Abstract
Aortic medial amyloid is a form of localized amyloid that occurs in virtually all individuals older than 60 years. The importance and impact of the amyloid deposits are unknown. In this study we have purified a 5.5-kDa aortic medial amyloid component, by size-exclusion chromatography and RP-HPLC, from three individuals, and we have shown by amino acid sequence analysis that the amyloid is derived from an integral proteolytic fragment of lactadherin. Lactadherin is a 364-aa glycoprotein, previously known to be expressed by mammary epithelial cells as a cell surface protein and secreted as part of the milk fat globule membrane. The multidomain protein has a C-terminal domain showing homology to blood coagulation factors V and VIII. We found that the main constituent of aortic medial amyloid is a 50-aa-long peptide, here called medin, that is positioned within the coagulation factor-like domain of lactadherin. Our result is supported by the specific labeling of aortic medial amyloid in light and electron microscopy with two rabbit antisera raised against two synthetic peptides corresponding to different parts of medin. By using in situ hybridization we have shown that lactadherin is expressed by aortic medial smooth muscle cells. Furthermore, one of the synthetic peptides forms amyloid-like fibrils in vitro. Lactadherin was not previously known to be an amyloid precursor protein or to be expressed in aortic tissue. The structure of lactadherin may implicate an important regulatory function in the aorta.
The localized amyloid deposits in some of the major age-related diseases have attracted immense interest in recent years. Investigations have focused mainly on Aβ and AIAPP,‡‡ the principal amyloid proteins in Alzheimer’s disease (2) and type 2 diabetes (3). Elucidation of the biochemical nature of these amyloid proteins not only has proved beneficial to provide important knowledge about the pathogenesis of the diseases but also has produced insight into the normal functions of the amyloid precursor proteins and into hitherto unknown pathological processes. Albeit not proven, it may be that all localized amyloidoses reflect primary pathological changes.
One extremely common form of senile localized amyloid is confined to the aortic media. This form of amyloid is biochemically different from that seen in association with atheromatous plaques, which is derived from apolipoprotein A1 (4, 5). In a previous study our group found aortic medial amyloid in 97% of the subjects above the age of 50 (6), and similar frequencies have been found in other studies (7). The impact of the amyloid deposits is unknown, but it is possible that they contribute to the age-related diminished elasticity of the vessels (8). Furthermore, the demonstration that amyloid fibrils can be toxic to cells has overthrown the past belief that localized small deposits are of little significance (9, 10). We therefore postulated that elucidation of the nature of the amyloid in the aortic media could increase the knowledge of the normal and pathological biology of the aorta.
Our previous study implicated that the aortic medial amyloid consisted of a protein component with a molecular mass less than 6 kDa. However, the aortic medial amyloid did not react with antisera to other known forms of amyloid (6), indicating that this form of amyloid is of previously unknown composition.
When two of us (J.N. and L.O.T.) were investigating potential Aβ peptide deposition in peripheral tissues from elderly individuals, an aortic protein component with a molecular mass less than 6 kDa was isolated. Amino acid sequence analysis of a tryptic peptide from this aortic protein component revealed the amino acid sequence NFGSVQFV, dissimilar to any part of the AβPP sequence. Interestingly, a rabbit antiserum (A172) against a synthetic peptide with this sequence labeled aortic medial amyloid specifically, indicating that the fragment originated from an amyloid protein. The antiserum was recognized as a tool for purifying and characterizing the aortic medial amyloid.
MATERIALS AND METHODS
Aortic Tissue.
Pieces of thoracic aorta from older persons (>70 years) were taken at autopsy and stored at −20°C. For light microscopy, pieces of the aortas were fixed in neutral buffered 4% formaldehyde solution and were paraffin-embedded. For electron microscopy, small aortic medial pieces were fixed in 2% paraformaldehyde and 0.25% glutaraldehyde in 0.05 M sodium phosphate buffer, pH 7.4, containing 0.15 M sodium chloride and embedded in Unicryl (British BioCell, Cardiff, U.K.) (6).
Aortic materials from three individuals, with extensive deposits of medial amyloid but with no intimal or adventitial amyloid, were selected for amyloid purification by examination of tissue sections stained with Congo red (11).
Extraction of Aortic Medial Amyloid Components.
