Abstract
Wound healing in epithelia requires coordinated cell migration and proliferation regulated by signaling mechanisms that are poorly understood. Here we show that epithelial cells expressing constitutively active or kinase-dead mutants of the Rac/Cdc42 effector Pak1 fail to undergo growth arrest upon wound closure. Strikingly, this phenotype is only observed when the Pak1 kinase mutants are expressed in cells possessing a free lateral surface, i.e. one that is not engaged in contact with neighboring cells. The Pak1 kinase mutants perturb contact inhibition by a mechanism that depends on the Pak-interacting Rac-GEF PIX. In control cells, endogenous activated Pak and PIX translocate from focal complexes to cell–cell contacts during wound closure. This process is abrogated in cells expressing Pak1 kinase mutants. In contrast, Pak1 mutants rendered defective in PIX binding do not impede translocation of activated Pak and PIX, and exhibit normal wound healing. Thus, recruitment of activated Pak and PIX to cell–cell contacts is pivotal to transduction of growth-inhibitory signals from neighboring cells in epithelial wound healing.
Keywords: contact inhibition/epithelial cells/p21-activated kinase 1/PIX/wound healing
Introduction
Polarized epithelial cells form barriers towards luminal surfaces of internal organs. This barrier function is pivotal to the homeostasis of the organism and it is therefore essential that polarized epithelia are able to undergo wound healing after injury. While repair processes have been studied extensively in skin and keratinocyte models (Martin, 1997), the molecular events underlying wound healing of monolayers of polarized epithelial cells (here referred to as polarized epithelia) have received little attention. Non-embryonic wounds in polarized epithelia are closed by coordinate cellular movements, in which membrane sheets, rather than individual cells, move into the denuded area. Cells adjacent to the wound migrate by extending protrusions into the wound, while pulling along cells located behind the wound edge (Jacinto et al., 2001). Moreover, in large wounds, lost cells are replaced by cell proliferation (Martin, 1997; Jacinto et al., 2001). However, the mechanisms that regulate changes in cell motility and proliferation in wound healing are not well understood. Presumably, multiple signals induced by wounding are involved, including mechanical cues, altered contact with extracellular matrix (ECM) components, and release of growth factors by damaged cells (Martin, 1997; Jacinto et al., 2001). Moreover, ‘contact inhibition’ by neighboring cells, a mechanism that inhibits cell motility and proliferation upon reaching high density and/or establishment of cell–cell contacts, is required upon wound closure. Although contact inhibition is a regulatory mechanism in development and its deregulation is thought to be crucial for invasion and metastasis of cancer cells, the signaling pathways mediating contact inhibition remain elusive (Fagotto and Gumbiner, 1996).
Rho family GTPases act as molecular switches in signal transduction pathways linking cell surface receptors to the actin cytoskeleton (Hall, 1998). Signals that induce activation of Rho GTPases include growth factors and integrin signaling as well as mechanical signals such as cell distortion and shear stress. Rho GTPases influence the shape, adhesion, motility and proliferation of cells, events that are all critical in wound healing (Van Aelst and Symons, 2002). Simple scrape wounding assays in cell monolayers have established roles for Rho proteins in wound healing in a variety of cell types (Nobes and Hall, 1999; Fenteany et al., 2000; Etienne-Manneville and Hall, 2001). Epithelial Madin–Darby canine kidney (MDCK) cells close wounds by extending lamellipodia and do not depend on the filopodia-based adhesion zippers described for keratinocytes (Vasioukhin and Fuchs, 2001). In MDCK cell monolayers, Rac1 is essential for the formation of lamellipodia and cell migration into the wound (Fenteany et al., 2000). Likewise, studies using fibroblasts and astrocytes demonstrate a pivotal role of Rac1 for protrusion formation and forward motion, whereas Cdc42 and RhoA regulate directionality of movement and maintenance of cell adhesion, respectively (Nobes and Hall, 1999; Etienne-Manneville and Hall, 2001). In polarized epithelial cells, Rho GTPases are moreover required for establishing epithelial polarity and junctions with neighboring cells. RhoA and Rac1 contribute to the generation and maintenance of adherence and tight junctions (Van Aelst and Symons, 2002). In addition, Rac1 and Cdc42 are involved in the establishment of apical and basolateral polarity, respectively (Van Aelst and Symons, 2002). Little is known, however, about the contribution of effectors of Rho GTPases in wound healing of epithelial cells.
