Abstract
A human disorder caused by mutation in nonmuscle actin has not been reported. We report here a variant of nonmuscle actin in a female patient with recurrent infections, photosensitivity, and mental retardation. She also had abnormalities in neutrophil chemotaxis, superoxide production, and membrane potential response. Two-dimensional PAGE analysis of proteins from neutrophils and other cell types from this patient demonstrated a unique protein spot migrating at 42 kDa with pI shifted slightly to neutral relative to normal β- and γ-actin. Digestion peptide mapping and Western blotting showed this spot to be an abnormal actin. A full-length cDNA library was constructed by using mRNA from patient’s cells and cDNA encoding the mutant β-actin molecule was identified by an in vitro translation method. Sequencing of the clones demonstrated a G-1174 to A substitution, predicting a glutamic acid-364 to lysine substitution in β-actin and eliminating a HinfI DNase restriction site found in normal β-actin sequence. By HinfI digestion and by sequencing, the mutation in one allele of patient’s genomic DNA was confirmed. Though no defect in cell-free polymerization of actin was detected, this defect lies in a domain important for binding to profilin and other actin-regulatory molecules. In fact, the mutant actin bound to profilin less efficiently than normal actin did. Heterozygous expression of mutant β-actin in neutrophils and other cells of this patient may act in a dominant–negative fashion to adversely affect cellular activities dependent on the function of nonmuscle actin.
Phagocytes such as neutrophils play profound roles in defense against microbial infection in the way that they migrate, ingest pathogens, generate reactive oxygen species, and release granule contents to phagosomes and to extracellular medium. The mechanism underlying these activities is associated with, and may require, rapid reorganization of the cytoskeleton. This involves a cyclic process that includes polymerization of G-actin to F-actin, crosslinking of the filaments to form the supramolecular assemblies anchored to membranes, and depolymerization of the F-actin to G-actin (1).
Various actin-binding proteins are known to regulate the dynamics of actin cytoskeleton. Schleicher et al. (2) classified these binding proteins into three categories. The first group of proteins, such as profilin and cofilin, are those that bind to monomeric G-actin and thus reversibly remove polymerizable actin from the equilibrium with F-actin. The second group contains the end-binding proteins or capping proteins, such as gelsolin and cap32/34. They inhibit further addition of monomers to actin polymers and keep filaments short. The third group contains proteins that bind along the side of actin filaments and either stabilize the filaments, crosslink filaments to form three-dimensional networks, anchor filaments to membranes, or work as motors. Binding proteins such as tropomyosin, filamin, spectrin, and myosin are examples of this family.
A variety of functional studies of actin and its associated proteins have been performed by using spontaneous or genetically engineered actin mutants in yeast, in amoebae of slime mold Dictyostelium, in the protozoan Tetrahymena, and in the indirect flight muscle of fruit fly Drosophila (3). However, in mammalian cells, the only studies of mutant actin that have been reported are from an in vitro model using a chemically transformed human fibroblast cell line (4). Although one clinical case was reported in which the patient’s symptoms were attributed to a defect in actin polymerization in neutrophils (5–7), a specific structural defect in the actin molecule has not been identified.
We now report here a human disorder caused by mutation in nonmuscle actin. The patient had repeated infections and other symptoms and had impairment of neutrophil functions. Neutrophils and other cells from the patient were found to contain abnormal β-actin together with normal β-actin. By sequencing the cDNA that encoded the abnormal actin, we found a single nucleotide substitution. The predicted mutation site in the actin molecule is in a binding site for certain actin-associated proteins such as profilin.
MATERIALS AND METHODS
Patient History.
