Abstract
Tissue factor (TF), a transmembrane receptor for coagulation factor VII/VIIa, is aberrantly expressed in human cancers. We demonstrated a significant correlation between TF and vascular endothelial growth factor (VEGF) production in 13 human malignant melanoma cell lines (r2 = 0.869, P < 0.0001). Two of these cell lines, RPMI-7951, a high TF and VEGF producer, and WM-115, a low TF and VEGF producer, were grown s.c. in severe combined immunodeficient mice. The high-producer cell line generated solid tumors characterized by intense vascularity, whereas the low producer generated relatively avascular tumors, as determined by immunohistologic staining of tumor vascular endothelial cells with anti-von Willebrand factor antibody. To investigate the structure-function relationship of TF and VEGF, a low-producer melanoma cell line (HT144) was transfected with a TF cDNA containing the full-length sequence, a cytoplasmic deletion mutant lacking the coding sequence for the distal three serine residues (potential substrates for protein kinase C), or an extracellular domain mutant, which has markedly diminished function for activation of factor X. Cells transfected with the full-length sequence produced increased levels of both TF and VEGF. Transfectants with the full-length sequence and the extracellular domain mutant produced approximately equal levels of VEGF mRNA. However, cells transfected with the cytoplasmic deletion mutant construct produced increased levels of TF, but little or no VEGF. Thus, the cytoplasmic tail of TF plays a role in the regulation of VEGF expression in some tumor cells.
Tissue factor (TF), a 47-kDa transmembrane protein, is the principal cell surface receptor for both the zymogen form of coagulation factor VII and the active enzyme form, factor VIIa. The coagulation cascade is triggered on the binding of VIIa to TF (1). In studies of normal human tissue, TF expression has been detected in situ principally in anatomic locations where it might serve in a protective role, or a so-called “barrier” function (e.g., pericytes in the central nervous system), which does not include the surface of vascular endothelial cells (VECs). Therefore, VECs in contact with flowing blood are not expected to express TF under usual circumstances (2). However, we have reported in situ localization of TF in tumor-associated VECs in patients with invasive breast cancer, a finding not duplicated in benign breast tumors (3). TF also was expressed by the tumor cells in these specimens (3). The potential importance of TF in tumor cell growth and metastasis has been suggested by demonstrating reduction of experimental tumor angiogenesis, growth, and/or metastases with one or more of the following interventions: (a) inhibition of tumor cell TF procoagulant activity with anti-TF antibodies (4–6) and active site inactivated factor VIIa (phenylalanyl-phenylalanyl-arginyl chloromethyl ketone-VIIa, FFR-ck-VIIa) (7); (b) transfection of tumor cells to express a procoagulant-active, but cytoplasmic mutant form of TF (7, 8); and (c) down-regulation of TF gene expression in murine tumor cells by transfection with TF antisense (9). One possible mechanism for this effect is the regulatory role shown for TF in the expression of vascular endothelial growth factor (VEGF) in murine tumor cells (9). Further support for the importance of this relationship comes from studies of the colocalization of TF and VEGF in human tumor cells from patients with either lung or breast cancer (10, 11). In addition, a statistically significant relationship was demonstrated recently between TF and VEGF expression as well as microvessel density in human nonsmall cell lung carcinoma (12). In addition, in human pancreatic carcinoma, expression of TF was found to correlate strongly with the degree of histologic differentiation (13). A significant linear trend was demonstrated with stronger TF expression observed in poorly differentiated tumors (13).
Structure-function studies of TF have focused on the extracellular domain as the key sequence for initiating blood coagulation on binding of factor VII (or VIIa) (1). However, recently published data have implicated the cytoplasmic domain of TF in the mediation of cell signal transduction (14–16), perhaps by a protein kinase C (PKC)-dependent mechanism involving phosphorylation of the three serine residues in the TF cytoplasmic tail. In the current study, we examined this structure-function relationship more closely in human tumor cells by measuring VEGF and TF production as endpoints. We transfected either a full-length TF cDNA, a truncated TF cDNA, or an extracellular domain mutant TF cDNA (17) in the sense orientation into HT144, a low TF/VEGF-producing human melanoma cell line. Transfectants with the full-length as well as the extracellular domain mutant TF cDNA produced increased levels of TF and VEGF, whereas transfectants with the truncated TF cDNA produced increased levels of TF, but negligible levels of VEGF. These results suggest that the cytoplasmic tail of TF may be involved in VEGF production in human tumors.
MATERIALS AND METHODS
Cell Lines and Cell Culture.