Among the most well established Rac effector molecules are the p21-activated kinases (Paks). Paks are highly conserved 62–68 kDa serine/threonine kinases that are activated by GTP-bound forms of Rac and Cdc42 (Bagrodia and Cerione, 1999). Paks are implicated in many Rac-mediated responses, including cell migration (Kiosses et al., 1999; Sells et al., 1999), neurite outgrowth (Daniels et al., 1998), cell transformation (Tang et al., 1997) and signaling downstream of the T cell receptor (Ku et al., 2001). In this work we have explored the role of Pak1 in epithelial wound healing. We demonstrate that MDCK cells expressing Pak1 kinase mutants do not undergo contact inhibition after closure of a wound, and are defective in redistributing endogenous activated Pak and the Rac GEF PIX (Manser et al., 1998) from focal contacts to lateral membranes upon establishment of cell–cell contacts. This phenotype is entirely dependent on the Pak1–PIX interaction and on inhibition of endogenous Pak1. Collectively, our results demonstrate that Pak1 and PIX play pivotal roles in relaying contact inhibition signals during epithelial wound healing.
Results
Deregulated wound healing in cells expressing constitutively active or kinase-dead Pak1
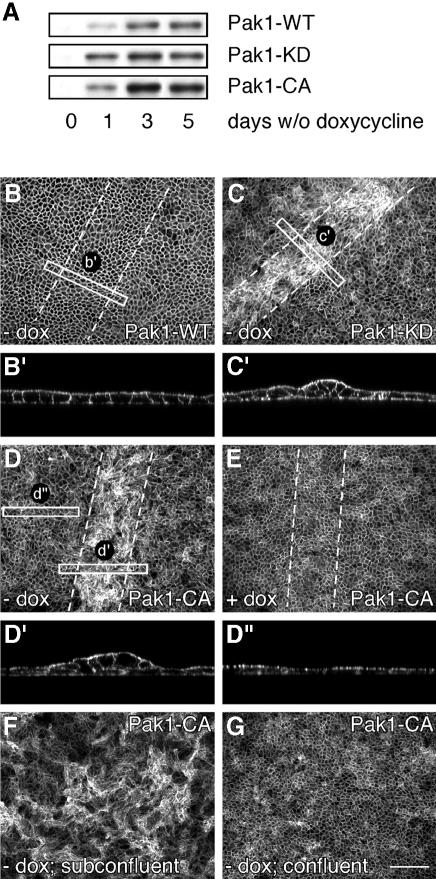
We established clonal MDCK cell lines expressing human wild-type Pak1, Pak1-WT, as well as constitutively active Pak1-CA(T423E) and kinase-dead Pak1-KD(K299R), mutants of Pak1 under the control of the tetracycline-controllable transactivator using the tet-off system (see Materials and methods). After 24 h induction, we observed robust expression of Pak1 in all cell lines, and maximum levels were obtained 2–3 days after induction (Figure 1A). To examine the role of Pak1 in wound healing in a polarized epithelium, cells were plated at high density on permeable Transwell filters and grown for 5 days in the presence of doxycycline. Under these conditions, a fully polarized monolayer, which is contact inhibited for growth, is formed. We then induced Pak1 expression in intact monolayers, or in monolayers that were scrape wounded with a micropipette. The cells were grown for another 48 h with or without doxycycline, which allowed for complete healing of the wound in monolayers of cells expressing Pak1-WT, leaving the site indistinguishable from the remaining non-wounded monolayer (Figure 1B and B′). In contrast, in Pak1-KD-expressing cells, the wound closed, but cells within the wound had lost their regular organization and exhibited extensive cell multilayering (Figure 1C). When the experiment was repeated in cells expressing Pak1-CA, a similar response was observed (Figure 1D). Interestingly, the remainder of the monolayer of Pak1-CA-expressing cells was indistinguishable from Pak1-WT-expressing cells or non-induced controls (compare Figure 1D′ and D′′ as well as B, D and E). Thus, expression of Pak1-CA or Pak1-KD, but not Pak1-WT, perturbs normal healing of a wounded monolayer of epithelial cells.
