Abstract
We designed, synthesized, and identified JE-2147, an allophenylnorstatine-containing dipeptide HIV protease inhibitor (PI), which is potent against a wide spectrum of HIV-1, HIV-2, simian immunodeficiency virus, and various clinical HIV-1 strains in vitro. Drug-resistant clinical HIV-1 strains, isolated from seven patients who had failed 9–11 different anti-HIV therapeutics after 32–83 months, had a variety of drug-resistance-related amino acid substitutions and were highly and invariably resistant to all of the currently available anti-HIV agents. JE-2147 was, however, extremely potent against all such drug-resistant strains, with IC50 values ranging from 13–41 nM (<2-fold changes in IC50 compared with that of wild-type HIV-1). The emergence of JE-2147-resistant HIV-1 variants in vitro was substantially delayed compared with that of HIV-1 resistant to another allophenylnorstatine-containing compound, KNI-272, and other related PIs. Structural analysis revealed that the presence of a flexible P2′ moiety is important for the potency of JE-2147 toward wild-type and mutant viruses. These data suggest that the use of flexible components may open a new avenue for designing PIs that resist the emergence of PI-resistant HIV-1. Further development of JE-2147 for treating patients harboring multi-PI-resistant HIV-1 is warranted.
In the last decade, a number of agents have become available for the treatment of individuals infected with HIV-1 (1), and combination chemotherapy with reverse transcriptase inhibitors and protease inhibitors (PIs) has been shown to suppress HIV-1 replication to an undetectable level in patients. However, accumulated data indicate that the development of HIV-1 variants with reduced susceptibility to reverse transcriptase inhibitors and PIs is directly related to clinical deterioration in those individuals receiving such therapy (2–7). Thus, the development of novel compounds that are active against multidrug-resistant HIV-1 variants and prevent or delay the emergence of resistant HIV-1 variants is urgently needed.
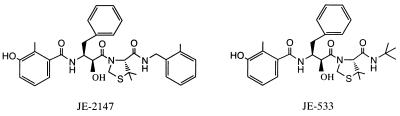
We designed and synthesized JE-2147 and its related compounds, which represent a class of transition-state mimetic dipeptide HIV protease inhibitors containing a unique unnatural amino acid, allophenylnorstatine [(2S, 3S)-3-amino-2-hydroxy-4-phenylbutyric acid], with a hydroxymethylcarbonyl isostere as an active moiety (8) (Fig. 1). In the present study, we report on the antiviral activities of JE-2147, its related compounds, and other PIs against a panel of HIV-1 and HIV-2 strains and highly PI-resistant clinical HIV-1 variants in vitro. We found that JE-2147 completely suppressed all HIV-1 and HIV-2 strains as well as clinical HIV-1 variants that were highly resistant against all currently available PIs including saquinavir (SQV), ritonavir (RTV), indinavir (IDV), nelfinavir (NFV), amprenavir (APV), and two experimental PIs, KNI-272 (9) and RS-346, which has the same structure as the experimental drug ABT-378 (10). We also selected JE-2147-resistant HIV-1 variants in vitro and determined the property of those variants. Using modeling and x-ray crystallography, we explain the resistance profile of JE-2147 and advance the hypothesis that flexible regions of JE-2147 are important for its potency against multi-PI-resistant HIV-1.
Figure 1.
Structures of JE-2147 and JE-533.
METHODS
Patients.
Patients with HIV-1 infection having a viral burden >50,000 RNA copies/ml by the branched-DNA assay (Chiron) were enrolled into an open-labeled clinical study of APV and abacavir (ABC) (Glaxo Wellcome) at the Clinical Center, National Institutes of Health. Seven patients were randomly chosen from enrollees who had failed the amprenavir-plus-ABC therapy and other existing regimens. The clinical characteristics of the patients at the time of inclusion in this study are illustrated in Table 1.
Table 1.
Profile of patients
| Patient | Sex | Age | CD4+, cells/mm3 | HIV-1 RNA*, copies/ml | Months on anti-viral therapy | Anti-HIV therapeutics received |
|---|---|---|---|---|---|---|
| 1 | M | 50 | 361 | 246,700 | 64 | AZT, ddl, ddC, d4T, 3TC, ABC, IDV, RTV, SQV, APV, DLV |
| 2 | M | 38 | 3 | 553,700 | 46 | AZT, ddl, ddC, d4T, 3TC, ABC, IDV, RTV, SQV, APV |
| 3 | M | 44 | 108 | 42,610 | 39 | AZT, ddl, ddC, d4T, 3TC, ABC, IDV, RTV, SQV, APV |
| 4 | M | 40 | 568 | 59,990 | 81 | AZT, ddl, ddC, d4T, 3TC, ABC, IDV, SQV, APV, DLV |
| 5 | M | 38 | 297 | 52,760 | 32 | AZT, ddl, ddC, d4T, 3TC, ABC, IDV, SQV, APV |
| 6 | M | 36 | 554 | 15,730 | 34 | AZT, ddl, ddC, d4T, 3TC, ABC, IDV, SQV, APV |
| 7 | M | 45 | 335 | 6,547 | 83 | AZT, ddl, ddC, d4T, 3TC, ABC, IDV, SQV, RTV, APV |
ddC, zalcitabine; d4T, stavudine; ABC, abacavir (1592U89); APV, amprenavir (VX-478/141W94); DLV, delavirdine; M, male.
Serum levels of HIV RNA were determined by using a branched-DNA assay.
Cells, Viruses, and Antiviral Agents.
