Abstract
The small GTPase Sar1p controls the assembly of the cytosolic COPII coat that mediates export from the endoplasmic reticulum (ER). Here we demonstrate that phospholipase D (PLD) activation is required to support COPII-mediated ER export. PLD activity by itself does not lead to the recruitment of COPII to the membranes or ER export. However, PLD activity is required to support Sar1p-dependent membrane tubulation, the subsequent Sar1p-dependent recruitment of Sec23/24 and Sec13/31 COPII complexes to ER export sites and ER export. Sar1p recruitment to the membrane is PLD independent, yet activation of Sar1p is required to stimulate PLD activity on ER membranes, thus PLD is temporally regulated to support ER export. Regulated modification of membrane lipid composition is required to support the cooperative interactions that enable selective transport, as we demonstrate here for the mammalian COPII coat.
Keywords: COPII/endoplasmic reticulum/PLD/Sar1p
Introduction
Cargo selection and export from the endoplasmic reticulum (ER) is mediated by the cytosolic COPII coat complex (Schekman and Orci, 1996; Antonny and Schekman, 2001). Activation of the small GTPase Sar1p leads to the formation of tubulated ER export sites (Aridor et al., 2001) and to the sequential recruitment of the cytosolic Sec23/24 and Sec13/31 COPII coat components from the cytosol to the membrane (Aridor et al., 1998; Matsuoka et al., 1998a). The assembled coat selects cargo for ER export (Dominguez et al., 1998; Aridor et al., 2001; Votsmeier and Gallwitz, 2001; Nufer et al., 2002), in part by interacting with export signals on cargo or cargo receptors (Aridor and Traub, 2002). Similar to the recognition of endocytic transport signals by the AP2 complex, these interactions are of relatively low affinity, as might be predicted from their dynamic and transient nature. Indeed, for Sec23/24 recognition of export motifs found on the SNARE protein Bet1, or on the cytoplasmic tail of the membrane vesicular stomatitis virus glycoprotein (VSV-G), a cooperative recruitment and formation of ternary complex with activated Sar1p-GTP is required to reveal low affinity recognition (Springer and Schekman, 1998; Aridor et al., 2001). The assembly of COPII coat on synthetic liposomes is markedly augmented by the presence of acidic phospholipids (Matsuoka et al., 1998a,b). Therefore, it may be that transient introduction of acidic phospholipids through regulated enzymatic activities can provide the required temporal increase in avidity that stabilizes the interactions between coat and cargo and supports selective vesicular transport.
Phospholipase D (PLD), which catalyzes the formation of phosphatidic acid (PA), may provide the required elevation in acidic lipid composition. Previous studies have shown that PLD activity is required to support transport to the Golgi in mammalian cells (Bi et al., 1997). Here, ADP-ribosylation factor 1 (ARF1) regulation of COPI sorting, a step that is required for the delivery of cargo from the intermediate compartment to the Golgi complex, was suggested to be the PLD-dependent step. ARF1 is a potent activator of PLD, and PLD activity has been implicated in supporting COPI function. However, the contribution of PLD activity to COPI function is yet to be determined, as intermediates in COPI-mediated cargo selection in the Golgi are yet to be defined. We have analyzed the role of PLD in supporting COPII assembly and cargo export from the ER. We demonstrate that Sar1p is a potent activator of PLD. We show that activation of PLD is required for membrane tubulation during export site formation. We demonstrate that PLD activity is required to support Sar1p-dependent recruitment of Sec23/24 and Sec13/31 to ER membranes, to enable cargo export from the ER.
Results
PLD activity is required to support ER export in vivo
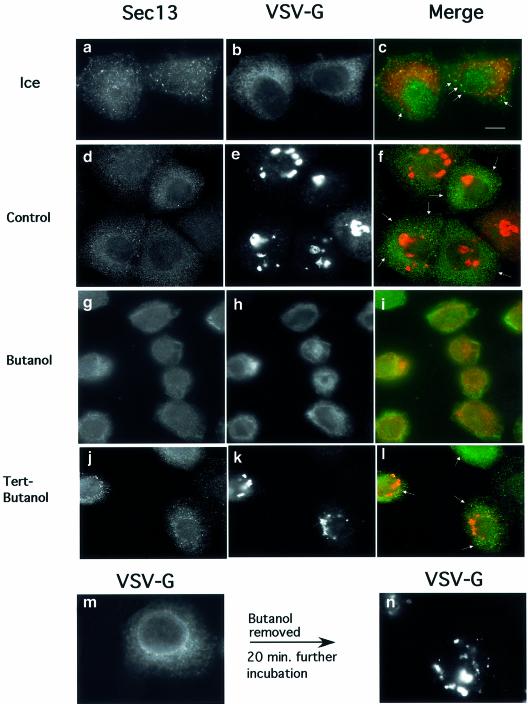
Previous studies have demonstrated that transport from the ER to the Golgi is blocked in vivo by inhibition of PLD (Bi et al., 1997; Siddhanta et al., 2000). We utilized a morphological assay to analyze a possible role for PLD in ER export in vivo. The assay follows the mobilization of a cargo reporter, the temperature-sensitive cargo protein TsO45 VSV-G, from the ER. NRK cells were infected with TsO45 VSV, to accumulate VSV-G in the ER at the restrictive temperature (40°C). Subsequently, the cells were incubated on ice or at 32°C for 20 min. At the end of incubations, the cells were transferred to ice, permeabilized, fixed and analyzed by immunofluorescence using VSV-G and Sec13-specific antibodies as indicated (Plutner et al., 1992). At the beginning of incubation, VSV-G largely resides in the ER (Figure 1b and c). At the end of incubations, the majority of cargo protein cleared the ER and is concentrated in the Golgi complex (Figure 1e). Incubation of cells with 1.5% 1-butanol, a potent inhibitor of PLD-mediated PA formation, led to inhibition of VSV-G transport to the Golgi (Figure 1h and i). Importantly, the majority of VSV-G is not present in pre-Golgi intermediates but rather resides in the ER, and is clearly visualized in the reticular ER and the nuclear envelope (Figure 1h and i). In contrast, incubation of cells in the presence of a similar concentration of tertiary alcohol, an inefficient inhibitor of PLD-mediated PA formation (tertiary butanol; tert-butanol), does not affect the kinetics (not shown) or efficiency of transport of VSV-G from the ER to the Golgi complex (Figure 1k and l). The inhibition of VSV-G export from the ER and transport to the Golgi was fully reversible. Removal of butanol from the treated cells led to efficient mobilization of VSV-G to the Golgi complex. (Figure 1m and n). The steady-state localization of COPII under these conditions was analyzed by following the localization of the COPII subunit Sec13. Sec13 is largely visualized as punctate structures throughout the cell, with some co-localization with the mobilized cargo protein (Figure 1a and c). In contrast, in the presence of butanol, a qualitative reduction in the punctate staining of Sec13 is observed (Figure 1g and i). This reduction is not observed in cells incubated with tert-butanol where efficient mobilization of VSV-G is observed and is correlated with elevated levels of membrane-associated COPII (Figure 1j and l).
Fig. 1. Phospholipase D is required to support ER export in vivo. Infected NRK cells expressing TsO45 VSV-G were incubated on ice (a–c) or at 32°C (d–n) in the absence (d–f) or presence (g–i) of 1-butanol (1.5%) or tert-butanol (1.5%) (j–l) for 20 min. At the end of incubations, the cells were transferred to ice, permeabilized, fixed and the localization of Sec13 (a, d, g and j) and VSV-G (b, e, h, k, m and n) was determined using indirect immunofluorescence. Export of VSV-G from the ER and delivery to the Golgi (compare h with e and k) and Sec13 binding to the membranes (compare a, d and j with g) were inhibited by 1-butanol but not by tert-butanol (the arrows in c, f and l indicate areas of punctate Sec13 staining). In (m), cells were incubated for 20 min in the presence of 1-butanol. At 20 min, the medium was replaced with fresh medium without butanol and the cells were incubated further for 20 min before fixation and analysis (n). Following butanol removal, VSV-G was delivered to the Golgi complex efficiently. The bar in (c) is 5 µm. A representative experiment is shown; similar results were obtained in three separate experiments.