Fig. 1. Expression of Pak1 mutants interferes with epithelial wound healing. (A) MDCK cells expressing human Pak1-WT, Pak1-KD and Pak1-CA were grown for the indicated periods of time with or without doxycycline (dox). Pak1 expression in cell lysates was assayed by western blotting using an anti-Pak1 antibody, which does not recognize canine Pak1. (B–E) MDCK cells expressing Pak1-WT (B), Pak1-KD (C) or Pak1-CA (D and E) were plated on filters and grown for 5 days in the presence of dox. The monolayers were then wounded by scraping away 30–50 rows of cells (dashed lines indicate wounded areas) and grown for 48 h without (B–D) or with (E) dox. Samples were fixed and stained for F-actin. In non-induced (E) and Pak1-WT-expressing cells (B and enlargement B′) the monolayer restores completely, while wounded areas of Pak1-KD- (C) and Pak1-CA-expressing cells (D) are characterized by cell multilayering (enlargements C′ and D′). Multilayering does not occur further back from the wound edge (enlargement D′′). (F) MDCK cells induced to express Pak1-CA before plating exhibit extensive cell multilayering throughout the culture, whereas no multilayering is observed when Pak1-CA expression is induced after a confluent monolayer is formed (G). Scale bar, 100 µm.
To characterize Pak1-dependent events that occur during wound closure in a uniform system, MDCK cells were plated in small clusters on permeable filters. Before reaching confluency, the cells were induced to express various Pak1 forms. The merging of cell islets to a confluent monolayer can be considered as the closure of a large number of small wounds. Under these conditions, the entire cell culture, after reaching confluency, exhibited the phenotype of cells within a closed wound. Thus, Pak1-CA- (Figure 1F) and Pak1-KD-expressing cells (data not shown) that were induced at subconfluent densities exhibited the cell multilayering phenotype, whereas Pak1-WT cells formed a monolayer similar to non-induced control cells (data not shown). In contrast, such effects of mutant Pak1 expression in MDCK cells that had already established a monolayer were virtually undetectable (Figure 1G). Thus, a free lateral surface, i.e. one that does not engage in cell–cell contact, is required for the effects elicited by expression of Pak1-CA or Pak1-KD in epithelial cells.
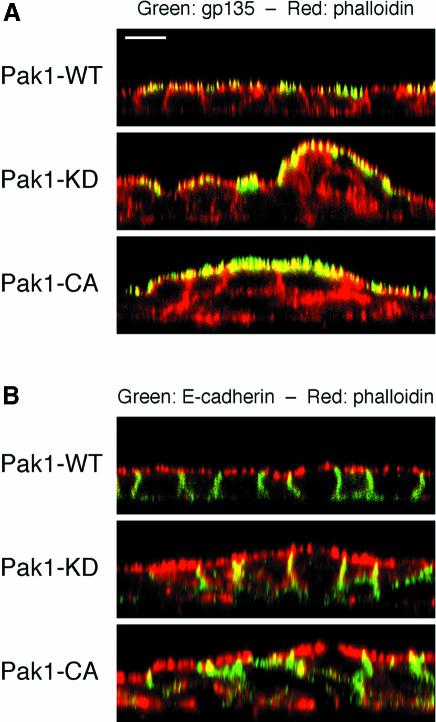
In spite of the grossly abnormal cell–cell arrangements in cultures induced to express Pak1 kinase mutants before plating, the cells were still able to segregate apical marker proteins such as gp135 and gp114 to the free apical surface (Figure 2A; data not shown). Similarly, the Pak1-CA and Pak1-KD mutants did not affect localization of E-cadherin and β-catenin to areas of cell–cell contact or the localization of the basolateral marker protein p58 to the basolateral surface (Figure 2B; data not shown). These results indicate that the ability of MDCK cells to polarize was not directly affected by expression of Pak1-CA and Pak1-KD mutants.