MT-2 cells were grown in an RPMI medium 1640-based culture medium supplemented with 15% FCS (HyClone), 50 units/ml of penicillin, and 50 μg/ml of streptomycin. The following HIV-1 viruses, which have been established elsewhere, were used for a drug susceptibility assay: HIV-1LAI (11), HIV-1Ba-L (12), HIV-2EHO (13), and HIV-1ERS104pre [a clinical HIV-1 strain isolated from a drug-naive AIDS patient (5)]. JE-533 and JE-2147, both of which are dipeptidic compounds containing allophenylnorstatine (Fig. 1), were designed and synthesized as described elsewhere (8). The properties of KNI-272 also containing allophenylnorstatine are described elsewhere (9). 3′-azido-2′,-dideoxythymidine (AZT or zidovudine) and 2′,3′-dideoxyinosine (ddI or didanosine) were purchased from Sigma and Calbiochem, respectively. 3′-thiacytidine (3TC or lamivudine) was a gift from R. F. Schinazi (Emory University, Atlanta). SQV and RTV were provided by Roche Products (Welwyn Garden City, U.K.) and Abbott, respectively. APV and ABC were gifts from Glaxo Wellcome. NFV and IDV were provided by Japan Energy (Tokyo). RS-346, which has the same structure as the experimental drug ABT-378, was synthesized by using published methods (10).
Virus Isolation.
Clinical HIV-1 strains were isolated as described (7) by culturing peripheral blood mononuclear cells (PBMCs) obtained from AIDS patients who had been treated with 9–11 anti-HIV-1 drugs for 32–83 months (Table 1). HIV-1 isolates were passaged once or twice in phytohemagglutinin (PHA)-stimulated PBMCs, and the culture supernatants served as a source of infectious virions. HIV-1-containing supernatants thus obtained were stored at −70°C until use.
Phenotypic Assays.
To determine whether HIV-1 isolates were syncytium-inducing (SI) or non-syncytium-inducing (NSI) phenotype, an aliquot of viral stock supernatant, containing 100 50% tissue culture infectious dose (TCID50) of the virus, was cultured with MT-2 cells (105) in 12-well culture plates. Cultures were maintained for 4 weeks and were examined under an inverted microscope for syncytium formation twice a week, as described by Liesnard et al. (14). The sensitivities of HIV-1LAI, HIV-1Ba-L, HIV-2EHO, and primary HIV-1 isolates against various drugs were determined as described with minor modifications (7, 15). In brief, MT-2 cells (2 × 104/ml) were exposed to 100 TCID50 of HIV-1LAI, HIV-1Ba-L, or HIV-2EHO in the presence of various concentrations of drugs in 96-well microculture plates and were incubated at 37°C for 7 days, and IC50s were determined by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. All assays were performed in duplicate (SD < 25%). PHA-PBMCs (1 × 106/ml) were exposed to 50 TCID50 of each primary HIV-1 isolate in the presence or absence of various concentrations of drugs in 10-fold serial dilutions in 96-well microculture plates. All assays were performed in triplicate. The amounts of p24 antigen produced by the cells were determined on day 7 of culture by using a commercially available radioimmunoassay kit (DuPont/NEN). Drug concentrations that resulted in 50% inhibition (IC50s) were determined by comparison with the p24 production level in drug-free control cell cultures (7, 15).
Determination of Nucleotide Sequences.
The primers used for the first PCR of the reverse transcriptase (RT)-encoding region were SA009 (5′-TTT AAA TTT TCC CAT TAG CCC TAT-3′) and SA015 (5′-ACT CCA TGT ACT GGT TCT TTT AGA-3′). The primers for the second PCR of the RT region were 881MF (5′-TGT AAA ACG ACG GCC AGT CCC GGG ATG GAT GGC CCA AAA GTT AAA CAA-3′) and 891MR (5′-CAG GAA ACA GCT ATG ACC GCT AGC CCA ATT CAA TTT TCC CAC TAA-3′), which included the M13 forward and reverse sequences, respectively. The primers for the first PCR of the protease (PR)-encoding region were PR-1 (5′-GAAG CAG GAG CCG ATA GAC AAG-3′) and PR-2 (5′-CAG TCT CAA TAG GGC TAA TGG G-3′). The primers for the second PCR of the PR region were PR3M (5′-TGT AAA ACG ACG GCC AGT GCC GAT AGA CAA GGA ACT GTA T-3′) and PR4M (5′-CAG GAA ACA GCT ATG ACC TAC TGG TAC AGT CTC AAT AGG G-3′), which included the M13 forward and reverse sequences, respectively. The nucleotide sequence of the various HIV-1 strains was determined by using cellular proviral DNA obtained from the infected PHA-PBMCs. The first PCR reaction mixture consisted of 5 μl of the proviral DNA solution, 50 mM KCl, 10 mM Tris⋅HCl (pH 8.3), 2 mM MgCl2, 0.01% gelatin, 0.2 mM dNTP, 2.0 units of Taq DNA polymerase (Perkin–Elmer), and 12.5 pmol of each of the first PCR primers in a total volume of 50 μl. The PCR conditions used were an initial 2 min at 94°C followed by 35 cycles of 30 sec at 94°C, 30 sec at 58°C, and 1 min at 72°C, with a final 8-min extension at 72°C. A 1-μl aliquot of the first PCR products then was amplified for 35 cycles, using the second PCR primers under the same reaction conditions. Second-round PCR products were directly sequenced by using both M13 forward and reverse dye-labeled primers on an Applied Biosystems Model 373 automated DNA sequencer.
Molecular Cloning.