At the time of initiation of these studies, this female patient was 12 years old, with a history of photosensitivity, recurrent stomatitis, and keratoconjunctivitis since age 3, thrombocytopenia (3 × 104/μl) at age 8, and tuberculous pneumonia, recurrent otitis media, iritis, furunculosis, and a polyathralgia with positive rheumatoid factor since age 9. She exhibited moderate intellectual impairment (IQ score: 54) and had short stature (141.5 cm: −1.4 SD). Laboratory studies showed leukopenia, hyper-IgE, and persistent high levels of C-reactive protein. Serum IgG, IgA, IgM, and IgE levels, respectively, were 3,200 mg/dl, 520 mg/dl, 173 mg/dl, and 2,526 units/ml. Serum protein was 8.8 g/dl, where percents of albumin, α1-, α2-, β-, and γ-globulin were 46.4, 4.3, 10.5, 8.5, and 29.8, respectively. Hemolytic complement activity was in normal range. Leukocyte counts at her admission were 3–5 × 103 cells/μl, where percent of segmented neutrophils, band form neutrophils, lymphocytes, and monocytes, respectively, were 5–15, 30–35, 30, and 20. The band forms were always the most abundant type of leukocyte in her blood samples, and their ratio to other cells did not change after steroid or epinephrine challenge. There was poor influx of leukocytes to skin window test sites. Subsets of lymphocytes were within the normal range, except OK-Ia1-positive cells, where percent of OKT-3-, OKT-4-, OKT-8-, OKT-10-, OK-Ia1-, and Leu-7-positive cells, respectively, were 64.1–73.9, 19.5–30.5, 10.2–17.4, 21.4–24.8, 54.8–54.4, and 2.6–7.3. Their responses to mitogens were normal. Mild anemia was found (437 × 104 RBC/μl; 9.5 g Hb/dl). Continuous thrombocytopenia was detected (8–10 × 104 cells/μl), with an increase of megakaryocytes in her bone marrow preparation. Otherwise, bone marrow showed normal cellularity, and percents of myeloblasts, myelocytes, metamyelocytes, band forms, segments, lymphocytes, and erythroblasts were 1.5, 2.5, 14.5, 45, 5.5, 13.5, and 13, respectively. Intradermal skin test gave a positive reaction to Candida antigen, purified protein derivitative, phytohemagglutinin, and streptokinase–streptodornase. Poryphirin metabolites and urinary amino acids were all within normal level. Erythema appeared on her arm after <30 sec of exposure of UV-B, though viability of her cultured fibroblast after exposure to UV light was normal. She was euthyroid, and her TSH value was normal. By 15 years of age, she had developed cardiomegaly, hepatomegaly, and hypothyroidism. At that time, she presented with persistent fevers, and, despite intensive therapy, she died from septicemia.
Preparation of Neutrophils and Other Cells.
Neutrophils and mononuclear cells were obtained as described by Böyum (8), and platelets were derived by centrifugation at 800 × g for 20 minutes from platelet-rich plasma. Biopsy-derived skin fibroblasts were grown in RPMI medium 1640 supplemented with 20% newborn calf serum. Epstein–Barr virus-transformed B cell lines were established from patient blood lymphocytes according to a method described previously (9).
Neutrophil Functions.
In vivo and in vitro assessment of phagocyte chemotaxis was determined by Rebuck’s skin window test (10) and Boyden chamber assay (11), respectively. Neutrophil superoxide generation was measured as superoxide dismutase-inhibitable reduction of ferricytochrome c (12). Membrane potential change of the cells (106 cells/ml) was assayed by using Di-O-C5-[3] (Nippon Kankoh Sikiso Kenkyusho, Okayama, Japan) (13).
Two-Dimensional (2D) PAGE Analysis and Immunoblotting.
2D PAGE analysis of proteins in cells was performed according to O’Farrel (14), with a slight modification. In brief, cells (2 × 107) were solubilized in 400 μl of lysis buffer containing 9.5 M urea, 2% NP40, 2% Pharmalyte (1.6% pH 5–8 and 0.4% pH 3–10), and 5% mercaptoethanol. Isoelectric focusing was performed at 500 V for 24 hours followed by the 2D SDS/PAGE. Proteins on the gel were stained with 2D Silver Stain II (Dai-ichi Pure Chemicals, Tokyo). For immunoblotting, proteins on the gel were transferred to poly(vinylidene difluoride) membrane and were stained with the anti-actin monoclonal antibody N350 (Amersham Pharmacia).
Construction of cDNA Library.
To construct a full-length cDNA library, the capping method (15) was used. In brief, mRNAs with Poly(A) tails were extracted from the patient’s B cell line and were purified by using poly (T) cellulose column. To remove the 5′ phosphate residues from degraded mRNAs, the samples were incubated with alkaline phosphatase. Then, the cap of an intact mRNA was removed by a tobacco acid pyrophosphatase treatment. To the generated 5′ phosphate group, a chimeric DNA-RNA oligonucleotide linker was ligated by using T4 RNA ligase. The resulting DNA-capped mRNA was annealed with dT-tailed pKA1 vector primer and was used as the template for cDNA synthesis by Super Script II reverse transcriptase (GIBCO/BRL). The product was digested by EcoRI at the EcoRI sites in the linker and in the vector primer I and was ligated with Escherichia coli DNA ligase. The second strand synthesis of the self-ligated product was performed by using RNase H and E. coli DNA pol. This library is designated as pKA1 library.
Cloning and Characterization of Actin cDNAs.
By using the pKA1 library, the patient’s actin cDNA clones were selected by hybridization with control γ-actin probe (15). The clones containing the EcoRI-NotI inserts with >1.8 kbp were selected and confirmed to contain the start codon by sequencing of 5′ region of the inserts. For in vitro translation of the actin cDNAs, TNT T7 coupled reticulocyte lysate system (Promega) was used. The translation products were labeled with [35S]methionine (Amersham Pharmacia) and were analyzed with 2D PAGE.