Fig. 2. Cells expressing Pak1 kinase mutants retain the capability to polarize. MDCK cells induced to express Pak1-WT, Pak1-KD and Pak1-CA before plating and grown to confluency were stained with phalloidin (red) to outline cell borders and immunolabeled (green) to detect either the apical gp135 marker (A) or E-cadherin (B). Note that, in spite of extensive multilayering, Pak1-KD- and Pak1-CA-expressing cells are still able to segregate gp135 to the free apical surface (A) and E-cadherin to membranes engaged in cell–cell contact (B). Scale bar, 10 µm.
Pak1-CA and Pak1-KD mutants perturb contact inhibition of cell migration and cell proliferation
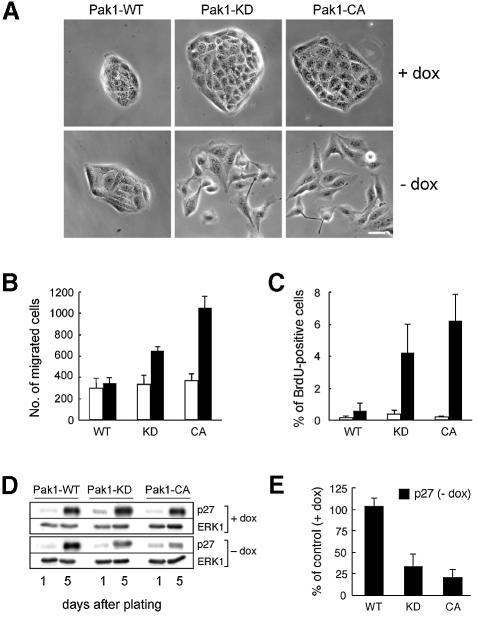
We next examined the effects of the Pak1-CA and Pak1-KD mutants on the rate of wound healing. To this end, we used classical scrape wounding assays as described in Materials and methods. In spite of the abnormal wound closure in cultures of cells expressing Pak1-CA or Pak1-KD, the rate of wound healing was identical to that of Pak1-WT-expressing and control cells (data not shown). Moreover, while the cells expressing Pak1 kinase mutants appeared more scattered at the wound edge, they did not detach from neighboring cells or move into the wound as single cells (data not shown). Microscopic examination revealed that sparsely seeded Pak1-WT cells behaved as non-induced control cells and grew in small clusters (Figure 3A). In contrast, cells expressing Pak1 kinase mutants exhibited a scattered phenotype and had more lamellipodia and cellular extensions than Pak1-WT-expressing cells (Figure 3A). Scattering in MDCK cells is suggestive of increased cell motility, supporting the notion that cells expressing Pak1-KD or Pak1-CA are more motile than Pak1-WT-expressing or non-induced control cells. One possible explanation why the increased motility of MDCK cells expressing Pak1-CA or Pak1-KD does not translate into elevated rates of migration into a wound is that these cells are defective in sensing a cue for directional migration represented by neighboring cells. If this defect is specific for cell contact-dependent signaling, one might predict that MDCK cells expressing Pak1-CA or Pak1-KD would exhibit increased rates of cell migration when exposed to other directional cues. To test this hypothesis, we performed Boyden chamber assays with HGF (hepatocyte growth factor/scatter factor) as chemoattractant. Indeed, we found that the rate of migration towards a gradient of HGF was increased in cells expressing Pak1-CA or Pak1-KD as compared with Pak1-WT-expressing cells (Figure 3B).