All human melanoma cell lines were purchased from the American Type Culture Collection. Each cell line was cultured in an appropriate medium according to the specifications of the American Type Culture Collection in a humidified incubator at 37°C in 5% CO2/95% air or no CO2. Cells were counted with a hemocytometer, and cell viability was determined by trypan blue dye exclusion. More than 90% of the cells were viable in all experiments.
Analysis of TF.
Cells were washed once with PBS, briefly trypsinized (0.05% porcine trypsin, 0.53 mM EDTA; GIBCO/BRL) for 2 min at 37°C, diluted 10-fold in PBS, and collected by centrifugation at 1,200 rpm for 5 min at 4°C. Cell pellets were resuspended to 106 cells/ml of PBS and sonicated by using a Biosonic IV (VWR Scientific; microprobe at 60-low for 20 sec at 4°C). TF procoagulant activity in the cell sonicates was determined in a one-stage clotting assay in the presence of excess phospholipid, by using pooled normal human plasma and either purified human brain TF (18) or recombinant TF (Innovin, Dade/Baxter Diagnostics, Deerfield, IL) as a standard. TF antigen in the cell sonicates was determined by using an ELISA (American Diagnostica, Greenwich, CT), according to the manufacturer’s directions. Because little or no TF was detected in cell supernatants, the data are reported only for cell sonicates.
Analysis of VEGF.
VEGF was measured in the culture supernatants by an ELISA (R & D Systems); in contrast to TF, levels of VEGF in the cell sonicates were negligible.
Human Melanoma Growth in Severe Combined Immunodeficient (SCID) Mice.
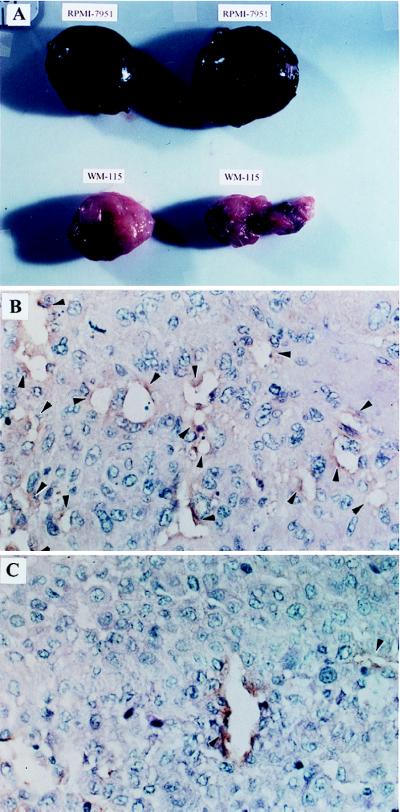
Six-week-old female CB17.SCID mice, obtained from the Emory University Animal Facility, were injected with asialo-ganglioside GM-1 antibody (anti-natural killer cell antibody, Wako Chemicals, Richmond, VA) on day 1, X-irradiated (200 rad), and inoculated with human melanoma cells, either RPMI-7951 or WM-115, 3 × 106 cells/mouse s.c. on day 3. All tumors were harvested at the same time (82 days after implantation) to compare accurately the growth rate of the WM-115 and RPMI-7951 tumors. WM-115 tumors grew approximately 0.4 g in this period of time, whereas RPMI-7951 tumors grew approximately 1.2 g (see Fig. 2A). Animal experiments were conducted in accordance with guidelines provided by Emory University Institutional Animal Care and Use Committee and the Centers for Disease Control and Prevention Animal Care and Use Committee.
Figure 2.
Human melanoma cell lines grown as xenogeneic tumors in SCID mice. RPMI-7951 melanoma cells (a high TF and VEGF producer) or WM-115 melanoma cells (a low TF and VEGF producer) were inoculated s.c. (3 × 106/mouse) on the same day. (A) The gross appearance of RPMI-7951 tumors was hemorrhagic, dark purplish in color, whereas the WM-115 tumors appeared pale and relatively avascular. The immunohistologic analysis of RPMI-7951 and WM-115 tumors from these SCID mice emphasized detection of VECs. (B) The RPMI-7951 tumor and (C) the WM-115 tumor were stained with rabbit anti-human von Willebrand factor, by using the standard immunoperoxidase method. The relative frequency of micro blood vessels is noted by arrow heads (magnifications: ×200 for B and C). The average number of blood vessels per a field for three RPMI-7951 tumors and five WM-115 tumors are 17.3 ± 4.0 (1 SD) and 3.2 ± 2.2, respectively.
Immunohistochemical Staining.