Three primary isolates were subjected to molecular cloning followed by sequence determination. The first-round PCR products (1 μl) of the RT and PR region were used directly in the second round of PCR with primers 881NMF (5′-ATG GAT GGC CCA AAA GTT AAA CAA-3′) and 891NMR (5′-CTG GCT AGC CCA ATT CAA TTT TCC CAC-3′); and PR3NM (5′-GCC GAT AGA CAA GGA ACT GTA T-3′) and PR4NM (3′-TAC TGG TAC AGT CTC AAT AGG G-3′), respectively. The PCR conditions used were the same as described above. The second-round PCR products were purified with spin columns (PCR select III, 5 Prime → 3 Prime), were cloned directly (PCR-Script Amp Cloning Kit), and were sequenced as described above.
Generation of JE-2147-Resistant HIV-1 in Vitro.
MT-2 cells (5 × 105) were exposed to 500 TCID50 of HIV-1LAI or HIV-1NL4–3/I84V and were cultured in the presence of KNI-272, JE-533, or JE-2147 at an initial concentration of 0.01–0.03 μM. When the virus began to propagate in the presence of the drug, the compound concentration was increased as described (15). Titers of passaged viral stocks were determined by using MT-2 cells, and the profiles of cross-resistance of selected viral stocks resistant to PIs were determined by using the MTT assay as described above. Proviral DNAs from the lysates of infected cells from several passages also were sequenced.
Crystallographical Analyses.
Cloning, overexpression, and purification of wild-type HIV protease have been described (16). Crystals of inhibitor wild-type HIV protease complex was grown by the hanging drop vapor diffusion method. Details of structural determination are found elsewhere (R.K. and J.E., unpublished work).
RESULTS
In Vitro Drug Sensitivity of HIV-1 Laboratory Isolates.
We designed, synthesized, and tested ≈300 compounds for antiviral activity against HIV-1 in vitro. Among them, JE-533 and JE-2147 were most potent against a wide spectrum of HIV-1 in vitro and were further examined in this study. We first tested JE-533 and JE-2147 against a lymphotropic HIV-1LAI and macrophage-tropic HIV-1Ba-L in PHA-PBMCs, and HIV-2EHO in MT-2 cells (Table 2). Antiviral activity of all PIs examined was comparable against both HIV-1LAI and HIV-1Ba-L. The antiviral activity of RTV, APV, and JE-533 against HIV-2EHO was 8- to 29-fold less potent compared with that against HIV-1LAI whereas IDV, SQV, NFV, and JE-2147 exhibited antiviral activity against HIV-2EHO comparable to that against other HIV-1 strains.
Table 2.
Sensitivities of HIV-1LAI, HIV-1Ba-L, and HIV-2EHO to various PIs, including JE-533 and JE-2147
| Virus | Phenotype | Cells | IC50, μM
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| RTV | IDV | APV | SQV | NFV | JE-533 | JE-2147 | |||
| HIV-1LAI | SI | PBMC* | 0.047 | 0.019 | 0.019 | 0.0054 | 0.0090 | 0.015 | 0.044 |
| HIV-1Ba-L | NSI | PBMC* | 0.018 | 0.0056 | 0.014 | 0.0037 | 0.0056 | 0.034 | 0.024 |
| HIV-1LAI | SI | MT-2† | 0.041 | 0.019 | 0.046 | 0.023 | 0.0050 | 0.020 | 0.035 |
| HIV-2EHO | SI | MT-2† | 0.37 | 0.006 | 0.55 | 0.0036 | 0.0050 | 0.50 | 0.047 |
IC50s were determined by using PHA-PBMCs exposed to HIV-1 (50 TCID50 dose, 105 PBMCs) and by using the inhibition of p24 Gag protein production as an endpoint on day 7 of culture. All assays were conducted in triplicate. The values shown are representatives of two or three separate experiments.
MT-2 cells (2 × 103) were exposed to 100 TCID50 of HIV-1LAI or HIV-2EHO and were cultured in the presence of various concentrations of reverse transcriptase inhibitors and PIs, and the IC50s were determined by using MTT assay on day 7 of culture. All assays were conducted in duplicate, and the values shown are representative of two or three repeated experiments.
Deduced Amino Acid Sequences of HIV-1 Isolated from Heavily Treated Patients.
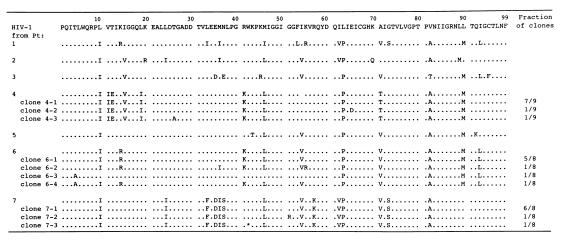
Proviral DNAs obtained from PHA-PBMCs exposed to the patients’ virus in the absence of drugs were directly sequenced. As illustrated in Fig. 2, 6–10 amino acid substitutions that have been reportedly associated with resistance against various PIs (RTV, IDV, SQV, APV, and NFV) (1–3, 17) were identified. Mutations seen in many of the isolates included L10I (7/7 isolates), M46I/L (6/7), I54V (5/7), L63P (7/7), A71V/T (6/7), V82A/T (7/7), and L90M (5/7). It is noteworthy that no isolates had an I84V mutation.
Figure 2.
Sequence analysis of the protease-encoding region of primary HIV-1 isolated from seven heavily drug-experienced patients. Deduced amino acid sequences (from direct sequencing) of the protease of primary HIV-1 isolates obtained from seven heavily drug-experienced patients are shown. Amino acid sequences of the protease of molecular clones generated from HIV-1 isolated from patients 4, 6 and 7, also are shown. The fractions of clones containing indicated mutations are shown on the right. The consensus B sequence cited from the Los Alamos database (24) is shown at the top. Identity with this sequence at individual amino acid positions is indicated by dots. An asterisk indicates a stop codon.