Fig. 3. Pak1 mutants promote cell scattering and migration towards HGF and perturb contact inhibition of proliferation. (A) MDCK cells were plated on culture dishes and induced to express Pak1-WT, Pak1-KD or Pak1-CA for 3 days. The images shown are representative of >99% of Pak1-WT-expressing cells, and of 70–90% of Pak1-KD- and Pak1-CA-expressing cells. Scale bar, 20 µm. (B) Migration towards HGF in the lower compartment of Transwell filters was determined as described in Materials and methods. White bars, non-induced controls; black bars, Pak1-expressing cells. In (C) and (D), cells were plated on filters and grown with or without dox. (C) Five days after plating, cell proliferation was assayed by BrdU incorporation as described in Materials and methods. The data represent mean ± SD of three independent experiments. White bars, non-induced controls; black bars, Pak1-expressing cells. (D and E) p27kip1 levels in cell lysates prepared from cells 1 and 5 days after plating were determined by western blotting using a monoclonal anti-p27kip1 antibody (D), and quantified by densitometry (E).
We next tested whether contact inhibition of cell proliferation is perturbed in MDCK cells expressing either Pak1-CA or Pak1-KD. Using a BrdU incorporation assay, we found that 5 days after plating at subconfluent densities Pak1-WT-expressing and non-induced control cells did not enter S-phase (Figure 3C). In contrast, cells expressing Pak1-CA or Pak1-KD continued to proliferate after reaching confluency (Figure 3C). Growth rates of subconfluent Pak1-expressing cells were identical to controls, thus ruling out the possibility that slower growth rates accounted for the observed effects on cell proliferation (data not shown). In epithelial cells, contact inhibition of cell proliferation is controlled by cadherin-mediated upregulation of the cdk2 inhibitor p27kip1 (St Croix et al., 1998; Levenberg et al., 1999). We therefore analyzed the protein levels of p27kip1 in subconfluent and confluent MDCK cells expressing wild-type or mutant Pak1. Whereas in control and Pak1-WT-expressing cells, p27kip1 levels increased strongly after reaching confluence, cells expressing either Pak1-CA or Pak1-KD had substantially diminished levels of p27kip1 as compared with non-induced controls (Figure 3D and E). This result further supports a role of Pak1 in regulating contact inhibition of cell proliferation. As observed for the wound healing process, the effects of Pak1 kinase mutants on BrdU incorporation and p27kip1 expression were only observed when Pak1 expression was induced in subconfluent cultures (data not shown), again indicating a requirement for a free lateral cell surface to elicit phenotypes of the mutant Pak1 proteins.
Since a role for integrin-mediated signaling has been invoked for contact inhibition (Huttenlocher et al., 1998), we next addressed the possibility that the defect in contact inhibition in Pak1-CA- or Pak1-KD-expressing cells was indirect, a consequence of altered deposition of ECM. MDCK cells express α2β1, receptor for collagen IV; α3β5, receptor for laminin V; αvβ3, receptor for fibronectin and vitronectin; and α6β4, receptor for laminin V (Schoenenberger et al., 1994; K.Matlin, personal communication). We thus visualized each of these ECM constituents in confluent cultures of cells where expression of wild-type or mutant Pak1 was previously induced at low cell density. No discernible alterations were detected in the patterns of collagen IV, laminin, fibronectin and vitronectin deposited by Pak1-CA- or Pak1-KD- as compared with Pak1-WT-expressing cells (see Supplementary figure 1 available at The EMBO Journal Online; data not shown). These data suggest that the defect in contact inhibition in Pak1 kinase mutant-expressing cells is intrinsic rather than secondary to aberrant deposition of ECM components.
Pak1-CA and Pak1-KD mutants perturb epithelial wound healing through sequestration of PIX
Pak is recruited into a complex that includes PIX (also known as ‘Cool’) (Bagrodia et al., 1998; Manser et al., 1998), the ARF GAPs PKL (Turner et al., 1999) or GIT/Cat (Bagrodia et al., 1999; Zhao et al., 2000b) and the focal adhesion adapter protein paxillin (Turner et al., 1999). The regulation of formation and disassembly of this complex is not well understood, but seems to involve a dynamic equilibrium between Pak autophosphorylation and inactivation (Zhao et al., 2000a). Since expression of Pak1-KD and Pak1-CA gave similar phenotypes, we suspected that both Pak1 kinase mutants might affect Pak1–PIX signaling. However, no effect of Pak1-KD or Pak1-CA expression was found on the association between Pak1 and PIX (β-PIX) in co-immunoprecipitation experiments. Thus, the amount of PIX associated with Pak1 and vice versa was the same in detergent-soluble lysates of cells expressing the Pak1 kinase mutants as in Pak1-WT-expressing cells. However, when we analyzed the detergent-insoluble cytoskeletal pellet, we found that expression of Pak1-KD and Pak1-CA mutants increased the amount of detergent-insoluble PIX by 3- and 5-fold, respectively (Supplementary figure 2), suggesting that the expression of Pak1 mutants resulted in sequestration of PIX into a cytoskeletal-associated fraction.