Tumors were fixed in 4% formalin/PBS and paraffin-embedded. Paraffin sections were stained with standard hematoxylin-eosin and anti-human von Willebrand factor antibody (Dako), by using the standard immunoperoxidase method (3).
Cloning of Full-Length, Truncated, and Extracellular Domain Mutant TF cDNA.
Human TF cDNA was a gift from J. Wilcox (Emory University) and was used as a template for creating the full-length and the truncated TF cDNA. TF cDNA containing the full-length coding sequence was made by PCR using the forward primer 5′-CACAAAGCTTCTCGCACTCCCTCTGGCCGGCCCA-3′ and the reverse primer 5′-GGTTGGATCCTCAAAAGTCCACCCAGGATTT-3′. The truncated TF cDNA with a distal cytoplasmic deletion of the coding region for amino acids 252–263, which ordinarily contains three serine residues at 253, 258, and 263, was produced by using the same forward primer but a different reverse primer (5′-GGTTGGATCCTTACCCCACTCCTGCCTTTCTAC-3′). The 5′ end of the forward primer was phosphorylated by T4 polynucleotide kinase. Both cDNAs produced by PCR were cloned into the pCR 3.1-Uni expression vector, by using the Eukaryotic TA Cloning kit, and were transformed into One Shot competent cells (Invitrogen). An extracellular domain mutant, TFmut (157/159), cDNA was made according to a published method (17). This TF mutant exhibits mutations at amino acids 157 (Y to A) and 159 (K to A), resulting in the failure to interact with factor X and loss of expression of procoagulant activity (17). TFmut (157/159) cDNA and a full-length human TF cDNA (containing 1,134 bp) were cloned in sense orientation into pCDNA3 (Invitrogen), resulting in plasmids TFmut (157/159) and TF sense, respectively.
Transfection.
In the experiments shown in Fig. 3 and Table 1, isolated plasmids were sequenced to confirm the presence and orientation of the cloned insert. Plasmids containing the full-length or truncated TF cDNA were transfected into the human melanoma cell line HT144 (a low TF and VEGF producer) by using the cationic liposome DOTAP (N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methylsulfate) (Boehringer Mannheim), according to the manufacturer’s instructions. Transfectants were selected by resistance to geneticin (500 μg/ml) (GIBCO/BRL) in the culture medium. In experiments shown in Fig. 4, transfectants containing plasmids TFmut (157/159), TF sense, or vector alone [pCDNA3] were selected in the presence of neomycin in 96-well plates. Positive clones from separate wells then were cultured in 24-well plates until the colonies reached a density of 3–5 × 105 cells. For each experiment more than seven colonies were tested, and the experiments have been repeated with colonies derived from separate transfections. Transfection with the plasmids TFmut (157/159) and TF sense was confirmed by performing PCR with a forward primer that recognizes sequences in the pCDNA3 vector (5′-CGACTCACTATAGGGAGACCCAAGC-3′) and a reverse primer that binds to the TF cDNA (5′-CCTTCACAATCTCGTCGGTGAGGTC-3′). This experimental design allows for distinguishing endogenous and transfected TF and only those single-cell derived colonies giving a positive signal for the TF-vector construct were used in the following studies.
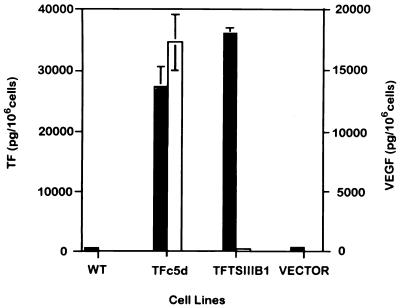
Figure 3.
Effects of transfection of TF cDNA, containing full-length sequence or the cytoplasmic tail deletion sequence into human melanoma cell line HT144. Filled bars represent the levels of TF and empty bars represent the levels of VEGF. The values are the mean ± 1 SD of triplicate assays from three separate experiments. Wild-type (WT) HT144 cells are low TF and VEGF producers. TF transfectants containing the full-length coding sequence (TFc5d) produced increased levels of both TF and VEGF. In contrast, TF transfectants containing the cytoplasmic tail deletion sequence (TFTSIIIB1) produced very little VEGF, although TF antigen production (and procoagulant activity, data not shown) was increased. Levels of VEGF in the nontransfected HT144 (WT) and HT144 transfected with the vector alone (Vector) were too low to illustrate on the graph, as was true for the vertical lines indicating SD for both TF and VEGF production by the WT cells and the cells containing vector, and for VEGF production by the TFTSIIIB-transfected cells.
Table 1.