Genomic Analysis of β-Actin Gene.
Genomic DNA was isolated from the patient’s B cell line. Thirty cycles of amplification by PCR using sets of primers shown in Table 1 (25 pmol/50 μl) were performed in thermal cycler PHC-3 (Techne Laboratories, Cambridge, U.K.) under the following conditions; denaturation at 96°C for 45 seconds, annealing at 56°C for 45 seconds, and extension at 72°C for 1 minute. The reaction mixture contained 50 mM Tris⋅HCl (pH 7.5), 50 mM KCl, 1.5 mM MgCl2, 0.2 mM dNTPs, 50 ng genomic DNA, and 5 units of recombinant Taq Polymerase and the primers. The products were separated with 2% agarose gel electrophoresis and were extracted from the gel by using Geneclean II kit (Funakoshi, Tokyo). The isolated product was treated with 5 units of HinfI restriction enzyme (Takara, Tokyo) at 37°C for 1 hour and was subjected to PAGE using 5% polyacrylamide gel. The PCR product was cloned into pCRII plasmid by using TA cloning kit (Invitrogen, San Diego) and was sequenced.
Table 1.
Oligonucleotide primers for cDNA sequencing and genomic amplification and sequencing
| No. | Primer sequence | Position, bp |
|---|---|---|
| DNA sequencing | ||
| s-1 | TGTAAAACGACGGCCAGT | pKA1 primer |
| s-2 | ATCGAGCACGGCATCGTCAC | 252–271 |
| s-3 | GGACCTGACTGACTACCTCA | 590–609 |
| s-4 | TGGAGAAGAGCTACGAG | 748–764 |
| s-5 | ACCATGTACCCTGGCATTGC | 951–970 |
| s-6 | CAAGTACTCCGTGTGGATCG | 1,046–1,065 |
| s-7 | TGACTTAGTTGCGTTACACC | 1,178–1,197 |
| s-8 | AACGGTGAAGGTGACAGCAG | 1,320–1,339 |
| s-9 | TGCATTGTTACAGGAAGTCC | 1,433–1,452 |
| s-10 | AACTTGAGATGTATGAAGGC | 1,656–1,675 |
| a-1 | CACATAGGAATCCTTCTGAC | 184–203 |
| a-2 | GAAGGTCTCAAACATGATCT | 403–422 |
| a-3 | TGAGGTAGTCAGTCAGGTCC | 590–609 |
| a-4 | CTCGTAGCTCTTCTCCAGGG | 744–763 |
| a-5 | CGGAACCGCTCATTGCCAAT | 789–808 |
| a-6 | GCAATGCCAGGGTACATGGT | 951–970 |
| a-7 | GGTGTAACGCAACTAAGTC | 1,179–1,197 |
| a-8 | GGACTTCCTGTAACAATGC | 1,434–1,452 |
| a-9 | CCTTCATACATCTCAAGTTG | 1,655–1,674 |
| a-10 | ATTAACCCTCACTAAAGGGA | T3 primer |
| Genomic amplification and sequencing | ||
| S-1 | CTCCATCATGAAGTGTGACGTG | 2,600–2,621 |
| S-2 | ACATCCGCAAAGACCTGTAC | 2,623–2,642 |
| A-1 | GTGTAACGCAACTAAGTCAT | 3,008–3,027 |
DNA Sequencing.
Cycle sequence method was done by using Taq Dye Deoxy Terminator Cycle Sequencing Kit (Perkin–Elmer), and the sequencing was performed with an autosequencer (ABI model 373A, Perkin–Elmer) by using primers listed in Table 1.
Tests for Polymerizing Ability of Mutant Actin and for Binding Ability to Profilin.
The patient’s B cell line (1.0 × 108) was labeled with 1 mCi [35S]methionine in 10 ml of a methionine-free RPMI medium 1640 supplemented with 10% dialyzed FCS at 37°C for 12 hours under 5% CO2. Actin polymerization study was done according to the method described by Gordon et al. for Acanthamoeba actin (16).
Binding of actin to profilin was performed by using glutathione S-transferase fusion protein with human profilin [glutathione S-transferase (GST)–profilin] coupled to glutathione Sepharose 4B beads (Amersham Pharmacia). For expression of the GST-profilin, human profilin I cDNA inserted to the BamHI site of pGEX-2T (17) was used. The 35S-labeled translation products of normal and mutant actin cDNAs and the GST-profilin-beads, both in a buffer containing 40 mM Tris⋅HCl (pH 7.5), 150 mM NaCl, 1 mM ethylendiaminetetraacetic acid, 0.5% Triton X-100, and 1 mM phenylmethanesulfonyl fluoride, were mixed and incubated at 4°C for 2 hours. As a control, the GST beads instead of the GST-profilin beads were used. From the beads, normal and mutant actin bond to profilin were recovered and analyzed by SDS/PAGE.