The inhibition of cargo export from the ER is in agreement with previous reports, which suggested that the transport from the ER and the Golgi in mammalian cells is dependent on PLD activity in vivo (Bi et al., 1997; Siddhanta et al., 2000). However, the inability of VSV-G to exit the ER suggests that transport inhibition may already be exerted at the ER export step. Two possibilities can explain the observed inhibition of ER export. In one, ARF-regulated PLD activity is required to support COPI function in mediating transport between Golgi compartments and retrograde transport from the Golgi to the ER. Therefore, the inhibition of PLD activity can lead indirectly to inhibition of ER export. Alternatively, PLD activation may be required directly during COPII assembly to support export from the ER. The arrest of VSV-G in the ER, together with the qualitative reduction in COPII punctate staining, supports the second possibility. Thus, while PLD may be required to support transport between pre-Golgi intermediates and the Golgi, it may also be required to support COPII assembly and ER export. In order to test this possibility, we utilized in vitro assays that directly reconstitute both COPII assembly and ER export, thus isolating the ER export step from subsequent transport steps within pre-Golgi and Golgi intermediates for analysis.
PLD activity is required to support COPII-mediated ER export in vitro
In order to analyze the role of PLD in ER export directly, we have utilized an in vitro budding assay containing ER microsomes incubated with purified COPII components Sar1p, Sec23/24 and Sec13/31 to focus on the ER export step (Rowe et al., 1996; Aridor et al., 1998). The assay follows the mobilization of VSV-G, which is released from the dense ER membranes into a slowly sedimenting COPII vesicles using differential centrifugation. As we previously reported, the addition of purified COPII proteins at physiological temperature led to efficient mobilization of VSV-G to the vesicle fraction (∼25%, Figure 2, lane 2, compare M with H). Incubation of the reactions with increasing concentrations of 1-butanol led to complete inhibition of cargo export from the ER [Figure 2, lanes 3 (1% butanol) and 4 (2% butanol); see also Supplementary figure 1-S, VSV-G and Bet1, available at The EMBO Journal Online]. In contrast, tert-butanol, while slightly inhibitory, does not block cargo export from the ER [Figure 2, lanes 5 (1% tert-butanol) and 6 (2% tert-butanol)]. Therefore, these results suggest that PLD activity is required to support COPII-mediated ER export.
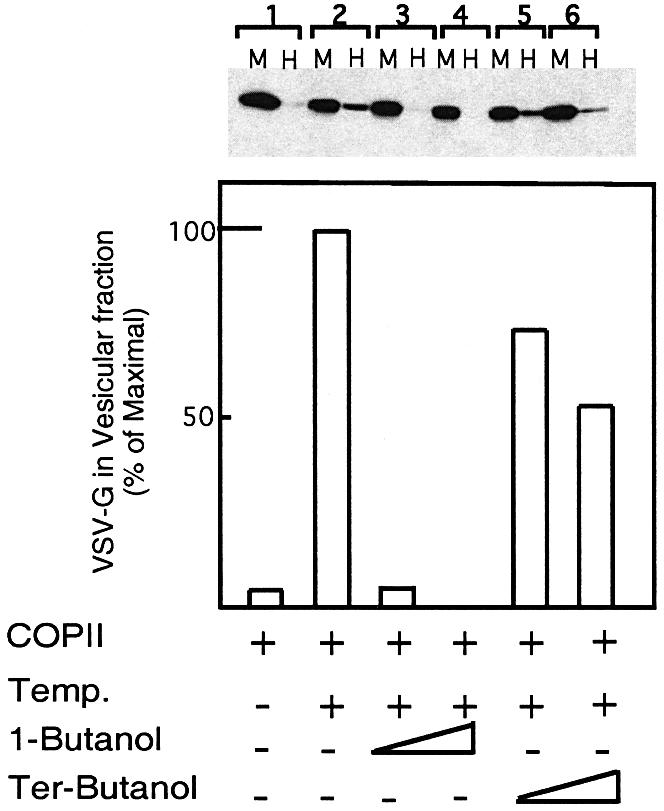
Fig. 2. PLD activity is required for COPII-mediated export from the ER. VSV-G-containing membranes were incubated with purified COPII components [Sar1p-H79G (2 µg) Sec23/24 (1 µg) and Sec13/31 (12 µg)] (Aridor et al., 1998) for 30 min on ice (lane 1), or at 32°C (lanes 2–6) in the absence (lanes 1 and 2) or presence of 1-butanol (lane 3, 1%; lane 4, 2%) or tert-butanol (lane 5, 1%; lane 6, 2%). At the end of the incubation, the vesicle fraction (H for high speed pellet) was separated from the donor membranes (M for medium speed pellet) by differential centrifugation, and the export of VSV-G into the vesicular fraction was determined by western blot analysis with anti-VSV-G antibody. The lower panel is a quantitative densitometry analysis of the amount of VSV-G in the vesicle fraction. Results are presented as a percentage of maximal budding under non-perturbed conditions (25% of total VSV-G in the starting membrane, lane 2). The experiment presented is representative of at least three independent experiments.
PLD activity is required to support export site formation and Sec13/31 recruitment
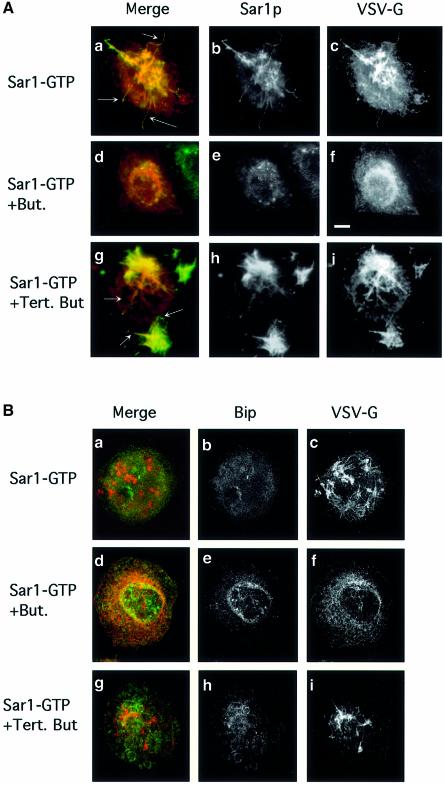
During the initiation of ER export, ER membranes assume the required curvature that leads to the formation of vesicular tubular structures. This tubulation can be initiated prior to coat recruitment through activation of Sar1p (Aridor et al., 2001). As membrane de-formation may require changes in lipid composition, we utilized an in vitro morphological assay to analyze the role of PLD in supporting Sar1p-dependent tubule formation, the first morphological intermediate in ER export in mammalian cells. NRK cells were infected with TsO45 VSV to accumulate VSV-G in the ER at the restrictive temperature. Subsequently, the cells were transferred to ice, permeabilized, washed and incubated in the absence of cytosol but in the presence of an activated Sar1p (Sar1p-GTP, H79G mutation) (Aridor et al., 2001) for 30 min at 32°C. The localization of Sar1p (Figure 3A, b, e and h) and VSV-G (Figure 3A, c, f and i, and B, c, f and i) was determined using indirect immunofluorescence (Aridor et al., 1995). Under these conditions, numerous tubules are formed on ER membranes of infected and non-infected cells [Figure 3A (epifluorescence) and B (confocal images) a and c, and not shown]. In infected cells, VSV-G is mobilized into the stabilized elongated tubules (as can be seen by the increased fluorescence) and is co-localized with Sar1p that is bound along the tubular structures (Figure 3A, b and a; see arrows in a). ER resident proteins such as Bip (Figure 3B, b and a; Aridor et al., 2001) are largely excluded from these ER export sites. Incubation of permeabilized cells with 1-butanol markedly inhibited the formation of the Sar1p-dependent tubular domains. VSV-G remains in a typical reticular stain characteristic of the ER, with increased co-localization with the ER resident chaperon Bip (Figure 3A and B, f and d). Sar1p can be detected on the membranes, but no tubulation can be visualized (Figure 3A, e and d). Therefore, Sar1p recruitment was not blocked by butanol. In contrast, incubation of cells with tert-butanol did not affect the formation or elongation of these domains significantly, as analyzed by Sar1p staining (Figure 3A, h and g). Likewise, tert-butanol did not inhibit the mobilization of VSV-G into these domains (Figure 3A and B, c and i). Incubation of permeabilized cells with purified PLD in the absence of Sar1p did not lead to cargo mobilization into tubular domains and VSV-G remained in a typical reticular ER (not shown). Therefore, while Sar1p activation is required to initiate tubular domain formation, the observed tubulation is dependent on PLD activity. Thus, we hypothesized that modulation of membrane lipid composition at ER export sites may support the subsequent assembly of the COPII coat to mediate ER export.