TF and VEGF production in clones of the HT144 cell line transfected with truncated TF cDNA
| Clone | TF, pg/106 cells | VEGF, pg/106 cells |
|---|---|---|
| HT144-TFTSIII-B1 | 36,400 | 123 |
| HT144-TFTSIII-B6 | 5,100 | 0 |
| HT144-TFTSIII-B9 | 613 | 0 |
| HT144-TFTSIII-B14 | 3,569 | 0 |
| HT144-TFTSIII-B51 | 424 | 0 |
| HT144-TFTSIII-2 | 1,392 | 9 |
| HT144-TFTSIII-20 | 6,121 | 81 |
| HT144-TFc5d | 27,367 | 17,252 |
| HT144-wild type | 506 | 0 |
| HT144-vector | 365 | 0 |
| Hs294T | 10,099 | 8,098 |
HT144-TFTSIII-B1, -B6, -B9, -B14, -B51, -2, and -20 are clones derived from the low TF- and VEGF-producing human melanoma cell line HT144, each of which was transfected with the truncated TF cDNA (TFTSIII). This TF cDNA lacks the coding region for the cytoplasmic tail serine residues, potential substrates for PKC (14). HT144-TFc5d is a clone derived from the HT144 cell line that was transfected with the full-length TF cDNA. The HT144 vector is derived from HT144 that was transfected with the vector alone. Hs294T is a constitutively high TF- and VEGF-producing human melanoma cell line. TF and VEGF levels were measured in duplicate by ELISA. The data are representative of three separate experiments.
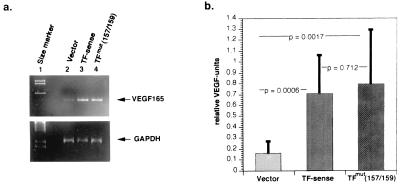
Figure 4.
Effects on VEGF transcription by transfection of TF cDNA, containing full-length sequence or the extracellular domain mutant sequence (Y157 → A157 and K159 → A159) into human melanoma cell line HT144. (a) A 2% agarose gel demonstrating RT-PCR products of VEGF-165 mRNA produced by transfectants with vector alone, TF sense, or TFmut (157/159) plasmids. (b) Relative levels of VEGF mRNA production by transfectants with vector alone, TF sense, or TFmut (157/159) plasmids. Relative levels of VEGF mRNA from each transfectant colony were determined by densitometric quantitation of RT-PCR products separated on 2% agarose gel as shown in a. Relative VEGF units were determined by dividing the intensity of densitometric values for VEGF by the intensity of densitometric values for GAPDH. Values of each bar graph represent mean ± 1 SD of at least seven separate transfectant colonies.
Reverse Transcriptase (RT)–PCR.
RT-PCR for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and VEGF was performed from total RNA of 3–5 × 105 cells for each clone. RNA was extracted by using the RNeasy-Minikit (Qiagen, Hilden, Germany) and resuspended in 20 ml of diethyl pyrocarbonate-H2O. cDNA was reverse-transcribed from 5 ml of total RNA with oligo(dt) primers (Amersham Pharmacia) and avian myeloblastosis virus-RT (Promega) followed by a first amplification for GAPDH that served as the basis for adjusting the input cDNA. After adjusting the cDNA by several dilutions in H2O (1:10 to 1:100), a second PCR for GAPDH was performed to confirm that equal amounts of cDNA were used. Thereafter VEGF-PCR was performed by using the same cDNA dilutions. PCR was done by using the following primer pairs: GAPDH forward 5′-TGAAGGTCGGAGTCAACGGATTTGGT-3′, GAPDH reverse 5′-CATGTGGGCCATGAGGTCCACCAC-3′; VEGF165 forward 5′-CTGCTGTCTTGGGTGCATTGGAG-3′, VEGF165 reverse 5′-CAAGGCCCACAGGGATTTTCTTGTC-3′; VEGF forward 5′-GAGTGTGTGCCCACTGAGGAGTCCAAC-3′, VEGF reverse 5′-CTCCTGCCCGGCTCACCGCCTCGGCTT-3′, and the following conditions 1× (94°C, 240 sec); 1× (94°C, 60 sec; 55°C, 60 sec; 72°C, 115 sec); 28× (94°C, 60 sec; 62°C, 30 sec; 72°C, 115 sec); 1× (72°C, 600 sec) for GAPDH and 1× (94°C, 240 sec); 30× (94°C, 60 sec; 55°C, 120 sec; 72°C, 120 sec); 1× (72°C, 600 sec) for VEGF. PCR products were separated onto 2% agarose gels. Verification of PCR products was performed by reamplification with different primers recognizing sequences internal to the amplification products. Reactions lacking template RNA or avian myeloblastosis virus-RT served as controls.