RESULTS
Functional Analyses of Patient’s Neutrophils.
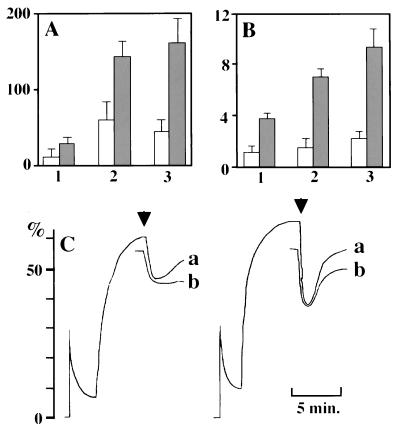
As seen in Fig. 1 A, B, and C, respectively, the patient’s neutrophils demonstrated depressed chemotactic responses, reduced stimulated superoxide generation, and weaker formyl methionyl-leucyl-phenylalanine (fMLP)-induced depolarization/repolarization relative to neutrophils from a normal control.
Figure 1.
Functional defects in neutrophils from the patient. (A) Chemotactic ability assessed by Boyden chamber method toward 10−8 (1) or 10−9 M fMLP (2) or 10% zymosan-activated serum (3). Vertical numbers indicate numbers of cells in a field. We used different concentrations of fMLP because we found previously that concentrations of fMLP necessary for maximum chemotactic ability were different between the cells from cord and adult blood. Chemotactic ability of cord blood neutrophils was 70–80% of normal adults at an optimum concentration of fMLP for adult cells. In each concentration, the cells from the patient exhibited significant chemotaxis deficiency even compared with the ability of the cells from cord blood. (B) Maximum rate of superoxide generation assayed on exposure to 2 × 10−7 M fMLP (1), 2 × 10−7 M fMLP plus 2.5 μg/ml cytochalasin D (2), or 2.5 μg/ml cytochalasin D plus 50 μg/ml Con A (3). Vertical numbers indicate nanomoles of superoxide/minute/106 neutrophils. Open column, patient; closed column, control. (C) Relative change of fluorescence as an indication of depolarization and repolarization of patient (Left) and control neutrophils (Right). Di-O-C5 [3] was used as the lipophilic probe, and 2 × 10−7 M fMLP was used as a stimulus (arrows). Neutrophils immediately after isolation (a) and those stored on ice for 1 hour (b) were used.
Characterization of Protein Profiles in Patient’s Neutrophils.
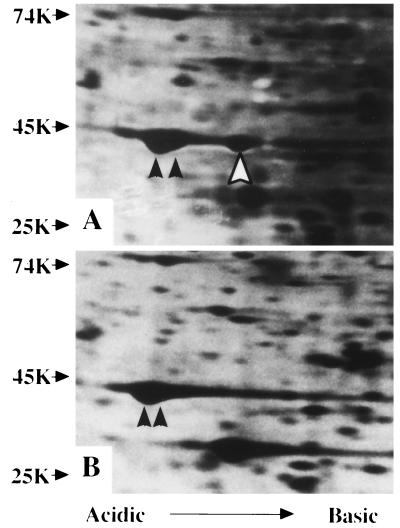
The protein profiles in patient’s neutrophils were compared with those of control (18) in 2D PAGE. As shown in Fig. 2, a unique spot that had the same molecular mass as β- and γ-actin molecules (42 kDa) but a slightly shifted pI value to cathode was found in the patient’s specimen. Such a spot was not found in neutrophils from control and in leukocytes from >100 individuals (ref. 18; data not shown) but did exist in leukocytes, platelets, fibroblasts, and the B cell line from the patient (data not shown).
Figure 2.
Analysis of proteins from the patient’s (A) and normal control neutrophils (B) with 2D PAGE. Closed arrows indicate β- (left) and γ-actin (right), and an open arrow indicates a unique spot. Molecular weight markers are indicated on the left.
The protein in the spot was reacted with a monoclonal anti-actin antibody and it’s fingerprint peptide graphic analysis after proteolytic digestion demonstrated high correlation with actin (data not shown). From these results, we concluded the patient’s cells had an abnormal, mutant actin together with normal β- and γ-actin. The amount of the protein expressed in the cells was comparable to that of β-actin molecules.
Cloning of Actin cDNAs and Their in Vitro Translation Analysis.