Fig. 3. PLD is required for export site formation. (A) PLD is required for Sar1p-induced tubule formation. VSV-infected NRK cells were permeabilized and incubated (Aridor et al., 2001) in the absence of cytosol in the presence of 9 µg of Sar1p-GTP (a–i), 1-butanol (d–f, 1.5%) or tert-butanol (g–i, 1.5%). The distribution of Sar1p (b, e and h) and VSV-G (c, f and i) was determined using indirect immunofluorescence (merged images are shown in a, d and g). Arrows in (a) indicate VSV-G-containing Sar1p-dependent tubules. Butanol inhibits tubule formation but does not block Sar1 binding (d–f). Tert-butanol does not inhibit the Sar1-dependent tubule formation (g–i; arrows in g indicate tubules on the ER; the bar in f is 5 µm. All images are representative of at least three independent experiments. (B) Cells were untreated or treated with butanol or tert-butanol as indicated (described above) and the localization of VSV-G (c, f and i) or Bip (b, e and h) was analyzed by confocal microscopy (merged images are shown in a, d and g).
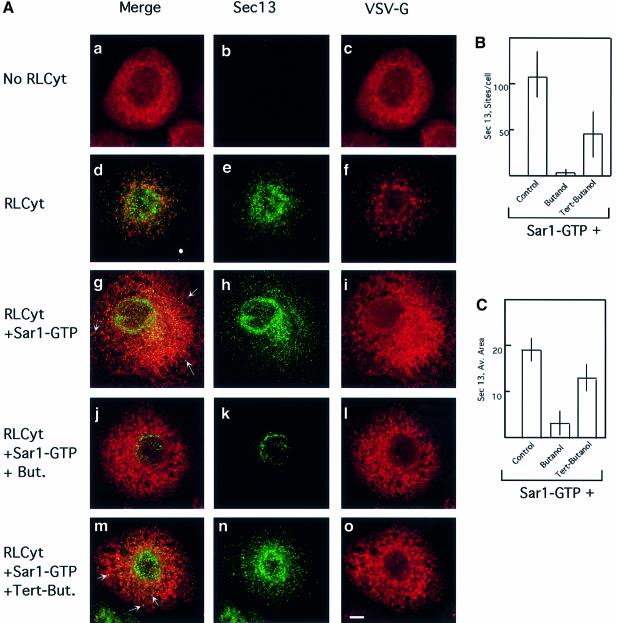
In order to test this prediction, we analyzed the assembly of COPII using the morphological in vitro assay. COPII is assembled sequentially, where the activation of Sar1p leads to the recruitment of the Sec23/24 complex, followed by the recruitment of Sec13/31 (Matsuoka et al., 1998b). VSV-G-containing cells were washed, permeabilized and incubated in the absence or presence of cytosol and activated Sar1p (Sar1p-GTP, H79G mutation) (Aridor et al., 1995) for 30 min at 32°C. The cells were fixed, and the localization of VSV-G (Figure 4A, c, f, i, l and o) and Sec13 (Figure 4A, b, e, h, k and n) was determined using indirect immunofluorescence (Plutner et al., 1992). VSV-G is mobilized from the ER to pre-Golgi vesicular tubular clusters (VTCs) in a cytosol-dependent manner (Figure 4A, compare a–c with d–f). Under these conditions, formed VTCs already passed the COPII-dependent step, and thus only partial co-localization between the mobilized VSV-G and Sec13 is detected (Figure 4A, e and d) (Aridor et al., 1995). Addition of Sar1p-GTP (H79G mutation) arrests the progression of transport between the ER and the Golgi at the COPII export stage, by locking the recruited COPII coat to the membranes (Aridor et al., 1995). Indeed, activated Sar1p led to stable recruitment of Sec13, which co-localized with VSV-G at export sites (Figure 4A, g and h; see arrows in g). Addition of 1-butanol (Figure 4A, j–l), but not tert-butanol (Figure 4A, m–o), inhibited Sec13 recruitment to the membranes (Figure 4A, k). We have quantified the effect of butanol on Sec13 recruitment in the morphological assay. Random fields of cells were acquired (see Materials and methods) and Sec13 recruitment was analyzed using computer software that measured the number of sites marked by Sec13 staining (Figure 4B) and the average area of Sec13-stained sites (Figure 4C). Butanol treatment led to a dramatic reduction in both Sec13 recruitment sites and their area compared with untreated or tert-butanol-treated cells (Figure 4B and C). Remarkably, results obtained from analyzing Sec13 recruitment morphologically were qualitatively similar to those obtained using in vitro budding assays following VSV-G export from the ER (compare Figure 2 with Figure 4B and C). Collectively, these results suggest that PLD activity may be required to support the assembly of COPII on export sites.
Fig. 4. PLD is required for COPII assembly. (A) PLD is required for COPII assembly and ER export: VSV-infected NRK cells were permeabilized and incubated as previously described (Plutner et al., 1992) in either the absence (a–c) or presence (d–f) of cytosol or cytosol and 9 µg of recombinant Sar1p-GTP (H79G mutation) (g–o), in the presence of 1-butanol (j–i) 1.5%) or tert-butanol (m–o 1.5%), in 220 ml for 30 min at 32°C. The distribution of VSV-G (c, f, i, l and o) and Sec13 (b, e, h, k and n) was determined using indirect immunofluorescence with specific antibodies (merged images are shown in a, d, g, j and m). Note the efficient mobilization of VSV-G to VTCs (compare c with f). In the presence of Sar1p-GTP (H79G), note the co-localization of Sec13 with VSV-G (arrows in g). Butanol (j–l) but not tert-butanol (m–o, see arrows indicating Sec 13 recruitment to VSV-G-containing export sites) inhibits COPII assembly and ER export (the bar in o is 5 µm). A representative experiment is shown; similar results were obtained in three separate experiments. (B) Analysis of the number of Sec13-positive sites per cell. Random fields of cells were captured and analyzed for Sec13-labeled sites/cell as described in Materials and methods. (C) Analysis of average area of Sec13-coated ER export sites. Random fields of cells were captured and the average area of Sec13 recruitment sites (in pixels) was determined as described in Materials and methods (averages ± SD are shown; control, n = 70; butanol n = 37; tert-butanol n = 67).