The RT sequences also were determined. HIV-1 isolates from four patients (patients 2, 5, 6, and 7) had the AZT resistance-associated M41L, D67N, L210W, and T215Y mutations (17) whereas three other patients (patients 1, 3, and 4) had K70R, T215F, and K219F (data not shown). The 3TC resistance-associated M184V mutation (17) was seen in all isolates. Three primary isolates (patients 4, 6, and 7) were cloned and sequenced. We found that the RT and PR sequences of the cloned HIV-1s were almost identical to those with the direct sequencing. It is of note that up to 15 amino acid substitutions were identified in a single protease in a number of clones (Fig. 2).
In Vitro Activity of JE-533 and JE-2147 Against Highly PI-Resistant Clinical HIV-1 Strains.
Clinical HIV-1 strains were isolated and propagated by culturing MT-2 cells with PBMCs from AIDS patients who received 9–11 anti-HIV-1 drugs in the past 32–83 months (Table 1). HIV-1 strains isolated from patients 1–4 induced syncytia (SI) whereas those isolated from patients 5–7 did not (NSI). As shown in Table 3, all isolates had a high level of resistance to AZT (19- to 400-fold), 3TC (>5000-fold), and ABC (16- to 32-fold) and a moderate level of resistance to ddI (3- to 7-fold) compared with those against a wild-type clinical isolate (HIV-1ERS104pre). All seven isolates were highly resistant to RTV and IDV by >17- to >71-fold as compared with those against HIV-1ERS104pre. Six of the seven primary isolates were also highly resistant to APV (8- to 32-fold) and NFV (6- to >56-fold). With regard to SQV, four of the seven isolates were highly resistant (13- to 31-fold), and three of them showed a moderate insensitivity (3- or 4-fold increase in IC50). All clinical HIV-1 strains were resistant to the experimental drug RS-346 (ABT-378) (10- to 33-fold). Five of the seven isolates were highly resistant to JE-533 (12- to 39-fold) whereas the other two had a moderate level of resistance (8- and 9-fold increase in IC50). In contrast, JE-2147 completely suppressed the replication of all seven isolates, with IC50 values ranging 0.013–0.041 μM (<2-fold changes in IC50 compared with that against HIV-1ERS104pre) (Table 3).
Table 3.
Sensitivities of HIV-1 isolated from heavily drug-experienced individuals to reverse transcriptase inhibitors and PIs, including JE-533 and JE-2147
| Patient/isolate code | Phenotype† | IC50*, μM
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase inhibitors
|
PIs
|
||||||||||||
| AZT | ddl | 3TC | ABC | RTV | IDV | APV | SQV | NFV | RS-346¶ | JE-533 | JE-2147 | ||
| HIV-1wt‡ | SI | 0.0015 | 0.61 | 0.02 | 0.18 | 0.032 | 0.014 | 0.013 | 0.0095 | 0.018 | 0.030 | 0.020 | 0.027 |
| (1×) | (1×) | (1×) | (1×) | (1×) | (1×) | (1×) | (1×) | (1×) | (1×) | (1×) | (1×) | ||
| 1 | SI | 0.37 | 3.0 | >100 | 2.8 | >1 | >1 | 0.29 | 0.29 | >1 | 0.39 | 0.45 | 0.024 |
| (247×)§ | (5×) | (>5,000×) | (16×) | (>31×) | (>71×) | (22×) | (31×) | (>56×) | (13×) | (23×) | (1×) | ||
| 2 | SI | 0.029 | 2.7 | 100 | 4.3 | >1 | 0.60 | 0.24 | 0.035 | 0.3 | 0.41 | 0.15 | 0.013 |
| (19×) | (4×) | (>5,000×) | (24×) | (>31×) | (43×) | (18×) | (4×) | (17×) | (14×) | (8×) | (1×) | ||
| 3 | SI | 0.6 | 4.0 | >100 | 3.2 | >1 | 0.46 | 0.33 | 0.036 | >1 | 0.7 | 0.78 | 0.019 |
| (400×) | (7×) | (>5,000×) | (18×) | (>31×) | (33×) | (25×) | (4×) | (>56×) | (23×) | (39×) | (1×) | ||
| 4 | SI | 0.54 | 4.0 | >100 | 3.3 | >1 | 0.24 | 0.4 | 0.033 | 0.1 | 0.31 | 0.17 | 0.03 |
| (360×) | (7×) | (>5,000×) | (18×) | (>31×) | (17×) | (31×) | (3×) | (6×) | (10×) | (9×) | (1×) | ||
| 5 | NSI | 0.22 | 2.3 | >100 | 2.8 | >1 | >1 | 0.28 | 0.24 | 0.33 | 0.31 | 0.33 | 0.021 |
| (147×) | (4×) | (>5,000×) | (16×) | (>31×) | (>71×) | (22×) | (25×) | (18×) | (10×) | (17×) | (1×) | ||
| 6 | NSI | 0.34 | 3.0 | >100 | 4.5 | >1 | 0.37 | 0.11 | 0.19 | >1 | 0.32 | 0.23 | 0.015 |
| (227×) | (5×) | (>5,000×) | (25×) | (>31×) | (26×) | (8×) | (20×) | (>56×) | (11×) | (12×) | (1×) | ||
| 7 | NSI | 0.53 | 2.1 | >100 | 5.7 | >1 | >1 | 0.42 | 0.12 | >1 | 0.98 | 0.40 | 0.041 |
| (353×) | (3×) | (>5,000×) | (32×) | (>31×) | (>71×) | (32×) | (13×) | (>56×) | (33×) | (20×) | (2×) | ||
IC50s were determined by using PHA-PBMC, exposed to HIV-1 (50 TCID50 dose, 105 PBMCs) as target cells and by using the inhibition of p24 Gag protein production as an endpoint. All assays were conducted in triplicate. The values shown are representatives of two or three separate experiments.