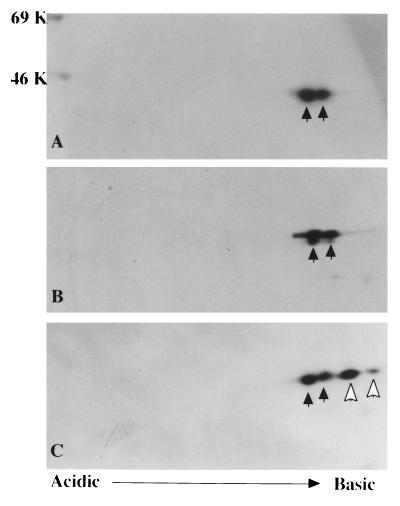
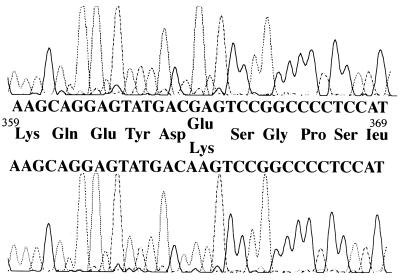
To determine the mutation responsible for generation of this abnormal actin, a full-length cDNA library constructed from the patient’s B cell line was used. By hybridization with control γ-actin cDNA and partial sequencing of 5′ends, eight clones, each encoding β- or γ-actin full-length cDNA, were selected. To identify which of these 16 clones coded the mutant actin, in vitro translation products of these clones were analyzed by 2D PAGE. As expected, the in vitro translation yielded both acetylated and nonacetylated products (19), and the products of five β-actin and all eight γ-actin clones migrated identically with normal β- and γ-actin protein (Fig. 3 A and B). However, the translation products of three β-actin-encoded clones showed a set of spots that had shifted pI value but the same molecular mass as control (Fig. 3C). DNA sequencing of these three clones demonstrated a mutation consisting of a G-1174-to-A base pair substitution (Fig. 4), predicting a glutamic acid 364-to-lysine substitution in the protein product.
Figure 3.
In vitro translation products of actin cDNAs cloned from patient’s cells and normal control cells. Products of control β-actin (A), γ-actin (B), and the mutant β-actin cDNAs together with control β-actin molecules (C) were subjected to 2D PAGE. Closed and open arrows indicate products of normal and mutant actin cDNA, respectively. Each set of spots includes both nonacetylated (right) and acetylated actin (left). Molecular weight markers are indicated on the left.
Figure 4.
Sequences around 1,174 of the actin cDNAs. The sense sequences and predicted amino acid sequences of control (Upper) and patient’s specimen (Lower) are shown.
Analysis of Patient’s Genomic DNA.
To confirm this mutation in the patient’s actin gene, genomic DNA was extracted from patient’s B cell line and was subjected to PCR to amplify the fragments predicted to contain the mutation site using the primers shown in Table 1. By using one set of these primers (S-2/A-1), the PCR fragment generated from control genomic DNA yielded products of 293 and 405 bp. By sequencing, the 293-bp product was found to be of a pseudo-actin gene that lacked intron 5, and the 405-bp product was of the true actin genomic sequence containing a part of exon 5, intron 5, and a part of exon 6, as expected.
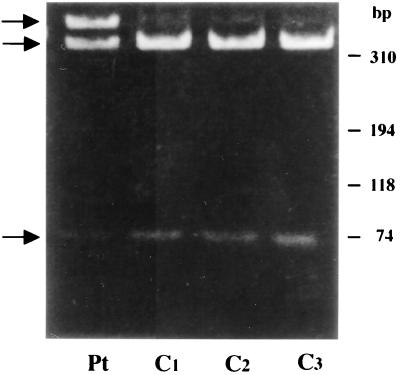
Because a HinfI DNase restriction site in the normal sequence was eliminated in the mutant sequence, the 405-bp fragment was isolated and digested with HinfI restriction enzyme. As shown in Fig. 5, both undigested 405-bp fragment and the 65- and 340-bp digestion fragments were generated from patient genomic DNA whereas only the 65- and 340-bp fragments were generated from normal control DNA. Similar results were obtained by the analysis of PCR product amplified by using another primer set (S-1/A-1). The results indicate that the normal and mutant alleles are present in the patient cells. The substitution of G-2962 with A in exon 6 was confirmed by sequencing the mutant 405-bp PCR product derived from the patient (20).
Figure 5.
HinfI digestion profile of PCR-generated, 405-bp fragment of β-actin gene from the patient (Pt) and Control (C1–3). Arrows from top to bottom indicate the position of 405-, 340-, and 65-bp fragments in this order. Mobility of each marker is indicated on the right.
Polymerizing Ability of the Mutant Actin and Binding Ability to Profilin.