PLD activity is required to support Sar1p-dependent Sec23/24 recruitment and enable ER export
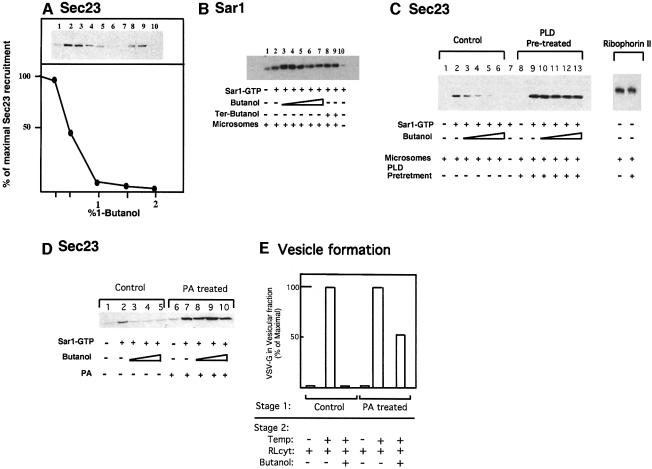
COPII assembly is initiated by the recruitment of Sar1p and Sec23/24 to the membrane. We therefore measured biochemically the recruitment of Sar1p and Sec23/24 to membranes (Aridor et al., 1995, 1998; Aridor and Balch, 2000). Membranes were incubated with cytosol in the presence or absence of Sar1p-GTP and ATP for 15 min at 32°C to enable COPII recruitment. Subsequently, membranes were salt washed, collected by centrifugation and Sec23/24 recruitment to the membranes was analyzed by western blots using specific antibody. Sar1p activation led to membrane binding of Sec23 as we previously described [Figure 5A, compare lane 2 (incubation with Sar1-GTP) with lane 1 (incubation without Sar1-GTP) and lane 10 (incubation with Sar1-GTP but without membranes]. 1-butanol inhibited Sar1p-dependent Sec23 binding in a dose-dependent manner (Figure 5A, lanes 3–7). Incubation with tert-butanol did not block Sec23 recruitment [Figure 5A, lanes 8 (1% tert-butanol) and 9 (2% tert-butanol)], attesting to the specificity of the observed inhibition.
Fig. 5. PLD is required for Sec23/24 recruitment and ER export. (A) Butanol inhibits Sec23/24 recruitment. Membranes (lanes 1–9) were incubated with cytosol, in the absence (lane 1) or presence of Sar1p-GTP (lanes 2–10) and in the presence of increasing concentrations of 1-butanol (lanes 3–7) or tert-butanol (lane 8, 1%; lane 9, 2%) as indicated. Lane 10 is incubation with Sar1p-GTP cytosol and no membranes. At the end of the incubations, membranes were washed and the recruitment of Sec23/24 was determined by western blotting with a Sec23-specific antibody. Quantitative densitometry of the upper gel is shown in the lower panel. (B) 1-butanol does not inhibit Sar1 recruitment. Membranes (lanes 1–9) were incubated with cytosol, in the absence (lane 1) or presence of Sar1p-GTP (lanes 2–10; lane 10 is incubation without membranes) and in the presence of increasing concentrations of 1-butanol (lanes 3, 0.25%; 4, 0.5%; 5, 1%; 6, 1.5%; 7, 2%) or tert-butanol (lane 8, 1%; lane 9, 2%) as indicated. At the end of the incubations, the membranes were layered on a 15% sucrose cushion and collected by centrifugation (Aridor and Balch, 2000). Sar1p recruitment was analyzed by western blotting with specific antibody. (C) PLD is required to support Sar1p-dependent Sec23/24 recruitment. Membranes were pre-treated with buffer (control, lanes 1–6) or peanut PLD (40 U/ml) for 20 min at 32°C (lanes 8–13); lane 7 is incubation with no membranes. After incubation, the membranes were transferred to ice and collected by centrifugation. The membranes were resuspended and incubated in the presence of purified Sec23/24 subunits (lanes 1–13), in the absence (lanes 1 and 8) or presence of Sar1p-GTP (lanes 2–7 and lanes 9–13) and increasing concentrations of 1-butanol (lanes 3–6 and 10–13). After incubation, the membranes were collected and the recruitment of Sec23 was determined as described in (A). In the right panel, ribophorin II present in PLD-pre-treated or untreated membranes is shown. (D) Phosphatidic acid is required to support Sar1p-dependent Sec23/24 recruitment. Membranes were incubated on ice in the presence or absence of phosphatidic acid-containing liposomes at a final concentration of 100 µM for 30 min. At the end of incubation, the membranes were collected by centrifugation and incubated with cytosol in the absence (lanes 1 and 6) or presence (lanes 2–5 and 7–10) of Sar1p-GTP and increasing concentrations of butanol (lanes 3–5 and 8–10; 1, 1.5 and 2% butanol, respectively). After incubation, the membranes were collected and the recruitment of Sec23/24 was determined. (E) Phosphatidic acid is required for ER export. VSV-G-containing membranes were incubated on ice in the presence of ATP and the presence or absence of phosphatidic acid-containing liposomes at a final concentration of 10 µM for 30 min (stage 1). At the end of incubation, the membranes were collected by centrifugation and incubated with cytosol on ice or at 32°C in the presence or absence of 1% 1-butanol as indicated (stage 2). The vesicular fraction was separated and the export of VSV-G was determined by western blot.
We analyzed the effects of PLD inhibition on Sar1p recruitment. For these experiments, membranes were incubated as above. At the end of incubation, the membranes were transferred to ice, layered on a sucrose cushion and collected by centrifugation for immunoblot analysis (Aridor and Balch, 2000). Typically, we found that low concentrations of 1-butanol tend to enhance Sar1p recruitment to the membranes (Figure 5B, lanes 3 and 4). Importantly, increasing concentrations of 1-butanol [up to 2%; Figure 5B, compare lane 2 (non-treated) and lane 7 (2% butanol)] failed to inhibit Sar1p recruitment. Similar results were obtained when the endogenous Sar1p was activated by GTP-γ-S using whole cytosol (not shown). These results are in agreement with morphological analysis (Figure 3A) and collectively suggest that PLD activity is required downstream from Sar1p recruitment.