MT-2 cells (105) were exposed to an aliquot of viral stock supernatant containing 100 TCID50 in 12-well culture plates. Cultures were maintained for 4 weeks and were examined for syncytium formation twice a week.
HIV-1wt used was a clinical HIV-1 strain, HIV-1ERS104pre (5).
Numbers in parentheses represent fold changes of IC50 values against each isolate compared to IC50 values against HIV-1wt.
RS-346 has the same structure as ABT-378.
Selection for JE-2147-Resistant HIV-1 in Vitro.
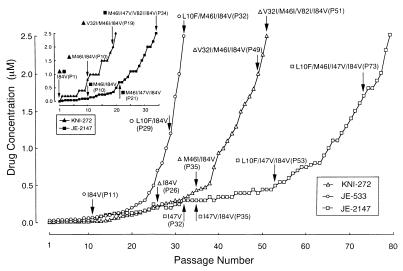
To induce resistant HIV-1 variants, HIV-1LAI was propagated in MT-2 cells in the presence of increasing concentrations of KNI-272, JE-533, or JE-2147. The virus was initially exposed to 0.01 μM KNI-272, 0.03 μM JE-533, or 0.01 μM JE-2147. Thirty-two and fifty-one passages were required to reach 2.5 μM (≈100-fold increase) for JE-533 and KNI-272, respectively, whereas 79 passages were needed to reach the same concentration for JE-2147 (Fig. 3). These results suggest that the emergence of JE-2147-resistant HIV-1 variants in vitro was substantially delayed as compared with that of KNI-272- and JE-533-resistant HIV-1 variants. HIV-1 was of a wild-type sequence in the PR-encoding region until passage 31, but an I47V substitution was identified at passage 32 (HIV-1JE-2147-P32) and beyond (Fig. 3). Viruses from various passages with JE-2147 were titered, and their susceptibility to JE-2147 was determined (Table 4). HIV-1JE-2147-P32 remained as sensitive to JE-2147 as the parental HIV-1LAI. HIV-1JE-2147-P35, which had acquired I47V/I84V, was only 2-fold more resistant to JE-2147 than was HIV-1LAI. However, HIV-1JE-2147-P53 carrying L10F/I47V/I84V and HIV-1JE-2147-P73 carrying L10F/M46I/I47V/I84V were substantially resistant to JE-2147 by 19-fold and 28-fold, respectively, compared with HIV-1LAI (Table 4). HIV-1JE-2147-P73 remained sensitive to SQV but was moderately resistant to IDV and RTV (3- to 9-fold increase in IC50) and, as expected, was insensitive to JE-2147, which HIV-1 was selected to (Table 5). HIV-1 passaged in the presence of increasing concentrations of JE-533 and KNI-272 (HIV-1JE-533-P32 and HIV-1KNI-272-P51, respectively) showed comparable resistance profiles, although these resistant variants lacked the I47V substitution (Table 5).
Figure 3.
In vitro selection of HIV-1 variants resistant to KNI-272, JE-533, or JE-2147. HIV-1LAI or HIV-1NL4–3/I84V was passaged in the presence of increasing concentrations of KNI-272, JE-533, or JE-2147 in MT-2 cells. The selection was carried out for a total of 20–79 passages with compound concentrations ranging from 0.01 to 2.5 μM. Proviral DNAs from the lysates of infected cells from several passages were sequenced. Drug susceptibility of HIV-1 propagated in the presence of JE-2147, JE-533, and KNI-272 at passages 73, 32, and 51, respectively, is illustrated in Table 5.
Table 4.
In vitro selection and phenotypic susceptibility of HIV-1 passaged with JE-2147
| Virus | Amino acid substitutions | JE-2147 concentration when examined | IC50, μM* | Fold resistance† |
|---|---|---|---|---|
| HIV-1LAI | Wild-type | None | 0.031 | 1 |
| HIV-1JE-2147-P32 | I47V | 0.3 | 0.041 | 1 |
| HIV-1JE-2147-P35 | I47V/I84V | 0.3 | 0.064 | 2 |
| HIV-1JE-2147-P53 | L10F/I47V/I84V | 0.5 | 0.60 | 19 |
| HIV-1JE-2147-P73 | L10F/M46I/I47V/I84V | 1.6 | 0.86 | 28 |
Sensitivity of each viral preparation selected to JE-214X was determined by using MT-2 cells and the MTT assay.
Fold resistance relative to the IC50 against HIV-1LAI.
Table 5.
Drug sensitivity of HIV-1 passaged in the presence of JE-2147, JE-533, or KNI-272
| Virus | Amino acid substitutions in PR | IC50, μM*
|
|||||
|---|---|---|---|---|---|---|---|
| JE-2147 | JE-533 | KNI-272 | SQV | IDV | RTV | ||
| HIV-1LAI | Wild-type | 0.031 (1×) | 0.033 (1×) | 0.045 (1×) | 0.020 (1×) | 0.025 (1×) | 0.036 (1×) |
| HIV-1JE-2147-P73 | L10F/M46I/I47V/I84V | 0.86 (28×) | 0.11 (3×) | 0.32 (7×) | 0.030 (1×) | 0.072 (3×) | 0.34 (9×) |
| HIV-1JE-533-P32 | L10F/K45I/M46I/I84V | 0.095 (3×) | 2.5 (76×) | 0.50 (11×) | 0.032 (1×) | 0.20 (8×) | 0.45 (13×) |
| HIV-1KNI-272-P51 | V32I/M46I/V82I/I84V | 0.040 (1×) | 0.42 (13×) | 1.2 (27×) | 0.025 (1×) | 0.041 (2×) | 0.31 (9×) |
MT-2 cells (2 × 103) were exposed to 100 TCID50 of HIV-1LAI, HIV-1JE-2147-P73, HIV-1JE-533-P32, or HIV-1KNI-272-P51 and were cultured in the presence of various concentrations of RTV, IDV, SQV, KNI-272, JE-533, and JE-2147. The IC50 values were determined by using the MTT assay on day 7 of culture. All assays were conducted in triplicate. The values shown are representatives of two or three separate experiments. The numbers in parentheses represent fold changes compared to IC50s against HIV-1LAI.