Purification of amyloid components was performed according to a previously described protocol (12) with minor modifications. Frozen aortic tissue (25–35 g) was thawed, and the media was dissected and cut in pieces. The small media pieces were homogenized vigorously with an electric homogenizer (model 17106; Omni International, Waterbury, CT) in 5 vol of buffer containing 1% SDS, 150 mM NaCl, and 50 mM Tris⋅HCl, pH 7.4 (TBS). SDS-insoluble material was collected by centrifugation at 100,000 × g (L-70 ultracentrifuge with Ti 55.2 rotor; Beckman) for 20 min. The pellet was rehomogenized in the same buffer, and the procedure was repeated at least three times. The resulting pellet was washed with distilled water, suspended in 30 ml of distilled water, and centrifuged at 100,000 × g for 20 min to minimize SDS content. The final pellet was transferred to a glass bottle and extracted with 30 ml of 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) for 30 min (22°C) on a shaker. Amyloid content was monitored throughout the procedure by staining smears of the homogenates and extracts on slides for 10 min in Congo red B solution (11). The HFIP extract was filtered through several layers of sterile gauze to remove coarse debris. The crude extract then was transferred to 1.5-ml Eppendorf tubes and dried in a vacuum centrifuge (SC 100; Savant). Each dried pellet was delipidated by tip sonication (Ultrasonics, Farmingdale, NY) in chloroform/methanol (2:1) (700 μl) followed by vortexing after the addition of 400 μl of distilled water. After centrifugation at 10,000 × g for 1 min, the aqueous supernatants were removed carefully. Methanol (500 μl) was added and proteins were pelleted by centrifugation at 10,000 × g. The supernatants were discarded and the pellets were dried rapidly in a vacuum centrifuge.
Size-Exclusion Chromatography.
The delipidated and dried pellet was suspended in 70% (vol/vol) formic acid by using a tip sonicator (Ultrasonics). Insoluble material was removed by centrifugation at 10,000 × g for 1 min. The supernatant, containing 2–5 mg of dissolved protein, was loaded onto a 10 × 300-mm Superose 12 HR column (Amersham Pharmacia) and developed with 70% formic acid at a flow rate of 200 μl/min. The effluent was monitored at 280 nm.
RP-HPLC.
Superose 12 HR fractions were subjected to slot-blot analysis (not shown) with antiserum A172. Reactive fractions (3- to 30-kDa range) were pooled, dried by vacuum centrifugation, and suspended in 60% formic acid. After 1 min of centrifugation at 10,000 × g, the supernatant was loaded onto a C4 column. The column was developed by using a 45-min linear gradient from 60% formic acid (vol/vol) (A solution) to 60% formic acid and 40% acetonitrile (vol/vol) (B solution). The flow rate was 700 μl/min, and the effluent was monitored at 280 nm. Two-minute fractions were collected. In one case, all material that eluted between 13.6 and 14.8 ml from the Superose 12 HR column was loaded directly onto the C4 column by multiple injections.
Electrophoresis and Western Blot Analysis.
Fractions from the RP-HPLC were dried by vacuum centrifugation after the addition of 2 μl of 10% SDS. The dried sample was dissolved in 9 M urea (100 μl), heated in a boiling-water bath for 5 min, and then mixed with 50 μl of 3× sample buffer consisting of 0.3 M Tris⋅HCl at pH 8.6, 30% (vol/vol) glycerol, 6% (wt/vol) SDS, and 6% (vol/vol) 2-mercaptoethanol and subjected to SDS/PAGE in a Tris-Tricine-buffered gel system (13). The proteins were electrotransferred in a semidry system (14) to a 0.45-μm nitrocellulose membrane (Hybond C; Amersham Pharmacia). Nonspecific binding of antibodies to the membrane was blocked for 1 h with 3% nonfat milk in TBS containing 0.05% Tween 20 (TBS-Tween). The membrane was incubated overnight at ambient temperature with the primary serum A172 diluted 1:500 in TBS. After three washes for 10 min in TBS-Tween, the membrane was incubated with horseradish peroxidase-conjugated swine anti-rabbit antibodies (Dako) diluted 1:1,500 in TBS. After washing as previously, labeling was visualized by enhanced chemiluminescence (ECL; Amersham Pharmacia) according to the manufacturer’s instructions.