Two possibilities can explain the requirement for PLD activity during COPII assembly. In one, PLD activity may be sufficient to drive COPII recruitment. Alternatively, PLD activation may be required together with Sar1p to provide the necessary avidity required for stabilization of the coat on the membrane. Therefore, to test directly the role of PLD in Sec23/24 recruitment and to control for the specificity of 1-butanol as a PLD inhibitor in our reactions, membranes were incubated in the presence or absence of peanut PLD for 20 min at 32°C. At the end of this first-stage incubation, membranes were isolated and further incubated in a second-stage incubation with purified Sec23/24 subunits, in the presence or absence of Sar1p-GTP, ATP and increasing concentrations of 1-butanol. The effect of PLD treatment on Sec23/24 recruitment was analyzed as above (Figure 5C). In control membranes, the recruitment of Sec 23/24 required Sar1p-GTP and was blocked by incubation with 1-butanol in a dose-dependent manner as observed for the recruitment of Sec23/24 subunits from cytosol (Figure 5C, compare lane 2 with lanes 3–6 and with Figure 5A). Therefore, PLD activity resides on the ER membranes. Pre-treatment of the membranes with PLD did not lead to Sec23/24 recruitment (Figure 5C, lane 8), nor did it affect ER morphology or ER export (not shown). Therefore, the increment in PA content per se does not support COPII recruitment. However, pre-treated membranes in general recruited higher levels of Sec23/24 in response to Sar1p activation (Figure 5C, compare lane 2 with 9 and membrane marker ribophorin II under both conditions). More importantly, PLD pre-treatment abolished the inhibition of Sar1p-dependent Sec23/24 recruitment by 1-butanol, providing direct evidence for a role for PLD in supporting Sec23/24 binding to membranes. Pre-incubation of membranes with peanut PLD, in the presence of butanol, abolished the protective effect of PLD pre-treatment, suggesting that PA formation by PLD is required to support Sec23 recruitment (not shown). In order to test the role of PA in Sec23/24 binding directly, membranes were pre-incubated in the presence or absence of dilauryl-PA liposomes for 30 min on ice, to introduce PA into the membranes (Bi et al., 1997). Subsequently, the membranes were isolated by centrifugation and incubated with cytosol in the presence or absence of Sar1p-GTP. The effect of PA addition on the inhibition of Sar1-mediated Sec23/24 recruitment by butanol was analyzed. Similar to the results obtained in PLD pre-treatment experiments, introduction of PA into the membrane did not lead to Sec23 recruitment (Figure 5D, lane 6). However, as observed with PLD pre-treatment, PA introduction into the membranes enhanced Sar1p-dependent Sec23/24 recruitment (Figure 5D, compare lanes 2 and 7) and abolished the inhibition of Sec23/24 binding by 1-butanol (Figure 5D, compare lanes 3–5 with 8–10). The specificity of Sec23/24 recruitment is therefore maintained by Sar1p activation regardless of PLD activity or PA addition. However, Sar1p-mediated Sec23/24 recruitment is dependent on PLD-mediated PA formation. These observations are in agreement with previous results in which we and others have demonstrated that protein–protein interactions participate in coat recruitment and cargo selection. Sec23/24 is co-isolated in detergents with activated Sar1p-GTP (but not with Sar1p-GDP) following membrane binding (Aridor et al., 1998; Kuehn et al., 1998). Sec23/24 recognizes export signals and assembles with cargo presented on liposomes or in detergent in response to Sar1p activation (Springer et al., 1999). Furthermore, activated Sar1p can interact directly with Sec23/24 in a reaction which stimulates Sar1p GTPase activity (Bi et al., 2002). However, while neither PA formation nor Sar1p binding to target membranes in the absence of PLD activation is sufficient to recruit COPII to the membranes under physiological conditions, both are required for stable coat recruitment.
We further tested if PA can support ER export in the presence of inhibitory concentrations of 1-butanol. For these experiments, VSV-G-containing membranes were incubated in the presence or absence of dilauryl-PA liposomes as described above, isolated and incubated with cytosol, and the effect of 1-butanol on mobilization of VSV-G from the ER to the vesicular fraction was determined. As observed with purified COPII components (Figure 2), incubation of control membranes and cytosol in the presence of 1% 1-butanol blocked VSV-G budding from the ER (Figure 5E). Pre-incubation of membranes with 10 µM PA liposomes (PA-treated) led to export of VSV-G in the presence of 1% butanol, with up to 50% of control levels being exported (Figure 5E). The combined results analyzing both COPII coat assembly and ER export indicate that the formation of PA by PLD activity is the target for 1-butanol inhibition, and suggest that PLD activity is required to support ER export.
Sar1p is a potent activator of PLD
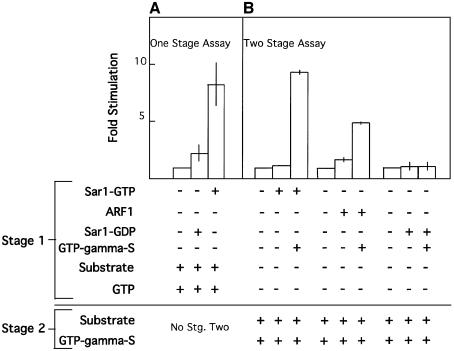
In order to provide temporal support to COPII coat assembly, PLD activation should be tightly coupled to COPII recruitment. Coupling can be achieved by placing the activation of PLD under regulation of the Sar1p GTPase. We therefore tested the ability of Sar1p to activate PLD in our membranes. We analyzed the ability of Sar1p to activate PLD using one- or two-stage assays. In a one-stage assay, we incubated membranes in the presence of Sar1-GTP or Sar1-GDP (T39N mutation, an inactive dominant-negative inhibitor of Sar1p that cannot bind GTP), in the presence of ATP, GTP, ethanol and a radiolabeled liposome substrate in an assay that is widely utilized to analyze the activity of membrane-associated PLD (Cockcroft et al., 2001). The activation of PLD was determined by following the formation of phosphatidylethanol (PET) using thin-layer chromatography (TLC) and autoradiography (Iyer and Kusner, 1999). Sar1-GTP, but not the inactive Sar1-GDP, stimulated the activity of PLD in our membrane preparations, with 8-fold stimulation observed (Figure 6A). Therefore, Sar1p activation leads to stimulation of PLD activity present on microsome membranes. We further utilized a two-stage assay to test the ability of Sar1p to activate PLD, and compared this activation with the activity of a known activator of PLD, ARF1. In these experiments, Sar1p (or ARF1 as control) was first activated and recruited to the membranes (stage 1) by incubation of the membranes with ATP, in the absence or presence of the guanine nucleotide, GTP-γ-S (to prevent GTP hydrolysis and consumption during incubation). The membranes were washed three times to remove inactive unbound ARF or Sar1 proteins. Washed membranes were incubated with radiolabeled substrate, ethanol and GTP-γ-S. GTP-γ-S was provided to all membranes in second-stage incubations, to control for possible activation of additional GTPases that may be present in the preparation. Sar1p-GTP strongly activated PET formation (10-fold) (Figure 6B). At maximal stimulation, incubations of microsome membranes with Sar1p led to production of 20–30 pmol PA/mg membrane protein/min (as compared with 1–3 pmol PA/mg membrane protein/min at basal activity). This rate of PLD-mediated PA formation is in agreement with previously reported rates of ARF1-stimulated PA production in Golgi membranes (Provost et al., 1996). The activity of Sar1-GTP is dependent on the provision of guanine nucleotides (Figure 6B). This dependency is in agreement with our previous findings, demonstrating that the provision of guanine nucleotides is required, together with ATP, to enable Sar1p recruitment and activation in assays that reconstitute coat binding or vesicle formation with purified COPII components (Aridor et al., 1998; Aridor and Balch, 2000). ARF1 also stimulated PET formation (5-fold), only when pre-incubated in the presence of GTP-γ-S. The difference in fold stimulation of PLD by Sar1p or ARF1 may be related to the myristoylation level of the recombinant ARF utilized, or to the enrichment of ER membranes in our preparation. Importantly, Sar1-GDP (T39N mutation), which cannot bind GTP-γ-S, did not stimulate the PLD activity present on the ER membranes. The results from one- or two-stage PLD assays demonstrate that Sar1p activation is required for the observed stimulation of PLD activity on ER membranes. Activation of PLD on ER membrane is therefore coupled to the initiation of coat assembly through Sar1p activation. We analyzed the intracellular localization of tagged PLD enzymes and found that PLD1 can associate with ER export sites in vivo. PLD1 is also found on Sar1-induced tubular domains in vitro (Supplementary figure 2-S). Future studies are required to determine if PLD1 is involved in regulating ER export and the mechanisms by which Sar1 may regulate its activity.