To potentially expedite the selection of JE-2147-resistant HIV-1 variants, an infectious clone carrying an I84V mutation (HIV-1I84V) also was exposed to JE-2147 (Fig. 3 Inset). In this experiment, 34 passages were required to reach 2.5 μM for JE-2147, and the resultant HIV-1 had M46I/I47V/V82I/I84V mutations and proved to be highly resistant to JE-2147 (IC50 was 0.9 μM, 33-fold). When HIV-1I84V was exposed to KNI-272, two other mutations appeared by passage 19. These data suggest that introduction of certain mutations in HIV-1 can expedite the induction of resistant HIV-1 variants in vitro and that M46I/I47V/I84V is highly associated with viral resistance to JE-2147.
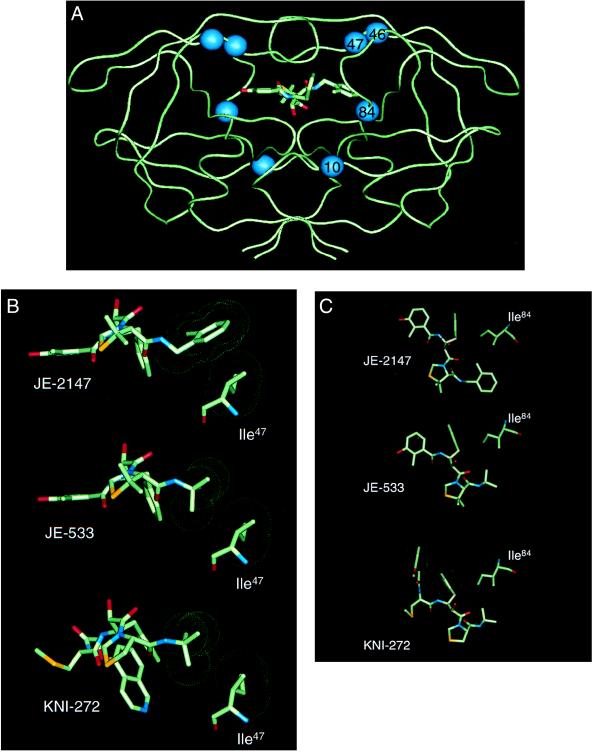
Structural Impact of Resistance Mutations on JE-2147 Interactions.
The locations of residues that participated in resistance to JE-2147 are shown in Fig. 4A. Three residues, Ile47, Val82, and Ile84, are active site residues whose side chains may be involved in making direct contacts with inhibitor atoms (18). In addition, Ile47 along with Met46 is located on the flap of HIV protease. Leu10 is located distal to the active site and cannot directly influence the active site structure. The side chain of Ile47 is localized to the S2′ subsite, where it contacts P2′ side chains of inhibitors (18). The crystal structure of the parent compound, KNI-272, shows that there are no interactions between Ile47 and the P2′ t-butylamide substituent (19). The same is true for JE-533, which has a similar P2′ group to KNI-272. On the other hand, JE-2147 contains a methylbenzamide group at the P2′ position. Preliminary crystallographic results indicate that Ile47 makes numerous close van der Waals contacts with this substituent (Fig. 4B). Thus, the mutation of Ile to the smaller Val residue is predicted to lead to reduced contacts with the methylbenzamide group and may explain the importance of this mutation for viral resistance to JE-2147. The I47V substitution is reportedly associated with viral resistance to APV and ABT-378 (10) as well, although this substitution does not confer detectable phenotypic resistance, as was also true in the case of JE-2147-resistant HIV-1 (Table 4).
Figure 4.
Crystallographic analysis of enzyme-inhibitor complexes. (A) Backbone representation of the dimeric HIV-1 protease with JE-2147-resistance-associated amino acid substitutions. A model of JE-2147 bound in the active site is shown. Location of residues involved in drug resistance attributable to the in vitro selection with JE-2147 are indicated by spheres. Two of these residues lie within the active site (residues 47 and 84) whereas the other two residues lie outside of the active site (residues 10 and 46). (B) Interactions between Ile47 of wild-type HIV protease and JE-2147, JE-533, or KNI-272. Neither JE-533 or KNI-272 have contacts with Ile47 whereas JE-2147 has a tight interaction with this residue. (C) Interactions between Ile84 of wild-type HIV protease and JE-2147, JE-533, or KNI-272. All three inhibitors make tight interactions with this residue. van der Waals surfaces are shown as dot surfaces; atoms are color-coded by type. The structure of KNI-272 is based on the published crystal structure [Protein Data Bank ID code 1HPX (19)]. The structures of JE-533 and JE-2147 are from unpublished crystal structures (R.K. and J.E., unpublished work).
We also examined the effect of the I84V substitution, which is important for JE-2147 resistance. The I84V mutation results in widespread biochemical cross-resistance to PIs (16) and is commonly observed in clinical resistance pathways, particularly with RTV (20). The I84V substitution has been shown to be important in the development of drug-resistance to KNI-272 (K.Y. and H. Mitsuya, unpublished work). This residue, along with the symmetry-related I84′, makes interactions across S2/S1′ and S1/S2′ subsites, respectively (18). Structural analysis with KNI-272 indicated that the I84V substitution will lead to loss of contacts with the P1′ thioproline P1 phenyl ring (19). Because both JE-533 and JE-2147 possess identical P1 and P1′ groups, they are expected to be adversely affected by the I84V substitution (Fig. 4C). However, crystallographic analysis revealed that JE-2147 undergoes a conformational rearrangement resulting in more favorable packing interactions elsewhere in the active site region (R.K. and J.E., unpublished work). Thus, on structural grounds, the I84V mutant is predicted to be less deleterious for JE-2147 binding than for either KNI-272 or JE-533, consistent with the phenotypic resistance results.