Amino Acid Sequence Analysis.
After separation in the Tris-Tricine system, proteins were transferred to a 0.2-μm poly(vinylidene difluoride) membrane (Bio-Rad) and stained with 0.025% Coomassie blue R250 (without acetic acid). The <6.5-kDa protein band, which was reactive with A172 in Western blot analysis, was cut out and applied directly to an automatic protein sequencer (Applied Biosystems 477A) connected to a 120-A phenylthiohydantoin Analyzer (Perkin–Elmer) as described (15). In one case, sequencing also was performed directly on RP-HPLC-separated samples dried in a vacuum centrifuge. N-terminal amino acid sequence analysis also was performed after cleavage in tryptophan residues with bromonitrophenylsulfenyl skatole (16).
In Situ Hybridization.
A pGEM vector with a 1.6-kb insert of cDNA [EST 05878 (17)] corresponding to the complete coding sequence for the milk fat globule protein gene was obtained from the American Type Culture Collection (ATCC number 84574, HIBAA24 construct). A 122-bp segment (coding for amino acid residues 106–146 of lactadherin) was cut out from the vector by using BamHI and EcoRI restriction enzymes (Boehringer Mannheim). The 122-bp segment was ligated with T4 DNA ligase (Boehringer Mannheim) into a pGEM4Z vector (Promega), cut with the same restriction enzymes. The vector was transfected into DH5α competent Escherichia coli (GIBCO/BRL). Riboprobes were transcribed from the plasmids with a digoxigenin RNA-labeling kit (T7/SP6; Boehringer Mannheim) according to the manufacturer’s instructions. The sense probe was used as a negative control. The probe sequence was verified by DNA sequencing, using the T7 Sequenase 2.0 sequencing kit (Amersham).
Sections were cut and placed on poly-l-lysine-treated slides under RNase-free conditions. In situ hybridization was performed as described (18, 19).
Synthetic Peptides and Antisera.
Two C-terminally amidated peptides with the sequences RLDKQGNFNAWV and NFGSVQFV, corresponding to the N- and C-terminal parts of the amyloid component (lactadherin, positions 245–256 and 286–293, respectively), were synthesized as described (20). The synthetic peptides were linked to keyhole limpet hemocyanin and used as immunogens for antisera production (nos. A172 and A177) in two rabbits as described (21).
ELISA.
ELISA was performed as described (22).
Light and Electron Microscopy.
Tissue sections (6 μm) were cut from the paraffin-embedded aortic tissue, stained with alkaline Congo red, and examined in a polarization microscope for green birefringence (11). For immunohistochemistry, sections were incubated with antisera diluted 1:200 in TBS, pH 7.4, for 16 h at room temperature followed by biotinylated swine anti-rabbit antibodies (Dako) diluted 1:200 and, subsequently, streptavidin (Dako) diluted 1:500. The reaction was visualized with diaminobenzidine.
For electron microscopy, ultrathin sections were immunolabeled as described (23) with antisera A172 and A177 diluted 1:200.
In Vitro Fibril Formation.
The N-terminal dodecapeptide was dissolved in 10% acetic acid (1 mg/ml) and incubated overnight at room temperature. The solution was inspected for gel formation, and 25% ammonia was added dropwise to neutralize the pH of the solution.
The C-terminal medin octapeptide was insoluble in acetic acid but soluble in DMSO after heating to near-boiling temperature. Distilled water was added to a final peptide concentration of 1 mg/ml, and the solution was inspected for gel formation.
Drops of the different peptide solutions were dried on microscopic slides and stained for 9 min with Congo red B solution and then examined for birefringence in polarized light. Drops of the solutions also were placed on coal-stabilized, Formvar-coated copper grids, negatively contrasted with 2% uranyl acetate, and examined in a JEOL 1200 TEM microscope operated at 80 kV.
RESULTS
Purification of the Aortic Medial Amyloid Component.
An amyloid component with an apparent molecular mass of less than 6.5 kDa eluted as a small, yet distinctive peak between 13.6 and 14.8 ml from the Superose 12 HR column (Fig. 1). This peak was exclusively positive when all fractions were tested for reactivity with antibody A172 in ELISA (not shown). RP-HPLC of the low-molecular-mass amyloid material revealed that this material was not homogenous. Several peaks were seen eluting in the vicinity of each other from the C4 column (Fig. 1 Inset). However, all the prominent peaks eluting between 17 and 20% of acetonitrile showed reactivity to antibody A172 when tested in ELISA (not shown).