Fig. 6. Sar1p activation stimulates PLD activity. (A) Membranes (75 µg) were incubated with ATP and GTP in the presence of recombinant Sar1p-GTP (H79G mutation) (8 µg), or recombinant Sar1p-GDP (T39N mutation) (8 µg), ethanol and radiolabeled liposomes for 60 min at 37°C. At the end of incubation, the lipids were extracted and the formation of phosphatidylethanol was determined using TLC (Iyer and Kusner, 1999). Results were averaged from two independent experiments and are presented as fold stimulation over control conditions set as 1. (B) Membranes (75 µg) were incubated with ATP in the presence of recombinant Sar1p-GTP (H79G mutation) (8 µg), recombinant myrARF1 (8 µg) or recombinant Sar1p-GDP (T39N mutation) (8 µg) in the presence or absence of GTP-γ-S (100 µM) for 30 min at 37°C as indicated. At the end of the incubation, the membranes were re-isolated by centrifugation, washed and resuspended in the presence of GTP-γ-S (100 µM provided to all samples), ethanol and radiolabeled liposomes, and further incubated for 60 min at 37°C. The lipids were then extracted and the formation of phosphatidylethanol was determined using TLC. Results were averaged from two independent experiments and are presented as fold stimulation over control conditions set as 1.
Discussion
Protein–protein interactions provide the specificity for cargo selection during export from the ER (Aridor and Traub, 2002). COPII interacts directly with activated Sar1p. This interaction provides the initial targeting and anchoring of the coat to the membrane (Bi et al., 2002). On the membrane, the Sec23/24 complex can bind directly to cargo and machinery proteins carrying ER export signals to select them for incorporation into COPII vesicles (Antonny and Schekman, 2001). However, the affinities between the coat, Sar1p and cargo may not be sufficient to enable coat assembly under physiological conditions (Aridor et al., 1998; Aridor and Balch, 2000; Lee and Linstedt, 2000). Indeed, activation of Sar1p will only support coat recruitment to synthetic liposomes which contain high mole percent concentrations of acidic phospholipids (Matsuoka et al., 1998a,b). In our current work, we demonstrate that PLD activity is required to support membrane tubulation, export site formation and COPII coat assembly on ER membranes. The regulation of PLD activity by Sar1 provides a required temporal control on membrane composition, elevating PA content to support ER export.
What roles can PA play in ER export? One role is to support the Sar1-dependent interactions of COPII Sec23/24 subunits with the membranes (Figures 4 and 5). Both Sec23 and Sec24 proteins expose basic surfaces to the ER membranes, where interactions with PA may coordinate the placement of the coat on the surface. PLD-mediated generation of supplementary low affinity binding sites on the lipid membrane can provide the required multivalent avidity that supports transient yet stable coat recruitment during cargo selection (Figures 4 and 5). Alternatively, initial coat–lipid interactions may be replaced by protein–protein interactions during cargo selection. It is also possible that the interaction of COPII with PA may play an allosteric role, modulating Sec23/24 to better recognize membrane cargo.
In addition to supporting coat assembly, modulation of the lipid layer by PLD and perhaps additional enzymatic activities during ER export is required to enable membrane curvature and support tubule and vesicle formation (Figure 3). The formed tubules may be intermediates in vesicle fission; thus, PA formation followed by cycles of de- and re-acylation of the formed PA may participate in vesicle membrane fission, as observed for Golgi and endosome membranes (Schmidt et al., 1999; Weigert et al., 1999). P125, a PA-specific phospholipase A1, was shown to interact specifically with COPII subunit Sec23 (Tani et al., 1999; Mizoguchi et al., 2000), suggesting that upon coat recruitment, further modulation of the lipid membrane may occur. Phospholipase A2 inhibitors or selected domains of diacyl glycerol kinase δ were also shown to inhibit ER to Golgi transport in mammalian cells (de Figueiredo et al., 1998, 2000; Nagaya et al., 2002). The site of action and possible contribution of these enzymatic activities in ER to Golgi transport remain to be determined.
Sar1p-controlled temporal modulation of the phospholipid membrane by PLD can also provide a binding surface for additional accessory proteins. These proteins may contribute, for example, to the coupling between the budded vesicles, cytoskeletal elements and motors, which enable ER to Golgi transport (Lippincott-Schwartz et al., 2000).
Of the characterized PLD enzymes, PLD1 is localized to vesicular tubular elements mostly of endocytic origin, but also to ER export sites (Supplementary figure 2-S). While it remains to be determined if PLD1 is regulated by Sar1p and what is the mechanism of such regulation, lipid modulation by PLD and other lipid-modifying enzymes can be viewed as part of the vesicle formation machinery that enables export. The yeast PLD homolog Spo14 is not required to support ER export in vivo (Rudge et al., 1998). As yeast COPII requires acidic phospholipids for assembly (Matsuoka et al., 1998a,b), it could be that constitutive and/or redundant enzymatic activities can provide the required lipid environment for coat assembly. In agreement with that, a role for Spo14 in transport in the Golgi is only revealed in cells harboring Sec14 mutations under conditions of compromised lipid homeostasis (Rudge et al., 2001). We have also found that additional lipid-modifying enzymes can support COPII assembly and ER export (A.Blumental-Perry and M.Aridor, unpublished). Therefore, a concerted disruption of lipid-modifying enzymes may reveal a role for PLD in yeast COPII function in the ER. The addition of temporal regulation of ER export may have evolved in higher organisms. Indeed, unlike their mammalian counterparts, yeast PLD activities are not regulated by ARF or GTP-binding proteins (Rudge et al., 1998). We and others have demonstrated that unlike yeast COPII, the recruitment and assembly of the mammalian coat is also regulated by membrane-associated kinases (Aridor and Balch, 2000; Lee and Linstedt, 2000). A signaling cascade that leads to Sar1p-activated modulation of membrane lipid composition may have evolved in higher organisms to regulate export from the ER. PA is only a minor transient component of ER and Golgi membranes (Allan, 1996). Therefore, regulated, induced (Figure 6) and localized production of acidic phospholipids such as PA may be required to support cargo selection at ER export sites.
Temporal regulation of lipid composition during assembly of cytosolic coats is observed during AP2 assembly at the plasma membrane. Lipid phosphatases and lipid-interacting accessory proteins are an integral part of the AP2-clathrin coat which selects cargo for endocytosis (Simonsen et al., 2001). ARF is a potent activator of both PLD and phosphatidylinositol-4 kinase (Godi et al., 1999; Liscovitch et al., 1999). The contribution of these activities to vesicle formation by ARF-regulated ARF-GAP-containing COPI coat or GGA-supported AP1 and AP3 coats is yet to be defined. We propose that the temporal control of membrane lipid composition may be generally required for the formation of cooperative interactions that enable selective vesicular transport, as we demonstrate here for the COPII coat.
Materials and methods
Materials
Sar1a proteins were expressed and purified as previously described (Rowe and Balch, 1995). Sec23/24 COPII coat subunits were purified from rat liver cytosol (Aridor et al., 1998). Sec13/31 coat subunits were co-expressed and purified from insect cells (Weissman et al., 2001). Antibodies to Sec23, Sec13, Sar1a and VSV-G were a generous gift from Dr W.E.Balch (TSRI, La Jolla, CA). Peanut PLD was obtained from Sigma (St Louis, MO). All phospholipids utilized were obtained from Avanti Polar Lipids (Albaster, AL).
Coat recruitment assays
Sec23 recruitment (recruitment assay with salt wash) and Sar1p binding assays (using a sucrose cushion) were performed using microsome membranes (20–40 µg), Sar1p-GTP (0.05–0.5 µg) and Sec23/24 (0.3 µg) or rat liver cytosol (200 µg), in a final volume of 60 µl as previously described (Aridor et al., 1995, 1998; Aridor and Balch, 2000). In some experiments, ATP (2 mM) replaced the ATP regeneration system to increase the sensitivity of the assay. All experiments presented were performed at least three times with identical results.