DISCUSSION
It is noteworthy that IC50 values of all PIs tested in this study, including new PIs under development, such as APV against clinical strains (e.g., those isolated from patients 1, 5, and 7), were ≥13-fold greater than those against HIV-1wt (Table 3). All clinical HIV-1 isolates were also resistant to RS-346 (ABT-378). JE-2147 was, however, uniformly potent; its IC50 values against all of the primary strains examined remained essentially the same (≤2-fold difference).
A variety of drug resistance mechanisms are at work with protease inhibitors (21). The most important ones from a purely drug-binding standpoint are mutations in the active site of the enzyme that lead to loss of anti-HIV-1 activity of the inhibitor. Resistance mutations have been seen in each of the unique specificity pockets, S3, S2, S1, and by symmetry, S1′, S2′, and S3′, whereas only a subset of all residues that constitute a particular subsite is preferentially selected in response to a particular drug. When HIV-1 was exposed to JE-2147, two resistance-associated mutations were found to map to the active site region: I84V and I47V (Fig. 4). These two subsite mutations effect hydrophobic and van der Waals interactions and can be considered to be “packing” mutants somewhat analogous to hydrophobic mutations in a protein core (19, 22). When HIV-1 containing the I84V mutation was subsequently exposed to JE-2147, a third active site mutation, V82I, also appeared. This mutation has been shown to be particularly effective in conferring resistance when combined with a second active site mutation, such as V32I (23). However, HIV-1 exposed to JE-2147 did not acquire the latter substitution (Fig. 3), possibly reflecting structural or biochemical constraints on the fitness of particular V32I-containing combinations.
In the present study, the wild-type protease was shown to have close contacts with JE-2147 at Ile47. In response to selection pressure from JE-2147, HIV-1 first mutated Ile47 to Val whereas the structurally related compounds JE-533 and KNI-272 selected other initial mutations (Figs. 3 and 4). The I47V substitution alone, however, showed no detectable resistance in conventional phenotypic assays (Table 4). Similar results with this mutation were found with APV and ABT-378, both of which select this mutant (10). It remains to be asked why the I47V mutation, which clearly leads to a structural prediction of reduced potency, does not give rise to a measurable reduction in antiviral potency. The fact that viruses containing this mutation can be propagated in the presence of 0.3 μM JE-2147, which is 10-fold above the IC50 for wild-type HIV-1LAI, suggests that there may be a sensitivity limitation of the phenotypic assay. In addition, the reduced contact with I47V may result in a minor change in inhibitor binding, which, although providing the mutant virus with a fitness advantage in the presence of drug, may be too small to be detected in standard assays. The L10F mutation also occurred in the presence of JE-2147. This mutation might act in concert with active site mutations by compensating for a functional deficit caused by the latter.
There are several strategies for the design of new protease inhibitors that may inhibit drug-resistant HIV-1 variants. One approach is to use accumulated knowledge of the three-dimensional structures of mutant HIV proteases to design inhibitors that bind tightly to key mutant enzymes. Another strategy is to design compounds that are more flexible in their interactions with the enzyme, so that the inhibitors can adapt to the structural changes in the mutant enzymes. Notably, JE-2147 has a P2′ benzylamide group that has two rotatable bonds between the amide and the phenyl ring. In contrast, both KNI-272 and JE-533 have a t-butylamide at this position, with only one rotatable bond. Furthermore, because of the symmetric nature of the t-butyl group and the limitations on energetically preferred torsion angles, rotation about the N-C bond in the latter compounds will not provide much variation in interacting surface to the enzyme. The above considerations suggest that the incorporation of a flexible P2′ substituent may be responsible for the superior potency of JE-2147 against drug-resistant HIV-1 variants. Preliminary crystallographic investigations of mutant and wild-type complexes with JE-2147 and JE-533 support this hypothesis (R.K. and J.E., unpublished work). From a pure binding standpoint, the design of inhibitors carrying molecularly flexible groups that can adapt to structurally altered active sites of mutant viral enzymes may represent a feasible strategy and open a new avenue to develop therapeutics that prevent or delay the emergence of drug-resistant HIV-1 variants.
Acknowledgments
We thank Drs. Robert E. Wittes, Carmen Allegra, and Edison Liu for helpful discussion and Dr. Ram Randad for preparing RS-346 (ABT-378). This work was supported in part by a Research for the Future Program (Grant JSPS-RFTF 97L00705) of Japan Society for the Promotion of Science and a Grant for Promotion of AIDS Research from the Ministry of Health and Welfare of Japan.