Evaluation of Signal Intensity.
To quantify the VEGF signal obtained from each colony derived from a single stable transfectant, the PCR signals obtained by VEGF and GAPDH were quantified by densitometry (Bio-Rad). The intensity of the signal obtained for VEGF was divided by the signal obtained for GAPDH, thus allowing normalization for variations in the input of total RNA and cDNA, respectively. The data are expressed as relative VEGF units and values of the independent clones are given as mean ± SD. Student’s two-tailed t test was used to determine significance.
Statistical Analysis.
ELISA data were analyzed by linear regression using the sigmastat program and Student’s two-tailed t test.
RESULTS
Coexpression of TF and VEGF in Human Melanoma Cell Lines.
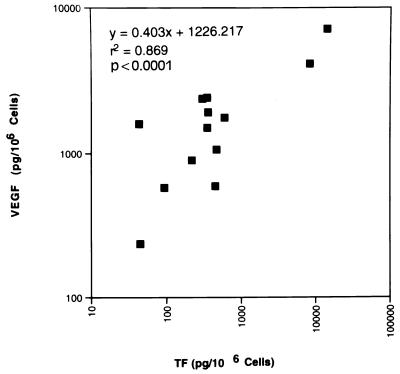
In an effort to determine whether or not TF regulates VEGF production in human tumor cells and therefore might stimulate angiogenesis, we compared TF and VEGF expression in 13 human melanoma cell lines. As illustrated in Fig. 1, a statistically significant correlation was demonstrated between the production of TF and VEGF in the tumor cell lines (r2 = 0.87, P < 0.0001).
Figure 1.
Coexpression of TF and VEGF in human melanoma cell lines. Levels of TF and VEGF were measured by ELISA (10) in triplicate wells of triplicate cultures. Each point represents the mean of the results of three determinations. Linear regression analysis demonstrates a significant correlation between TF and VEGF production in these 13 melanoma cell lines (r2 = 0.869, P < 0.0001). The TF was fully functional, as measured in a one-stage assay for procoagulant activity (PCA) (data not shown) (16), and the correlation between TF PCA and antigen expression was highly statistically significant (r2 = 0.94, P < 0.0001).
Angiogenesis by Human Melanoma Tumors Grown in SCID Mice.
To examine the potential relevance of this in vitro observation to tumor behavior in vivo, we inoculated SCID mice with either a high TF- and VEGF-producer human melanoma cell line (RPMI-7951) or a low TF and VEGF producer (WM-115). Xenogeneic tumors recovered from animals injected with the high-producer cell line, RPMI-7951, were quite hemorrhagic on gross inspection (Fig. 2A) and were characterized microscopically by the presence of more prominent blood vessels (as determined by immunohistochemical staining for von Willebrand factor) (Fig. 2B). In contrast, the low-producer cell line, WM-115, was found to generate rather avascular tumors on gross inspection (Fig. 2A) with scant numbers of visible blood vessels on immunohistochemical analysis, as indicated by rare staining of endothelial cells for von Willebrand factor (Fig. 2C). The average number of blood vessels in the RPMI-7951 tumors and in the WM-115 tumors per microscopic field (at ×200 magnification) was 17.3 ± 4.0 (1 SD) and 3.2 ± 2.2, respectively. These results suggested a parallel between production of VEGF in vitro and angiogenesis in vivo.
Effects of Transfection of the Full-Length, the Truncated, or the Extracellular Domain Mutated TF cDNA(s).
To test the hypothesis that TF in cancer cells may regulate VEGF expression via its cytoplasmic domain, we transfected TF cDNA in the sense orientation into a low-producer human melanoma cell line, HT144. We used TF cDNA containing either the full-length coding sequence or a truncated coding sequence lacking the distal cytoplasmic domain. Transfectants with the full-length TF cDNA (TFc5d) produced increased levels of both TF and VEGF in vitro (Fig. 3). The TF produced by these cells was fully functional in one-stage clotting assays (data not shown). In contrast, transfectants containing the truncated sequence (TFTSIIIB-1), which produced increased levels of TF (also fully functional), produced little or no VEGF. The untransfected, wild-type HT144 cells and HT144 cells transfected with a vector only produced low levels of TF and very low levels of VEGF (Fig. 3). To demonstrate that this depressed VEGF expression in cells transfected with the truncated TF cDNA sequence was not caused by a clonal artifact, we measured TF and VEGF levels by ELISA in six other clones from the melanoma cell line (HT144) transfected similarly with the truncated TF cDNA, as well as in two different control clones. All clones revealed the same pattern of depressed VEGF and increased TF, whereas the controls demonstrated the typical coordinate pattern of increased VEGF and TF production (Table 1).