In vitro vesicle formation assay
The in vitro vesicle formation assay was performed with purified COPII components utilizing Sar1p-GTP (2 µg), Sec23/24 (1 µg) and Sec13/31 (12 µg) or rat liver cytosol as previously described (Aridor et al., 1998). All experiments presented were performed at least three times with identical results.
Immunofluorescence labeling
Permeabilized cells were prepared and incubated under indicated conditions as described in the figure legends (Plutner et al., 1992). At the end of the incubations, the cells were fixed with 2% formaldehyde in phosphate-buffered saline (PBS) and analyzed as previously described (Aridor et al., 1995). For Sar1p-dependent tubule formation, cells were incubated as indicated and fixed with cold methanol (–20°C) (Aridor et al., 2001).
Morphological analysis and quantitation
To quantify vesicle formation within cells, confocal images through the midplane of the cells were collected with fixed laser illumination and pinhole size using a 60× 1.4 NA plan apochromat objective on an Olympus Fluoview 500 confocal microscope. This ensures that the sample volume is the same in all images. Using Metamorph (UIC, Downington, PA), individual cells were delineated, thresholded and the number, size and signal intensity within the vesicles measured in each cell. No significant difference was detectable in the gray scale values for each voxel within the vesicles in any of the cells or any of the conditions. Thus, our quantification is restricted to the number and size of the vesicles within the cells, which were measured automatically, based upon threshold segmentation in Metamorph.
Phospholipase D and phosphatidic acid treatment
For PLD pre-treatments, microsome membranes were incubated in buffer A (20 mM HEPES, 70 mM KoAc, 2 mM MgoAc, 250 mM sorbitol) and a cocktail of protease inhibitors at 32°C for 20 min in the presence of 40 U/ml purified peanut PLD. At the end of incubations, the membranes were collected by centrifugation at 12 000 g for 3 min and resuspended for coat binding as described above.
In order to introduce PA into ER membranes, dilauryl-PA was dried from chloroform and introduced into transport buffer A supplemented with 0.5% fatty acid-free bovine serum albumin at a final concentration of 50–500 µM. The hydrated lipids were sonicated for 5 min in a bath sonicator. Resulting liposomes were diluted 1/5 to a final concentration of 10–100 µM and incubated with microsome membranes for 30 min in transport buffer on ice [an ATP-regenerating system (Rowe et al., 1996) was added during pre-incubations for vesicle formation assays]. At the end of incubation, the membranes were collected by centrifugation at 12 000 g for 3 min and the vesicle formation assay was performed as described above.
Phospholipase D assay
PLD activity on microsome membranes was determined as previously described (Shome et al., 1998; Iyer and Kusner, 1999). Microsome membranes (75 µg) were incubated in the presence of ATP, GTP, Sar1p-GTP (H79G) or Sar1p-GDP (T39N) (as indicated) for 60 min at 37°C, in the presence of radiolabeled substrate liposome preparations composed of phosphatidylethanolamine (PE), phosphatidylinositol-(4,5)-bisphosphate [(4,5)PIP2] phosphatidylcholine (PC) (molar ratio 16:1.4:1), [3H]dipalmitoyl PC (5 µCi/sample) and ethanol (1.5%). In a two-stage assay, microsome membranes (75 µg) were first incubated in the presence of ATP, Sar1p-GTP (H79G), Sar1p-GDP (T39N) or wild-type ARF1 (8 µg of each), and GTP-γ-S (when indicated), for 30 min at 37°C. At the end of the first incubation, the membranes were collected by centrifugation, washed and incubated further in the presence of radiolabeled substrate liposomes, ethanol and GTP-γ-S, which was provided now to all samples. Reactions for both one- and two-stage assays were terminated at 60 min by the addition of chloroform:methanol. Lipids were extracted and dried, and the formation of PET was determined on TLC plates developed in ethyl acetate:trimethylpentane: acetic acid (9:5:2 by vol.) (Shome et al., 1998; Iyer and Kusner, 1999).
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Dr D.Meyer (UCLA, CA) for providing ribophorin II antibodies, Dr W.E.Balch (TSRI, CA) for providing recombinant Sec13/31 subunits and COPII antibodies, and Dr Michael Frohman (SUNY, Stony Brook, NY) for valuable discussions. A.B. is supported by a post-doctoral fellowship from the Osteogenesis Imperfecta foundation. M.A. is supported by grants from the Competitive Medical Research Fund (CMRF) of the University of Pittsburgh Medical Center and by the Edward Mallinckrodt Jr Foundation. K.S. and G.R. are supported by NIH grants DK51183, DK54782 and DK02465. This work is dedicated with love and admiration to the memory of my father, Mr Edward Aridor.
References
- Allan D. (1996) Mapping the lipid distribution in the membranes of BHK cells (mini-review). Mol. Membr. Biol., 13, 81–84. [DOI] [PubMed] [Google Scholar]
- Antonny B. and Schekman,R. (2001) ER export: public transportation by the COPII coach. Curr. Opin. Cell Biol., 13, 438–443. [DOI] [PubMed] [Google Scholar]
- Aridor M. and Balch,W.E. (2000) Kinase signaling initiates coat complex II (COPII) recruitment and export from the mammalian endoplasmic reticulum. J. Biol. Chem., 275, 35673–35676. [DOI] [PubMed] [Google Scholar]
- Aridor M. and Traub,L.M. (2002) Cargo selection in vesicular transport: the making and breaking of a coat. Traffic, 3, 537–546. [DOI] [PubMed] [Google Scholar]
- Aridor M., Bannykh S., Rowe,T. and Balch,W.E. (1995) Sequential coupling between COPII and COPI vesicle coats in endoplasmic reticulum to Golgi transport. J. Cell Biol., 131, 875–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridor M., Weissman,J., Bannykh,S., Nuoffer,C. and Balch,W.E. (1998) Cargo selection by the COPII budding machinery during export from the ER. J. Cell Biol., 141, 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridor M., Fish,K.N., Bannykh,S., Weissman,J., Roberts,T.H., Lippincott-Schwartz,J. and Balch,W.E. (2001) The Sar1 GTPase coordinates biosynthetic cargo selection with endoplasmic reticulum export site assembly. J. Cell Biol., 152, 213–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi K., Roth,M.G. and Ktistakis,N.T. (1997) Phosphatidic acid formation by phospholipase D is required for transport from the endoplasmic reticulum to the Golgi complex. Curr. Biol., 7, 301–307. [DOI] [PubMed] [Google Scholar]
- Bi X., Corpina,R.A. and Goldberg,J. (2002) Structure of the Sec23/24-Sar1 pre-budding complex of the COPII vesicle coat. Nature, 419, 271–277. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Bi,K., Ktistakis,N.T. and Roth,M.G. (2001) Biological properties and measurement of phospholipase D activation by ADP-ribosylation factor (ARF). Methods Enzymol., 329, 355–372. [DOI] [PubMed] [Google Scholar]