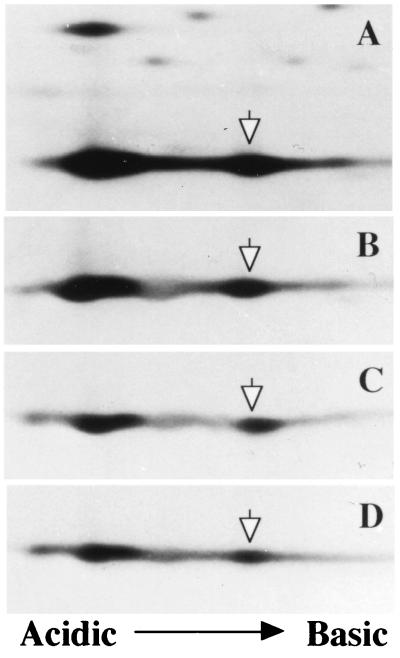
To know whether the mutant actin molecule has polymerizing ability as normal actin, a cytosole fraction from 35S-labeled patient’s B cell line was exposed to polymerization conditions (high magnesium and potassium salts) and was centrifuged. A portion of the resulting precipitate was analyzed by 2D PAGE. The other portion was dialyzed to cause depolymerization. These procedures were repeated three times. As shown in Fig. 6, the ratios of the mutant and normal actin molecules did not change at each cycle of polymerization, suggesting that the mutant actin can polymeized and depolymerize similar to the normal actin.
Figure 6.
Copolymerization of mutant and normal actin molecules from the patient’ B cell line. The cytosolic fraction from [35S]methionine-labeled patient’s cell (A) were polymerized and depolymerized as described in the text. The procedures were repeated three times, and, each time after centrifugation, the precipitate was analyzed by 2D PAGE (B–D). Open arrows indicate the mutant actin.
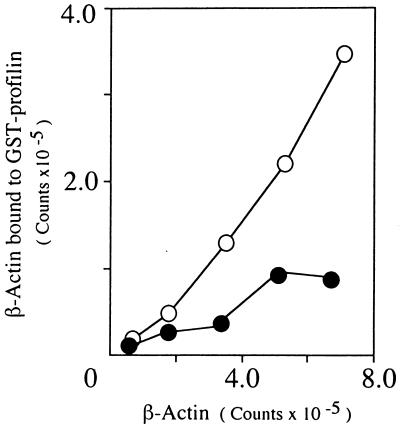
Binding ability of mutant actin to human profilin was examined by using GST-profilin beads. As shown in Fig. 7, efficiency of binding of mutant actin to profilin was significantly lower than that of normal actin.
Figure 7.
Binding of actin to profilin. The 35S-labeled translation products of normal and mutant actin cDNAs were incubated with the GST-profilin-beads or GST-beads at 4°C for 2 hours. Recovered labeled actin from the beads was subjected to SDS/PAGE. The gel was dried and exposed to Fuji imaging plate, and radioactivity of the actin band was determined by using Bas 2000 (Fuji). The values after subtraction of those obtained by using GST-beads (<10%) are plotted. Open circle, control β-actin; closed circle, mutant β-actin.
DISCUSSION
In the present study, we showed that the patient suffering from recurrent infections had the mutant β-actin in her neutrophils and other cells together with normal β-actin. An actin mutation has not been observed previously in vertebrates, except for a mutation induced in a chemically transformed human fibroblast cell line (4). By the analysis of the mutant actin cDNA, a substitution of G-1174 to A was found in the coding region of the β-actin molecule, and the mutation was confirmed by examining her genomic DNA. This mutation corresponds to the amino acid transition of glutamic acid-364 to lysine (E364K).
The primary structure of actin is highly conserved in various organisms, implying high biological constraint on structure. The mutation site is situated in a region of actin that is highly conserved. An amino acid substitution at the identical site was reported in actin molecules of Saccharomyces cerevisiae (E364A) (21), Dictyostelium (E364H) (22), and in the indirect flight muscle-specific Act88F actin in Drosophila melanogaster (E364K) (3). Like the mutant actin in our patient, the flight muscle mutant actin occurring in the flightless strain of Drosophila was relatively stable and demonstrated normal in vitro polymerization.
Glutamic acid at residue 364 and its vicinity in actin molecule (residues 355–375) has been shown to be important for binding several proteins that modify behavior of the molecule. These proteins include profilin, myosin, cofilin, α-actinin, and gelsolin (23). In particular, the mutation in the flight muscle actin of Drosophila was shown to affect binding of this mutant actin to profilin. We also showed in this paper that the mutation affected binding of the mutant actin to proflilin. Among the actin binding proteins, profilin, which forms a 1:1 complex with actin, has precisely been studied by mutation (24), cross-linking (25), and atomic models derived from crystallized complex with actin (26). Specifically, Acanthamoeba actin residue E364 can be shown to form a chemical cross-link with lysine-115 of Acanthamoeba profilin (25) and bovine actin residue E364 to form an intermolecular electrostatic interaction with lysine-125 of bovine profilin, which is homologous to lysine-115 of Acanthamoeba profilin in sequence alignment (25).