Studies published by others using a model system of experimental hematogenous metastasis indicate that both signaling of the tumor cell through the thrombin receptor (4, 5, 7) and the cytoplasmic tail of TF (7, 8) are necessary for survival of metastatic tumor cells. Therefore, to resolve the issue regarding whether the extracellular (procoagulant) domain of TF or (only) the cytoplasmic tail is necessary for VEGF production, we performed the following experiments. When high TF- and VEGF-producing human melanoma and human breast cancer cell lines were cultured in a serum-free medium in the absence or presence of factor VIIa (100 or 300 nM), increased VEGF production could not be demonstrated (data not shown). Likewise, when TF-sense transfectant TFc5d (a high TF and VEGF producer) was cultured in a serum-free medium in the absence or presence of either factor VIIa (30, 100, or 300 nM) or active site inactivated factor VIIa (phenylalanyl-phenylalanyl-arginyl-chloromethyl ketone modified recombinant human factor VIIa, FFR-ck-VIIa) (17) (30, 100, or 300 nM), neither enhanced nor inhibited VEGF production was observed (data not shown). Finally, to test whether the proteolytic function of bound factor VIIa is required, a TF- and VEGF-producing human melanoma cell line was cultured in 10% FBS in the presence or absence of factor VIIa (100 or 300 nM) and in the presence or absence of hirudin (0.5 units/ml), a specific thrombin inhibitor (9). Hirudin failed to inhibit VEGF production, indicating that thrombin generation was not required (data not shown).
Finally, we transfected HT144 cells with TF containing an extracellular domain in which amino acids 157 (Y to A) and 159 (K to A) were mutated. Total RNA was isolated from 3–5 × 105 cells derived from each single clone of either vector-, TF-sense-, or TFmut (157/159)-transfected human HT-144 cells and was subjected to RT-PCR for VEGF. A parallel RT-PCR for GAPDH served as a positive control in each experiment and allowed for normalization of the VEGF signal. When primers specific for VEGF 165 (Fig. 4) or for all three VEGF isoforms (121, 165, and 189, data not shown) were used for amplification, vector-transfected cells demonstrated only low levels of VEGF mRNA. In contrast, strong VEGF transcription was observed in TF-sense-transfected clones as well as in clones stably transfected with the procoagulant-defective TF mutant, TFmut (157/159) (Fig. 4). The mean relative VEGF units (intensity of VEGF signals/intensity of GAPDH signals) for vector, TF sense, and TFmut (157/159) are 0.162 ± 0.105 (1 SD), 0.710 ± 0.348, and 0.798 ± 0.499, respectively. These data indicate that the TF cytoplasmic tail is sufficient to induce VEGF transcription and that factor VIIa/factor X interactions are not required, because this extracellular TF mutant lacks the ability to act as a cofactor for the proteolytic activation of factor X, and thus lacks procoagulant activity. These data were confirmed by the observation that addition of recombinant factor VIIa (100 nM) to melanoma cells stably transfected with TF sense or TFmut (157/159) did not increase VEGF transcription (data not shown).
DISCUSSION
In this report, we have demonstrated a significant correlation between TF and VEGF production in human melanoma cell lines, which correlated with tumor angiogenesis in vivo. Transfection of a low-producer cell with a cytoplasmic tail deletion mutant of TF cDNA resulted in a significant reduction of VEGF production, but no reduction of TF synthesis in the tumor cells. Only the cytoplasmic domain of TF appears to be necessary for VEGF production. We demonstrated unequivocally that in this system factor VIIa binding to TF and the subsequent generation of thrombin is not required. This hypothesis is supported by several lines of evidence. We demonstrated that transfectants containing the extracellular domain mutant TFmut (157/159), which has markedly diminished function for activation of factor X (17), produced essentially the same levels of VEGF mRNA as those containing the full-length TF cDNA. In experiments reported elsewhere, incubating tumor cells with a 50-fold excess of either procoagulant activity blocking anti-TF antibody or with an anti-VEGF antibody did not inhibit VEGF production (10). The anti-VEGF antibody was used, because it has been demonstrated that VEGF induces TF expression in VECs and monocytes (19). Neither factor VIIa nor FFR-ck-VIIa affected VEGF production. Hirudin also failed to inhibit VEGF production, which is consistent with previous results in a murine model of methylcholantrene A-induced fibrosarcoma, in which equal levels of VEGF were produced by tumor cells in vitro regardless of the presence or absence of this potent and specific thrombin inhibitor (9).