Figure 1.
Purification of medin from aortic medial amyloid. The enriched amyloid material, dissolved in 70% formic acid, was subjected to size-exclusion chromatography. The material containing most of the immunoreactive material (indicated with a bar) was purified further by RP-HPLC (Inset).
Western Blot of Aortic Medial Amyloid Material.
Western blot analysis with A172 after Tris-Tricine gel separation of the RP-HPLC fractions from the Superose 12-separated material (<30 kDa) showed labeling of a 4.5- to 6-kDa protein band (Fig. 2). There was also labeling of a protein band of a slightly larger molecular mass that was not visible with ordinary Coomassie blue staining.
Figure 2.
(A) Tris-Tricine system separation of material eluting at 16–18% acetonitrile in a RP-HPLC purification of Superose 12 HR-separated material (<30 kDa). (B) Western blot with an antiserum (A172) to a synthetic peptide corresponding to positions 286–293 of lactadherin of the same material.
Amino Acid Sequence Analysis of Aortic Medial Amyloid Material.
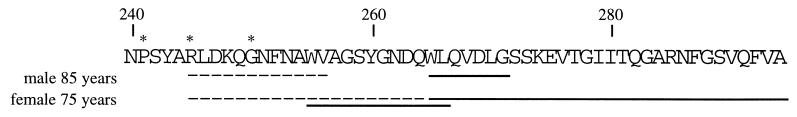
The results of the amino acid sequence analyses of the different aortic materials are shown in Fig. 3. The amyloid fibril protein, which we call medin, had a ragged N terminus, but the main component consisted of a protein of 50 aa residues. The elucidated amino acid sequence is identical with positions 245–294 of lactadherin. Some minor species starting at 241 and 250 of lactadherin also were identified. No amino acid substitution was detected. Partial medin sequences were obtained from an 85-year-old male, and the full medin sequence was obtained from a 75-year-old female. The first tryptic peptide was isolated from forensic material of an individual known only as more than 80 years old.
Figure 3.
The observed amino acid sequence of medin in material purified from aortic medial amyloid of two individuals (male, 85 years old, and female, 75 years old). The medin sequence is identical to lactadherin; positions 245–294. Minor peptides (∗) started at positions 241 and 250 of lactadherin. N-terminal amino acid sequences obtained from the purified proteins are indicated with a dashed line, and sequences obtained after cleavage with BNPS-skatole are indicated with a solid line.
In Vitro Fibril Formation.
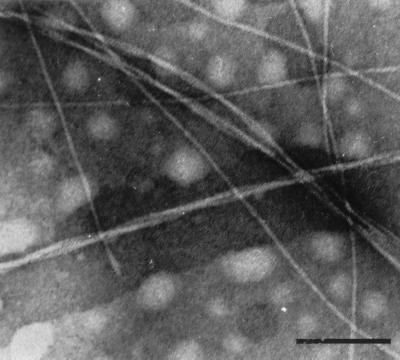
The synthetic peptide with the sequence NFGSVQFV solubilized in DMSO instantly formed a gel-like aggregate when a small aliquot of water was added (final concentration, 1 mg/ml). This aggregate had typical amyloid properties, was Congo red-positive, and showed intense green birefringence under polarized light. Electron microscopy of negatively stained material confirmed the presence of fibrils with an amyloid-like appearance (Fig. 4). The 12-residue N-terminal peptide did not form any fibrils under the conditions tested.
Figure 4.
Amyloid-like fibrils formed by the synthetic octapeptide corresponding to the C terminus of medin. The fibrils were long and about 7 nm in diameter. Prolonged incubation resulted in heavier aggregation, with longer and thicker fibrils consisting of thinner fibrils twisted around or parallel to each other. The fibrils were Congo red-positive and exhibited green birefringence in polarized light. (Bar = 100 nm.)
Immunohistochemical and Immunoelectron Microscopical Findings.