ABBREVIATIONS
- PI
protease inhibitor
- SQV
saquinavir
- RTV
ritonavir
- IDV
indinavir
- NFV
nelfinavir, APV, amprenavir
- ABC
abacavir
- AZT
3′-azido-2′,-dideoxythymidine
- ddI
2′,3′-dideoxyinosine
- 3TC
3′-thiacytidine
- PBMC
peripheral blood mononuclear cell
- PHA
phytohemagglutinin
- SI
syncytium-inducing
- NSI
non-syncytium-inducing
- TCID50
50% tissue culture infectious dose
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- RT
reverse transcriptase
- PR
protease
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Mitsuya H, Erickson J. In: Textbook of AIDS Medicine. Merigan T C, Bartlet J G, Bolognesi D, editors. Baltimore: Williams & Wilkins; 1999. pp. 751–780. [Google Scholar]
- 2.Condra J H, Schleif W A, Blahy O M, Gabryelski L J, Graham D J, Quintero J C, Rhodes A, Robbins H L, Roth E, Shivaprakash M, et al. Nature (London) 1995;374:569–571. doi: 10.1038/374569a0. [DOI] [PubMed] [Google Scholar]
- 3.Molla A, Korneyeva M, Gao Q, Vasavanonda S, Schipper P J, Mo H M, Markowitz M, Chernyavskiy T, Niu P, Lyons N, et al. Nat Med. 1996;2:760–766. doi: 10.1038/nm0796-760. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Imamichi H, Imamichi T, Lane H, Falloon J, Vasudevachari M, Salzman N. J Virol. 1997;71:6662–6670. doi: 10.1128/jvi.71.9.6662-6670.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shirasaka T, Yarchoan R, O’Brien M C, Husson R N, Anderson B D, Kojima E, Shimada T, Broder S, Mitsuya H. Proc Natl Acad Sci USA. 1993;90:562–566. doi: 10.1073/pnas.90.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shafer R W, Kozal M J, Winters M A, Iversen A K, Katzenstein D A, Ragni M V, Meyer W A R, Gupta P, Rasheed S, et al. J Infect Dis. 1994;169:722–729. doi: 10.1093/infdis/169.4.722. [DOI] [PubMed] [Google Scholar]
- 7.Shirasaka T, Kavlick M F, Ueno T, Gao W Y, Kojima E, Alcaide M L, Chokekijchai S, Roy B M, Arnold E, Yarchoan R, et al. Proc Natl Acad Sci USA. 1995;92:2398–2402. doi: 10.1073/pnas.92.6.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mimoto T, Kato R, Takaku H, Nojima S, Terashima K, Misawa S, Fukazawa T, Ueno T, Sato H, Shintani M, et al. J Med Chem. 1999;42:1789–1802. doi: 10.1021/jm980637h. [DOI] [PubMed] [Google Scholar]
- 9.Kageyama S, Mimoto T, Murakawa Y, Nomizu M, Ford H, Jr, Shirasaka T, Gulnick S, Erickson J, Takada K, Hayashi H, et al. Antimicrob Agents Chemother. 1993;37:810–817. doi: 10.1128/aac.37.4.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrillo A, Stewart K, Sham H, Norbeck D, Kohlbrenner W, Leonard J, Kempf D, Molla A. J Virol. 1998;72:7532–7541. doi: 10.1128/jvi.72.9.7532-7541.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clavel F, Guetard D, Brun-Vezinet F, Chamaret S, Rey M A, Santos-Ferreira M O, Laurent A G, Dauguet C, Katlama C, Rouzious C, et al. Science. 1986;233:343–346. doi: 10.1126/science.2425430. [DOI] [PubMed] [Google Scholar]
- 12.Gartner S, Markovits P, Markovitz D M, Kaplan M H, Gallo R C. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 13.Rey-Cuille M, Galabru J, Laurent-Crawford A, Krust B M L, Hovanessian A. Virology. 1994;202:471–476. doi: 10.1006/viro.1994.1364. [DOI] [PubMed] [Google Scholar]
- 14.Liesnard C, Delforge M, Tchetcheroff M, De Maertelaer V, Farber C, Van Vooren J. J Virol Methods. 1997;64:137–145. doi: 10.1016/s0166-0934(96)02152-0. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka M, Srinivas R V, Ueno T, Kavlick M F, Hui F K, Fridland A, Driscoll J S, Mitsuya H. Antimicrob Agents Chemother. 1997;41:1313–1318. doi: 10.1128/aac.41.6.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gulnik S V, Suvorov L I, Liu B, Yu B, Anderson B, Mitsuya H, Erickson J W. Biochemistry. 1995;34:9282–9287. doi: 10.1021/bi00029a002. [DOI] [PubMed] [Google Scholar]
- 17.Schinazi R F, Larder B A, Mellors J W. Int Antiviral News. 1997;5:129–142. [Google Scholar]
- 18.Wlodawer A, Erickson J W. Annu Rev Biochem. 1993;62:543–585. doi: 10.1146/annurev.bi.62.070193.002551. [DOI] [PubMed] [Google Scholar]
- 19.Baldwin E T, Bhat T N, Gulnik S, Liu B, Topol I A, Kiso Y, Mimoto T, Mitsuya H, Erickson J W. Structure (London) 1995;3:581–590. doi: 10.1016/s0969-2126(01)00192-7. [DOI] [PubMed] [Google Scholar]
- 20.Xie, D., Gulnik, S. & Erickson, J. W. (1999) Vit. Horm. (San Diego), in press.
- 21.Erickson J W. Nat Struct Biol. 1995;2:523–529. doi: 10.1038/nsb0795-523. [DOI] [PubMed] [Google Scholar]
- 22.Markowitz M, Mo H, Kempf D J, Norbeck D W, Bhat T N, Erickson J W, Ho D D. J Virol. 1995;69:701–706. doi: 10.1128/jvi.69.2.701-706.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan A H, Michael S F, Wehbie R S, Knigge M F, Paul D A, Everitt L, Kempf D J, Norbeck D W, Erickson J W, Swanstrom R. Proc Natl Acad Sci USA. 1994;91:5597–5601. doi: 10.1073/pnas.91.12.5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myers G, Korber B, Hahn B H, Jeang K-T, Mellors J W, McCutchan F E, Henderson L E, Pavlakis G N. Human Retroviruses and AIDS. Los Alamos, NM: Los Alamos National Laboratory; 1995. [Google Scholar]