In summary, the evidence to date indicates that TF procoagulant function and the proteolytic function of bound factor VIIa are not required for regulation of VEGF production in human tumor cells. In further support of this concept, it has been demonstrated in TF-transfected baby hamster kidney cells that binding of factor VIIa to TF induces phosphorylation of the p44/42 mitogen-activated protein kinase without requiring the generation of factor Xa (20). Therefore, we hypothesize that TF-mediated VEGF synthesis in cancer cells is caused by endogenous (autonomous) stimuli generated, which transduce a signal for VEGF production via phosphorylation of the cytoplasmic serine residues of TF. Because all of our experiments were performed under normal oxygen tension, this mechanism must be distinct from the well-characterized pathway of hypoxia-induced VEGF production, although it has been demonstrated recently that hypoxia further enhances TF production (21, 22).
In contrast to our results in human melanoma cells, TF-dependent VEGF production in human lung fibroblasts has been shown to depend on factor VIIa binding to TF (23). Furthermore, active site inactivated VIIa (dansyl-glutaminyl-glycinyl-arginyl chloromethyl ketone-VIIa, DEGR-ck-VIIa) inhibited VEGF production in this cell system (23). However, we presume that normal human lung fibroblasts do not possess the autonomous pathway for TF and VEGF synthesis we are describing in human cancer cells. Thus, normal tissue cells most likely depend on exogenous stimuli like lipopolysaccharide (24) or factor VIIa for the induction of TF-mediated VEGF production. However, in both cases, we postulate that the signals must be mediated by the cytoplasmic tail of TF. Our cytoplasmic deletion TF mutant is missing serine residues at positions 253, 258, and 263. Phorbol ester-induced phosphorylation of serine residues 253 and 258 in the TF cytoplasmic tail has been demonstrated previously by others (14). Because the deletion of the presumed PKC substrate portion of the tail in our TF mutant tumor cells greatly reduced VEGF production (Fig. 3; Table 1), one might speculate that TF-mediated VEGF production may be PKC dependent. Indeed, we and others (data not shown; ref. 23) have demonstrated that specific antagonists of PKC and tyrosine kinase inhibit TF-mediated VEGF production. In another model system, for example, PKC-α, which directly phosphorylated and activated Raf-1 kinase (25), was able to mediate VEGF expression (26).
In contrast to the structural requirements for TF-mediated VEGF synthesis in vitro and angiogenesis in vivo in the human tumor model, the formation of lung colonies after i.v. injection of tumor cells (an experimental model of metastasis) appears to depend on both the procoagulant function of TF, which resides in the extracellular factor VIIa-binding domain of the protein and leads to thrombin generation (4, 5, 7), and the cytoplasmic serine residues of the protein (7, 8), which may be mediating signal transduction (7, 8). Thus, TF may prove to be a multifunctional protein that, in addition to its traditional role in blood coagulation, can regulate the proadhesive and proangiogenic properties of tumor cells. The structure-function studies published to date suggest that it is the cytoplasmic tail of TF rather than its extracellular domain that subserves these important additional functions.
Acknowledgments
We gratefully acknowledge that factor VIIa and FFR-ck-VIIa are generous gifts from Drs. Ulla Hedner and Mirella Ezban, Novo Nordisk, Denmark. We thank Mr. James D. Gathany for the illustrations. This research was supported by Grant CA22202 from the Department of Health and Human Services (F.R.R. and M.S.), Grant Na 138 from Deutsche Forschungsgemeinschaft (P.P.N.), and a Schilling professorship (P.P.N.).