- de Figueiredo P., Drecktrah,D., Katzenellenbogen,J.A., Strang,M. and Brown,W.J. (1998) Evidence that phospholipase A2 activity is required for Golgi complex and trans Golgi network membrane tubulation. Proc. Natl Acad. Sci. USA, 95, 8642–8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Figueiredo P., Drecktrah,D., Polizotto,R.S., Cole,N.B., Lippincott-Schwartz,J. and Brown,W.J. (2000) Phospholipase A2 antagonists inhibit constitutive retrograde membrane traffic to the endoplasmic reticulum. Traffic, 1, 504–511. [DOI] [PubMed] [Google Scholar]
- Dominguez M., Dejgaard,K., Fullekrug,J., Dahan,S., Fazel,A., Paccaud,J.P., Thomas,D.Y., Bergeron,J.J. and Nilsson,T. (1998) gp25L/emp24/p24 protein family members of the cis-Golgi network bind both COP I and II coatomer. J. Cell Biol., 140, 751–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godi A., Pertile,P., Meyers,R., Marra,P., Di Tullio,G., Iurisci,C., Luini,A., Corda,D. and De Matteis,M.A. (1999) ARF mediates recruitment of PtdIns-4-OH kinase-β and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat. Cell Biol., 1, 280–287. [DOI] [PubMed] [Google Scholar]
- Iyer S.S. and Kusner,D.J. (1999) Association of phospholipase D activity with the detergent-insoluble cytoskeleton of U937 promonocytic leukocytes. J. Biol. Chem., 274, 2350–2359. [DOI] [PubMed] [Google Scholar]
- Kuehn M.J., Herrmann,J.M. and Schekman,R. (1998) COPII–cargo interactions direct protein sorting into ER-derived transport vesicles. Nature, 391, 187–190. [DOI] [PubMed] [Google Scholar]
- Lee T.H. and Linstedt,A.D. (2000) Potential role for protein kinases in regulation of bidirectional endoplasmic reticulum-to-Golgi transport revealed by protein kinase inhibitor H89. Mol. Biol. Cell, 11, 2577–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Roberts,T.H. and Hirschberg,K. (2000) Secretory protein trafficking and organelle dynamics in living cells. Annu. Rev. Cell Dev. Biol., 16, 557–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscovitch M., Czarny,M., Fiucci,G., Lavie,Y. and Tang,X. (1999) Localization and possible functions of phospholipase D isozymes. Biochim. Biophys. Acta, 1439, 245–263. [DOI] [PubMed] [Google Scholar]
- Matsuoka K., Morimitsu,Y., Uchida,K. and Schekman,R. (1998a) Coat assembly directs v-SNARE concentration into synthetic COPII vesicles. Mol. Cell, 2, 703–708. [DOI] [PubMed] [Google Scholar]
- Matsuoka K., Orci,L., Amherdt,M., Bednarek,S.Y., Hamamoto,S., Schekman,R. and Yeung,T. (1998b) COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell, 93, 263–275. [DOI] [PubMed] [Google Scholar]
- Mizoguchi T., Nakajima,K., Hatsuzawa,K., Nagahama,M., Hauri,H.P., Tagaya,M. and Tani,K. (2000) Determination of functional regions of p125, a novel mammalian Sec23p-interacting protein. Biochem. Biophys. Res. Commun., 279, 144–149. [DOI] [PubMed] [Google Scholar]
- Nagaya H., Wada,I., Jia,Y.J. and Kanoh,H. (2002) Diacylglycerol kinase δ suppresses ER-to-Golgi traffic via its SAM and PH domains. Mol. Biol. Cell, 13, 302–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nufer O., Guldbrandsen,S., Degen,M., Kappeler,F., Paccaud,J.P., Tani,K. and Hauri,H.P. (2002) Role of cytoplasmic C-terminal amino acids of membrane proteins in ER export. J. Cell Sci., 115, 619–628. [DOI] [PubMed] [Google Scholar]
- Plutner H., Davidson,H.W., Saraste,J. and Balch,W.E. (1992) Morphological analysis of protein transport from the ER to Golgi membranes in digitonin-permeabilized cells: role of the P58 containing compartment. J. Cell Biol., 119, 1097–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provost J.J., Fudge,J., Israelit,S., Siddiqi,A.R. and Exton,J.H. (1996) Tissue-specific distribution and subcellular distribution of phospholipase D in rat: evidence for distinct RhoA- and ADP-ribosylation factor (ARF)-regulated isoenzymes. Biochem. J., 319, 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe T. and Balch,W.E. (1995) Expression and purification of mammalian Sarl. Methods Enzymol., 257, 49–53. [DOI] [PubMed] [Google Scholar]
- Rowe T., Aridor,M., McCaffery,J.M., Plutner,H., Nuoffer,C. and Balch,W.E. (1996) COPII vesicles derived from mammalian endoplasmic reticulum microsomes recruit COPI. J. Cell Biol., 135, 895–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudge S.A., Cavenagh,M.M., Kamath,R., Sciorra,V.A., Morris,A.J., Kahn,R.A. and Engebrecht,J. (1998) ADP-ribosylation factors do not activate yeast phospholipase Ds but are required for sporulation. Mol. Biol. Cell, 9, 2025–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudge S.A., Pettitt,T.R., Zhou,C., Wakelam,M.J. and Engebrecht,J.A. (2001) SPO14 separation-of-function mutations define unique roles for phospholipase D in secretion and cellular differentiation in Saccharomyces cerevisiae. Genetics, 158, 1431–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schekman R. and Orci,L. (1996) Coat proteins and vesicle budding. Science, 271, 1526–1533. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Wolde,M., Thiele,C., Fest,W., Kratzin,H., Podtelejnikov,A.V., Witke,W., Huttner,W.B. and Soling,H.D. (1999) Endophilin I mediates synaptic vesicle formation by transfer of arachidonate to lysophosphatidic acid. Nature, 401, 133–141. [DOI] [PubMed] [Google Scholar]
- Shome K., Nie,Y. and Romero,G. (1998) ADP-ribosylation factor proteins mediate agonist-induced activation of phospholipase D. J. Biol. Chem., 273, 30836–30841. [DOI] [PubMed] [Google Scholar]
- Siddhanta A., Backer,J.M. and Shields,D. (2000) Inhibition of phosphatidic acid synthesis alters the structure of the Golgi apparatus and inhibits secretion in endocrine cells. J. Biol. Chem., 275, 12023–12031. [DOI] [PubMed] [Google Scholar]
- Simonsen A., Wurmser,A.E., Emr,S.D. and Stenmark,H. (2001) The role of phosphoinositides in membrane transport. Curr. Opin. Cell Biol., 13, 485–492. [DOI] [PubMed] [Google Scholar]
- Springer S. and Schekman,R. (1998) Nucleation of COPII vesicular coat complex by endoplasmic reticulum to Golgi vesicle SNAREs. Science, 281, 698–700. [DOI] [PubMed] [Google Scholar]
- Springer S., Spang,A. and Schekman,R. (1999) A primer on vesicle budding. Cell, 97, 145–148. [DOI] [PubMed] [Google Scholar]
- Tani K., Mizoguchi,T., Iwamatsu,A., Hatsuzawa,K. and Tagaya,M. (1999) p125 is a novel mammalian Sec23p-interacting protein with structural similarity to phospholipid-modifying proteins. J. Biol. Chem., 274, 20505–20512. [DOI] [PubMed] [Google Scholar]
- Votsmeier C. and Gallwitz,D. (2001) An acidic sequence of a putative yeast Golgi membrane protein binds COPII and facilitates ER export. EMBO J., 20, 6742–6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert R. et al. (1999) CtBP/BARS induces fission of Golgi membranes by acylating lysophosphatidic acid. Nature, 402, 429–433. [DOI] [PubMed] [Google Scholar]
- Weissman J.T., Plutner,H. and Balch,W.E. (2001) The mammalian guanine nucleotide exchange factor mSec12 is essential for activation of the Sar1 GTPase directing endoplasmic reticulum export. Traffic, 2, 465–475. [DOI] [PubMed] [Google Scholar]