The mutant actin in the indirect flight muscle was antimorphic for flight ability of Drosophila when expressed in a null strain together with wild-type actin and could not form myofibrils when homozygously expressed in the null strain. By analogy, it is likely that mutant actin molecules of the patient in this study also interfere functions of normal β-actin molecules in a dominant–negative manner. It is of note that the mutant β-actin described in a chemically transformed human fibroblast line (4) was also present together with normal a β-actin molecule and exerted a dominant negative effect on function (4).
It is interesting to note that not only functions directly connected to actin such as chemotaxis but also superoxide generating ability in neutrophils were affected by the mutation. Epstein–Barr virus-transformed B cell line from the patient also had a comparable amount of mutant β-actin molecules to that of normal β-actin and reduced superoxide generating ability (data not shown). The superoxide-generating system in these cells consists of two membrane proteins designated as gp91-phox and p22-phox that form cytochrome b558 and three cytosolic proteins, namely p47-phox, p67-phox (27–29), and a small GTP binding protein Rac-p21. The system is in a dormant state in resting neutrophils but is activated on stimulation, where p47-phox and other cytosolic proteins translocate to the membrane and associate to the cytochrome (30) to form a functionally active enzyme complex on the plasma membrane. Nauseef et al. suggested that p47-phox phospholyrated during activation is more effectively transferred to the membrane as a result of binding to actin (31). The abnormality in our patient’s neutrophils would support a role for actin in the activation of the superoxide-generating phagocyte NADPH oxidase.
Clinical symptoms of the present patient included photosensitivity and intellectual impairment in addition to the recurrent infections resulting from the neutrophil dysfunction. She did not demonstrate more profound defects in other organ systems. This patient died from infection before our subsequent molecular identification of her defect, precluding additional investigation of her clinical phenotype or defects in cells other than her fibroblast line, B cell line, or previously frozen materials. However, our study suggests that directed mutation at the same site in an engineered mouse or other target animal would generate a viable phenotype and an informative model for further study of the function of nonmuscle actin in higher organisms.
Acknowledgments
We were indebted to Dr. E. D. Korn (National Heart, Lung and Blood Institute, National Institutes of Health) for his valuable suggestions and gift of purified Acanthamoeba actin, Dr. T. Takenawa (Institute of Medical Science, University of Tokyo) for providing profilin I cDNA inserted to pGEX-2T, Mr. S. Sekine (Sagami Chemical Research Center) for helping us to construct full-length c-DNA, and Drs. T. Oohira, Y. Yanabe, S. Higuchi, I. Akahosi (Department of Pediatrics, Kumamoto University Medical School), and A. Furuse (Department of Pediatrics, National Sanitarium of Beppu) for their support in the patient’s care. A part of this work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan.
ABBREVIATIONS
- 2D
two-dimensional
- fMLP
formyl methionyl-leucyl-phenylalanine
- GST
glutathione S-transferase
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Moraczewska J, Strzelecka-Golaszewska H, Moens P D, dos Remedios C G. Biochem J. 1996;317:605–611. doi: 10.1042/bj3170605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schleicher M, Andre B, Andreoli C, Eichinger L, Haugwitz M, Hofmann A, Karakesisoglou J, Stockelhuber M, Noegel A A. FEBS Lett. 1995;369:38–42. doi: 10.1016/0014-5793(95)00579-x. [DOI] [PubMed] [Google Scholar]
- 3.Drummond D R, Hennessey E S, Sparrow J C. Mol Gen Genet. 1991;226:70–80. doi: 10.1007/BF00273589. [DOI] [PubMed] [Google Scholar]
