greenberg P D, Riddell S R. Blood. 1998;91:2197–2207. [PubMed] [Google Scholar]
- 18.Goulmy E. Immunol Rev. 1997;157:125–140. doi: 10.1111/j.1600-065x.1997.tb00978.x. [DOI] [PubMed] [Google Scholar]
- 19.Warren E H, Gavin M, Greenberg P D, Riddell S R. Curr Opin Hematol. 1998;5:429–433. doi: 10.1097/00062752-199811000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Mutis T, Verdijk R, Schrama E, Esendam B, Brand A, Goulmy E. Blood. 1999;93:2336–2341. [PubMed] [Google Scholar]
- 21.van der Harst D, Goulmy E, Falkenburg J H, Kooij-Winkelaar Y M, van Luxemburg-Heijs S A, Goselink H M, Brand A. Blood. 1994;83:1060–1066. [PubMed] [Google Scholar]
- 22.Falkenburg J H, Goselink H M, van der Harst D, van Luxemburg-Heijs S A, Kooij-Winkelaar Y M, Faber L M, de Kroon J, Brand A, Fibbe W E, Willemze R, et al. J Exp Med. 1991;174:27–33. doi: 10.1084/jem.174.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niederwieser D, Grassegger A, Aubock J, Herold M, Nachbaur D, Rosenmayr A, Gachter A, Nussbaumer W, Gaggl S, Ritter M, et al. Blood. 1993;81:2200–2208. [PubMed] [Google Scholar]
- 24.Faber L M, van der Hoeven J, Goulmy E, Hooftman-den Otter A L, van Luxemburg-Heijs S A, Willemze R, Falkenburg J H. J Clin Invest. 1995;96:877–883. doi: 10.1172/JCI118134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri M A, Dick J E. Nature (London) 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 26.Bonnet D, Dick J E. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 27.Bennett J M, Catovsky D, Daniel M T, Flandrin G, Galton D A, Gralnick H R, Sultan C. Br J Haematol. 1976;33:451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 28.Brodie S J, Lewinsohn D A, Patterson B K, Jiyamapa D, Krieger J, Corey L, Greenberg P D, Riddell S R. Nat Med. 1999;5:34–41. doi: 10.1038/4716. [DOI] [PubMed] [Google Scholar]
- 29.Waye J S, Willard H F. Mol Cell Biol. 1986;6:3156–3165. doi: 10.1128/mcb.6.9.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiden P L, Flournoy N, Thomas E D, Prentice R, Fefer A, Buckner C D, Storb R. N Engl J Med. 1979;300:1068–1073. doi: 10.1056/NEJM197905103001902. [DOI] [PubMed] [Google Scholar]
- 31.Weiden P L, Sullivan K M, Flournoy N, Storb R, Thomas E D. N Engl J Med. 1981;304:1529–1533. doi: 10.1056/NEJM198106183042507. [DOI] [PubMed] [Google Scholar]
- 32.Butturini A, Bortin M M, Gale R P. Bone Marrow Transplant. 1987;2:233–242. [PubMed] [Google Scholar]
- 33.Fefer A, Sullivan K M, Weiden P, Buckner C D, Schoch G, Storb R, Thomas E D. Prog Clin Biol Res. 1987;244:401–408. [PubMed] [Google Scholar]
- 34.Horowitz M M, Gale R P, Sondel P M, Goldman J M, Kersey J, Kolb H J, Rimm A A, Ringden O, Rozman C, Speck B, et al. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- 35.Gale R P, Horowitz M M, Ash R C, Champlin R E, Goldman J M, Rimm A A, Ringden O, Stone J A, Bortin M M. Ann Intern Med. 1994;120:646–652. doi: 10.7326/0003-4819-120-8-199404150-00004. [DOI] [PubMed] [Google Scholar]
- 36.Mosier D E, Gulizia R J, MacIsaac P D, Corey L, Greenberg P D. Proc Natl Acad Sci USA. 1993;90:2443–2447. doi: 10.1073/pnas.90.6.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Kroon J F, van Bergen C A, de Paus R A, Kluin-Nelemans H C, Willemze R, Falkenburg J H. J Immunother. 1997;20:101–110. doi: 10.1097/00002371-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Bortin M M, Truitt R L, Rimm A A, Bach F H. Nature (London) 1979;281:490–491. doi: 10.1038/281490a0. [DOI] [PubMed] [Google Scholar]