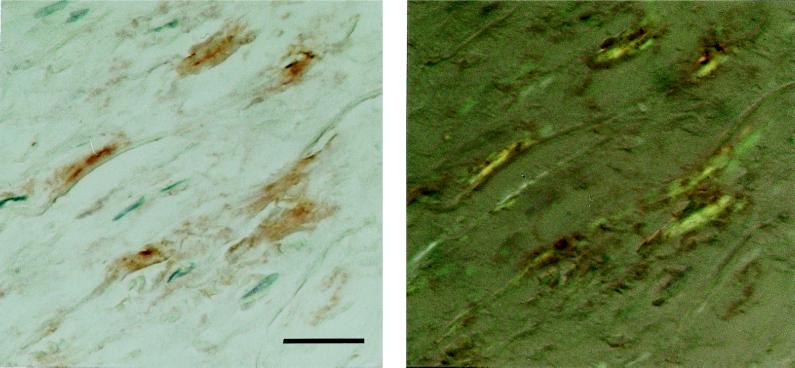
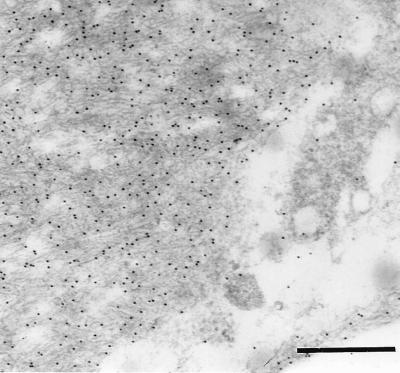
The two antisera (A172 and A177) against synthetic peptides corresponding to two different parts of the medin sequence showed specific labeling that colocalized almost completely with the Congo red-positive amyloid deposits of the aortic media (Fig. 5). Part of the deposits were, to some extent, concentrated close to or around small vessels entering the aortic wall. Some staining also could be found outside amyloid deposits in the media but usually not in aortic intima or adventitia, although in some cases there were scarce amounts of aortic medial amyloid in the intima. Absorption of the antisera with an excess of the corresponding synthetic peptides abolished labeling entirely. Moreover, both antisera A172 and A177 showed specific labeling of the aortic medial amyloid on an ultrastructural level (Fig. 6). When the antisera were absorbed with an excess of synthetic peptide, all specific labeling of the aortic medial amyloid visible in the electron microscope was abolished.
Figure 5.
Section of aortic media, immunolabeled with antibody (A172) against a synthetic peptide corresponding to positions 286–293 of lactadherin. The reaction was visualized with diaminobenzidine (Left). The section subsequently was stained with Congo red and studied in polarized light (Right). The specific immunolabeling of birefringent amyloid is evident. (Bar = 25 μm.)
Figure 6.
Electron microscopical picture of amyloid fibrils in the aortic media, labeled with antiserum A172 to a synthetic peptide corresponding to positions 286–293 of lactadherin. (Bar = 500 nm.)
Expression of Lactadherin in the Aortic Media.
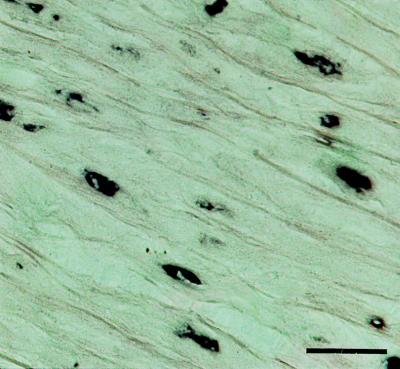
The expression of lactadherin in the aortic media was investigated with the aid of a digoxigenized riboprobe corresponding to the codons translated into amino acids 106–146 of the protein. In situ hybridization on sections of the aorta of a patient with extensive aortic media amyloid resulted in a strong and specific labeling of smooth muscle cells in the aortic media (Fig. 7) and in the endothelium of small medial vessels (not shown). The probe used corresponded to a part of the mRNA that is not included in the amyloid fibril protein. Control sections with the sense probe were negative.
Figure 7.
In situ hybridization showing expression of lactadherin in aortic medial smooth muscle cells. (Bar = 25 μm.)
DISCUSSION
By amino acid sequence analysis of aortic medial amyloid from three individuals and immunolabeling we have shown that the principal amyloid fibril protein in aortic medial amyloid is identical with positions 245–294 of the milk fat globule protein, lactadherin. Thus, the aortic medial amyloid protein is an internal cleavage product of the much larger lactadherin. Internal cleavage products are not uncommon as amyloid fibril proteins and are also seen with, for instance, Aβ derived from AβPP protein (24) and with gelsolin-derived amyloid (25).