- 4.Taniguchi S, Sagara J, Kakunaga T. J Biol Chem. 1988;103:707–713. doi: 10.1093/oxfordjournals.jbchem.a122333. [DOI] [PubMed] [Google Scholar]
- 5.Boxer L A, Hedley-Whyte E T, Stossel T P. N Engl J Med. 1974;291:1093–1099. doi: 10.1056/NEJM197411212912101. [DOI] [PubMed] [Google Scholar]
- 6.Southwick F S, Dabiri G A, Stossel T P. J Clin Invest. 1988;82:1525–1531. doi: 10.1172/JCI113761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Southwick F S, Howard T H, Holbrook T. Blood. 1989;73:1973–1979. [PubMed] [Google Scholar]
- 8.Boyum A. J Clin Lab Invest. 1968;21:77–89. [PubMed] [Google Scholar]
- 9.Iwata M, Nunoi H, Yamazaki H, Nakano T, Niwa H, Tsuruta S, Ohga S, Ohmi S, Kanegasaki S, Matsuda I. Biochim Biophys Res Commun. 1994;199:1372–1377. doi: 10.1006/bbrc.1994.1382. [DOI] [PubMed] [Google Scholar]
- 10.Rebuck J W, Crowley J H. Ann NY Acad Sci. 1955;59:757–805. doi: 10.1111/j.1749-6632.1955.tb45983.x. [DOI] [PubMed] [Google Scholar]
- 11.Rice J E, Bignold L P. J Immunol Methods. 1992;149:121–125. doi: 10.1016/s0022-1759(12)80056-1. [DOI] [PubMed] [Google Scholar]
- 12.Nunoi H, Iwata M, Tatsuzawa S, Onoe Y S, Shimizu S, Kanegasaki S, Matsuda I. Blood. 1995;86:329–333. [PubMed] [Google Scholar]
- 13.Seligmann B E, Gallin J I. J Clin Invest. 1980;66:493–503. doi: 10.1172/JCI109880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Farrell P H. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 15.Kato S, Sekine S, Oh S W, Kim N S, Umezawa Y, Abe N, Yokoyama-Kobayashi M, Aoki T. Gene. 1988;150:243–250. doi: 10.1016/0378-1119(94)90433-2. [DOI] [PubMed] [Google Scholar]
- 16.Gordon D J, Eisenberg E, Korn E D. J Biol Chem. 1976;251:4778–4786. [PubMed] [Google Scholar]
- 17.Suetsugu S, Miki H, Takenawa T. EMBO J. 1998;17:6516–6526. doi: 10.1093/emboj/17.22.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuchiya H, Migita M, Nunoi H, Adachi N, Matsuda I, Takeda T. Acta Haematol. 1987;77:65–71. doi: 10.1159/000205956. [DOI] [PubMed] [Google Scholar]
- 19.Sheff D R, Rubenstein P A. J Biol Chem. 1992;267:2671–2678. [PubMed] [Google Scholar]
- 20.Nakajima-Iijima S, Hamada H, Reddy P, Kakunaga T. Proc Natl Acad Sci USA. 1985;82:6133–6137. doi: 10.1073/pnas.82.18.6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wertman K, Drubin D G, Botstein D. Genetics. 1992;132:337–350. doi: 10.1093/genetics/132.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johara M, Toyoshima Y Y, Ishijima A, Kojima H, Yanagida T, Sutoh K. Proc Natl Acad Sci USA. 1993;90:2127–2131. doi: 10.1073/pnas.90.6.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheterline P, Sparrow J. In: Actin Structure, Protein Profile, Actin. Sheterline P, editor. London: Academic; 1994. pp. pp.1–62. [Google Scholar]
- 24.Drummond D R, Hennessey E S, Sparrow J C. Eur J Biochem. 1992;209:171–179. doi: 10.1111/j.1432-1033.1992.tb17274.x. [DOI] [PubMed] [Google Scholar]
- 25.Vandekerckhove J S, Kaiser D A, Pollard T D. J Cell Biol. 1989;109:619–626. doi: 10.1083/jcb.109.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schutt C E, Myslik J C, Rozycki M D, Goonesekere N C, Lindberg U, Lindberg U. Nature (London) 1993;365:810–816. doi: 10.1038/365810a0. [DOI] [PubMed] [Google Scholar]
- 27.Nunoi H, Rotrosen D, Gallin J I, Malech H L. Science. 1988;242:1298–1301. doi: 10.1126/science.2848319. [DOI] [PubMed] [Google Scholar]
- 28.Volpp B D, Nauseef W M, Clark R A. Science. 1988;242:1295–1297. doi: 10.1126/science.2848318. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi S, Imajoh-Ohmi S, Kuribayashi F, Nunoi H, Nakamura M, Kanegasaki S. J Biochem. 1995;117:758–765. doi: 10.1093/oxfordjournals.jbchem.a124773. [DOI] [PubMed] [Google Scholar]
- 30.de Mendez I, Adams A G, Sokolic R A, Malech H L, Leto T L. EMBO J. 1996;15:1211–1220. [PMC free article] [PubMed] [Google Scholar]
- 31.Nauseef W M, Volpp B D, McCormick S, Leidal K G, Clark R A. J Biol Chem. 1991;266:5911–5917. [PubMed] [Google Scholar]
- 32.Ponte P, Ng S Y, Engel J, Gunning P, Kedes L. Nucleic Acid Res. 1984;12:1687–1696. doi: 10.1093/nar/12.3.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]