ABBREVIATIONS
- TF
tissue factor
- VEGF
vascular endothelial growth factor
- VEC
vascular endothelial cell
- SCID
severe combined immunodeficient
- PKC
protein kinase C
- RT
reverse transcriptase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Edgington T S, Mackman N, Brand K, Ruf W. Thromb Haemostasis. 1991;66:67–79. [PubMed] [Google Scholar]
- 2.Drake T A, Morrissey J H, Edgington T S. Am J Pathol. 1989;134:1087–1097. [PMC free article] [PubMed] [Google Scholar]
- 3.Contrino J, Hair G, Kreutzer D L, Rickles F R. Nat Med. 1996;2:209–215. doi: 10.1038/nm0296-209. [DOI] [PubMed] [Google Scholar]
- 4.Mueller B M, Reisfeld R A, Edgington T S, Ruf W. Proc Natl Acad Sci USA. 1992;89:11832–11836. doi: 10.1073/pnas.89.24.11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher E G, Ruf W, Mueller B M. Cancer Res. 1995;55:1629–1632. [PubMed] [Google Scholar]
- 6.Huang X, Molema G, King S, Watkins L, Edgington T S, Thorpe P E. Science. 1997;275:547–550. doi: 10.1126/science.275.5299.547. [DOI] [PubMed] [Google Scholar]
- 7.Mueller B M, Ruf W. J Clin Invest. 1998;101:1372–1378. doi: 10.1172/JCI930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bromberg M E, Konigsberg W H, Madison J F, Pawashe A. Proc Natl Acad Sci USA. 1995;92:8205–8209. doi: 10.1073/pnas.92.18.8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Deng Y, Luther T, Muller M, Ziegler R, Waldherr R, Stern D M, Nawroth P P. J Clin Invest. 1994;94:1320–1327. doi: 10.1172/JCI117451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shoji M, Hancock W W, Abe K, Micko C, Casper K A, Baine R M, Wilcox J N, Danave I, Dillehay D L, Matthews E, et al. Am J Pathol. 1998;152:399–411. [PMC free article] [PubMed] [Google Scholar]
- 11.Shoji M, Abe K, Nawroth P P, Rickles F R. Trends Cardiovascu Med. 1997;7:52–59. doi: 10.1016/S1050-1738(96)00142-9. [DOI] [PubMed] [Google Scholar]
- 12.Koomagi M, Volm M. Int J Cancer. 1998;79:19–22. doi: 10.1002/(sici)1097-0215(19980220)79:1<19::aid-ijc4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 13.Kakkar A K, Lemoine N R, Scully M F, Tebbutt S, Williamson C N. Br J Surg. 1995;82:1101–1104. doi: 10.1002/bjs.1800820831. [DOI] [PubMed] [Google Scholar]
- 14.Zioncheck T F, Roy S, Vehar G A. J Biol Chem. 1992;267:3561–3564. [PubMed] [Google Scholar]
- 15.Rottingen J A, Enden T, Camerer E, Iversen J G, Prydz H. J Biol Chem. 1995;270:4650–4660. doi: 10.1074/jbc.270.9.4650. [DOI] [PubMed] [Google Scholar]
- 16.Camerer E, Rottingen J A, Iversen J G, Prydz H. J Biol Chem. 1996;271:29034–29042. doi: 10.1074/jbc.271.46.29034. [DOI] [PubMed] [Google Scholar]
- 17.Ruf W, Miles D J, Rehemetulla A, Edgington T S. J Biol Chem. 1992;267:22206–22210. [PubMed] [Google Scholar]
- 18.Ewan V A, Edwards R L, Rickles F R. J Lab Clin Med. 1983;101:401–410. [PubMed] [Google Scholar]
- 19.Clauss M, Gerlach M, Gerlach H, Brett J, Wang F, Familletti P C, Pan Y C E, Olander J V, Connolly D T, Stern D. J Exp Med. 1990;172:1535–1545. doi: 10.1084/jem.172.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poulsen L K, Jacobsen N, Sorensen B B, Bergenhem N C H, Kelly J D, Foster D C, Thastrup O, Ezban M, Petersen L C. J Biol Chem. 1998;273:6228–6232. doi: 10.1074/jbc.273.11.6228. [DOI] [PubMed] [Google Scholar]
- 21.Amirkhosravi A, Meyer T, Warnes G, Amaya M, Malik Z, Biggerstaff J P, Siddiqui F A, Sherman P, Francis J L. Thromb Haemostasis. 1998;80:598–602. [PubMed] [Google Scholar]
- 22.Yan S-F, Zou Y S, Gao Y, Zhai C, Mackman N, Lee S L, Milbrandt J, Pinsky D, Kisiel W, Stern D. Proc Natl Acad Sci USA. 1998;95:8298–8303. doi: 10.1073/pnas.95.14.8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ollivier V, Bentlila S, Chabbat J, Hakim J, de Prost D. Blood. 1998;91:2698–2703. [PubMed] [Google Scholar]
- 24.Mackman N, Brand K, Edgington T S. J Exp Med. 1991;174:1517–1526. doi: 10.1084/jem.174.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolch W, Heidecker G, Kochs G, Hummel R, Vahidi H, Mischak H, Finkenzeller G, Marme D, Rapp U R. Nature (London) 1993;364:249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- 26.Finkenzeller G, Marme D, Weich H A, Hug H. Cancer Res. 1992;52:4821–4823. [PubMed] [Google Scholar]