This study also showed the presence of lactadherin mRNA in aortic medial cells, strongly suggesting that lactadherin is produced locally in the aortic media. This was an anticipated result considering the strict, local distribution of aortic medial amyloid (6) and also because most localized amyloid forms are derived from precursors expressed by cells close to the deposition sites (26).
Lactadherin is known to be expressed by mammary epithelial cells and has been used as a mammary tumor cell marker (27). To find lactadherin as the precursor protein to localized amyloid in the aortic media therefore was highly unexpected. Earlier studies have focused on lactadherin expression in breast and tumor cells; there is also considerable variability in tissue expression of proteins corresponding to lactadherin in other species [i.e., milk fat globule erythrocyte growth factor 8 (MFG-E8)], although the aorta has not been studied (28, 29).
Lactadherin is a glycosylated, multidomain protein, expressed as a 387-aa-long, single polypeptide chain from which a 23-aa signal peptide is cleaved. The normal function of lactadherin is not known but it has been suggested to be of importance for resistance to rotavirus infection (30, 31). Lactadherin has an epidermal growth factor-like N terminus that contains an integrin αvβ3-binding Arg-Gly-Asp motif that promotes cell adhesion (32). The C-terminal part of lactadherin contains a tandem repeated domain with 43% and 38% identity, respectively, to the similarly positioned C1 and C2 domains of blood coagulation factors V and VIII (27). Medin is positioned within the C2-like domain of lactadherin, which comprises amino acid positions 206–364. The C2 domains are necessary for the procoagulant activity of factors V (33) and VIII, and mutations in the C2 domain of factor VIII may cause hemophilia (34, 35). Given the strong homology between functionally important parts of factors V and VIII and the C1C2 domains of lactadherin, it is tempting to speculate that expression of lactadherin in the aortic media is of importance for the regulation of blood coagulation in the vessel walls. However, further investigation is required to establish this protein’s normal function in the aortic media.
The mechanism for assembly of protein molecules into amyloid fibrils is insufficiently understood. When in sufficient concentration and under certain conditions, amyloid fibril proteins have an intrinsic tendency to form amyloid fibrils by interaction between β-sheets (36). Overexpression of the precursor protein or increased concentration of protein-split products by alternative processing is commonly believed to be involved in amyloidogenesis.
Studies of other amyloid proteins have shown that they contain segments with strong amyloid fibril-formation capacity. We found that the 8-aa-residue-long synthetic peptide corresponding to the C-terminal part of the amyloid component had a strong tendency to form amyloid-like fibrils in vitro. A multiple-alignment model of a family of proteins with domains closely related to the C2 domain suggests three conserved β-strands present within the medin peptide (37). One of the conserved β-strands is positioned in the C-terminal flank of medin, corresponding to the octapeptide susceptible to fibril formation.
Based on comparisons with a predicted model for factor V C2 folding and the conserved, overall three-dimensional folding of homologous domains of lactadherin, a solvent-exposed area available for enzymatic cleavage could exist between residues 206 and 258 (38). Interestingly, a highly abundant, 30-kDa truncated variant, corresponding to the C-terminal 180 aa residues of lactadherin, has been reported in the milk fat globule membrane (39). This variant is N-terminally cleaved at a site in the molecule, with an amino acid sequence very similar to that of the N-terminal cleavage point of the 50-aa residue’s main component of the aortic medial amyloid. In the present study, we found a ragged N terminus that also points to enzymatic cleavage rather than a result of alternative splicing. We propose that the ≈50-aa-residue medin fragment released by cleavage in the lactadherin protein exposes the described β-strand segments (37) that interact with each other to form amyloid fibrils. Normally, these segments may be hidden within the larger protein molecule.
The possible impact of the aortic medial amyloid deposits as well as the mechanisms leading to the amyloid fibril formation of medin must be studied further. These mechanisms may encompass an age-related expression and/or metabolic change of lactadherin as well as local environmental factors affecting the protein conformational properties. In the latter respect, our characterization of aortic medial amyloid also may contribute to the understanding of the increasing number of diseases recognized lately as conformational diseases (40, 41).
The homology of lactadherin with other proteins implicates that this protein is involved in important cell surface-mediated regulatory events. Thus, the amyloid fibril formation may reflect an important age-related primary pathological change.
Acknowledgments
We are indebted to Ulf Hellman for the amino acid sequence analysis of the first tryptic peptide. We thank Marie-Louise Eskilsson and Christer Bergman for skilled technical assistance and Dr. Jon Wall for reading the manuscript. This work was supported by the Swedish Medical Research Council (Project No. 5941) and The Swedish Society for Medical Research.
Footnotes
Nomenclature according to recommendations by the International Nomenclature Committee on Amyloidosis (1).
References
- 1.International Nomenclature Committee on Amyloidosis. Bull W H O. 1993;71:105–108. [Google Scholar]
- 2.Selkoe D J. Neuron. 1991;6:487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- 3.Westermark P, Johnson K H, O’Brien T D, Betsholtz C. Diabetologia. 1992;35:297–303. doi: 10.1007/BF00401195. [DOI] [PubMed] [Google Scholar]
- 4.Westermark P, Mucchiano G, Marthin T, Johnson K H, Sletten K. Am J Pathol. 1995;147:1186–1192. [PMC free article] [PubMed] [Google Scholar]
- 5.Amarzguioui M, Mucchiano G, Häggqvist B, Westermark P, Kavlie A, Sletten K, Prydz H. Biochem Biophys Res Commun. 1998;242:534–539. doi: 10.1006/bbrc.1997.8005. [DOI] [PubMed] [Google Scholar]
- 6.Mucchiano G, Cornwell G G, III, Westermark P. Am J Pathol. 1992;140:871–877. [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz P. Amyloidosis, Cause and Manifestation of Senile Deterioration. Springfield, IL: Thomas; 1970. [Google Scholar]
- 8.Wuyts F L, Vanhuyse V J, Langewouters G J, Decraemer W F, Raman E R, Buyle S. Phys Med Biol. 1995;40:1577–1597. doi: 10.1088/0031-9155/40/10/002. [DOI] [PubMed] [Google Scholar]
- 9.Yankner B A, Mesulam M-M. N Engl J Med. 1991;325:1849–1857. doi: 10.1056/NEJM199112263252605. [DOI] [PubMed] [Google Scholar]
- 10.Schubert D, Behl C, Lesley R, Brack A, Dagusch R, Sagara Y, Kimura H. Proc Natl Acad Sci USA. 1995;92:1989–1993. doi: 10.1073/pnas.92.6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puchtler H, Sweat F, Levine M. J Histochem Cytochem. 1962;10:355–364. [Google Scholar]
- 12.Näslund J, Thyberg J, Tjernberg L O, Wernstedt C, Karlström A R, Bogdanovic N, Gandy S E, Lannfelt L, Terenius L, Nordstedt C. Neuron. 1995;15:219–228. doi: 10.1016/0896-6273(95)90079-9. [DOI] [PubMed] [Google Scholar]
- 13.Schagger H, von Jagow G. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 14.Towbin H, Staehelin T, Gordon J. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engvig J P, Olsen K E, Gislefoss R E, Sletten K, Wahlström O, Westermark P. Scand J Immunol. 1998;48:92–98. doi: 10.1046/j.1365-3083.1998.00352.x. [DOI] [PubMed] [Google Scholar]
- 16.Fontana A. Methods Enzymol. 1972;25:419–423. doi: 10.1016/S0076-6879(72)25037-6. [DOI] [PubMed] [Google Scholar]
- 17.Adams M D, Soares M B, Kerlavage A R, Fields C, Venter J C. Nat Genet. 1993;4:373–380. doi: 10.1038/ng0893-373. [DOI] [PubMed] [Google Scholar]
- 18.Westermark G T, Christmanson L, Terenghi G, Permert J, Betsholtz C, Larsson J, Polak J M, Westermark P. Diabetologia. 1993;36:323–328. doi: 10.1007/BF00400235. [DOI] [PubMed] [Google Scholar]
- 19.Bergenwald C, Westermark G T, Sander B. Cancer Immunol Immunother. 1997;44:335–340. doi: 10.1007/s002620050391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Z, Westermark G T, Li Z-C, Engström U, Westermark P. Diabetologia. 1997;40:793–801. doi: 10.1007/s001250050751. [DOI] [PubMed] [Google Scholar]
- 21.Gustavsson Å, Engström U, Westermark P. Am J Pathol. 1994;144:1301–1311. [PMC free article] [PubMed] [Google Scholar]
- 22.Olsen K E, Sletten K, Westermark P. Am J Clin Pathol. 1999;111:355–362. doi: 10.1093/ajcp/111.3.355. [DOI] [PubMed] [Google Scholar]
- 23.Johan K, Westermark G T, Engström U, Gustavsson Å, Hultman P, Westermark P. Proc Natl Acad Sci USA. 1998;95:2558–2563. doi: 10.1073/pnas.95.5.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evin G, Beyreuther K, Masters C L. Amyloid. 1994;1:263–280. [Google Scholar]
- 25.Maury C P J. J Clin Invest. 1991;87:1195–1199. doi: 10.1172/JCI115118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westermark P. Amyloid. 1994;1:47–60. [Google Scholar]
- 27.Larocca D, Peterson J A, Urrea R, Kuniyoshi J, Bistrain A M, Ceriani R L. Cancer Res. 1991;51:4994–4998. [PubMed] [Google Scholar]
- 28.Aoki N, Ishii T, Ohira S, Yamaguchi Y, Negi M, Adachi T, Nakamura R, Matsuda T. Biochim Biophys Acta. 1997;1334:182–190. doi: 10.1016/s0304-4165(96)00091-8. [DOI] [PubMed] [Google Scholar]
- 29.Ogura K, Nara K, Watanabe Y, Kohno K, Tai T, Sanai Y. Biochem Biophys Res Commun. 1996;225:932–938. doi: 10.1006/bbrc.1996.1274. [DOI] [PubMed] [Google Scholar]
- 30.Yolken R H, Peterson J A, Vonderfecht S L, Fouts E T, Midthun K, Newburg D S. J Clin Invest. 1992;90:1984–1991. doi: 10.1172/JCI116078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newburg D S, Peterson J A, Ruiz-Palacios G M, Matson D O, Morrow A L, Shults J, Guerrero M L, Chaturvedi P, Newburg S O, Scallan C D, et al. Lancet. 1998;351:1160–1164. doi: 10.1016/s0140-6736(97)10322-1. [DOI] [PubMed] [Google Scholar]
- 32.Taylor M R, Couto J R, Scallan C D, Ceriani R L, Peterson J A. DNA Cell Biol. 1997;16:861–869. doi: 10.1089/dna.1997.16.861. [DOI] [PubMed] [Google Scholar]
- 33.Ortel T L, Moore K D, Quinn-Allen M A, Okamura T, Sinclair A J, Lazarchick J, Govindan R, Carmagnol F, Kane W H. Blood. 1998;91:4188–4196. [PubMed] [Google Scholar]
- 34.Levinson B, Janco R, Phillips J, III, Gitschier J. Nucleic Acids Res. 1987;15:9797–9805. doi: 10.1093/nar/15.23.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan V, Chan T K, Tong T M, Todd D. Blood. 1989;74:2688–2691. [PubMed] [Google Scholar]
- 36.Jarrett J T, Lansbury P T. Cell. 1993;73:1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- 37.Baumgartner S, Hofmann K, Chiquet-Ehrismann R, Bucher P. Protein Sci. 1998;7:1626–1631. doi: 10.1002/pro.5560070717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villoutreix B O, Bucher P, Hofmann K, Baumgartner S, Dahlbäck B. J Mol Model. 1998;4:268–275. [Google Scholar]
- 39.Giuffrida M G, Cavaletto M, Giunta C, Conti A, Godovac-Zimmermann J. J Protein Chem. 1998;17:143–148. doi: 10.1023/a:1022531500370. [DOI] [PubMed] [Google Scholar]
- 40.Carrell R W, Lomas D A. Lancet. 1997;350:134–138. doi: 10.1016/S0140-6736(97)02073-4. [DOI] [PubMed] [Google Scholar]
- 41.Wisniewski T, Aucouturier P, Soto C, Frangione B. Amyloid. 1998;5:212–224. doi: 10.3109/13506129809003848. [DOI] [PubMed] [Google Scholar]