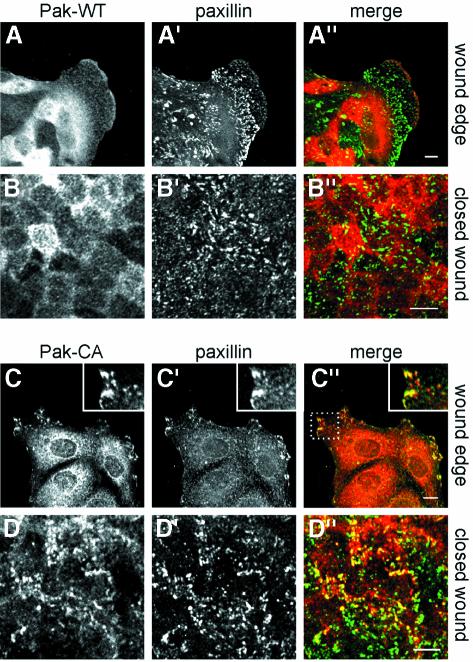
We next examined the effects of Pak1 kinase mutants on Pak1 and PIX localization during closure of scrape wounds in MDCK cell monolayers. Following induction of Pak1 expression for 48 h, the cells were fixed and double-labeled to detect Pak1 and paxillin, or Pak1 and PIX. At the wound edge, Pak1-WT accumulated in membrane ruffles, and co-localization with paxillin was limited to focal complexes within areas of cell ruffling (Figure 4A–A′′). In contrast, Pak1-CA (Figure 4C–C′′) and Pak1-KD (data not shown) co-localized extensively with paxillin in focal contacts (larger matured focal complexes) in the periphery of cells at the wound edge. After wound closure, Pak1-WT partly accumulated at areas of cell–cell contact in confluent cells and co-localization with paxillin was virtually absent (Figure 4B–B′′). Contrary to Pak1-WT, no Pak1-CA (Figure 4D–D′′) or Pak1-KD (data not shown) accumulated around sites of cell–cell contact after wound closure. Instead, both Pak1 mutants were retained in focal contact-like structures as evidenced by extensive co-localization with paxillin at the base of cells (Figure 4D–D′′).
Fig. 4. Pak1 mutants fail to redistribute from focal complexes after wound closure. The localization of Pak1 and paxillin in MDCK cells was analyzed before and after wound closure. Images show staining for Pak1 (A–D), paxillin (A′–D′) and merged images (A′′–D′′: Pak1 in red; paxillin in green). In all samples, a diffusely stained intracellular pool of Pak1 is observed. At the wound edge, WT-Pak1 is also found in cell ruffles (A), where limited co-localization with paxillin is observed (A′ and A′′). In contrast, Pak1-CA (C) extensively co-localizes with paxillin (C′ and C′′), although the number of paxillin-containing focal complexes is diminished. Co-localization of Pak1-CA and paxillin is highlighted in the insert in (C′′), which represents an enlargement of the area in the dashed rectangle. In closed wounds, Pak1-WT (B) does not co-localize with paxillin (B′), whereas Pak1-CA (D) remains co-localized with paxillin (D′ and D′′). Pak1-expressing cells comprised ∼80% of all cells, as determined by immunofluoresence. The extent of co-localization of Pak1 and paxillin in Pak1-expressing cells was similar in all Pak1-expressing cells at the indicated sites. Scale bars, 10 µm.
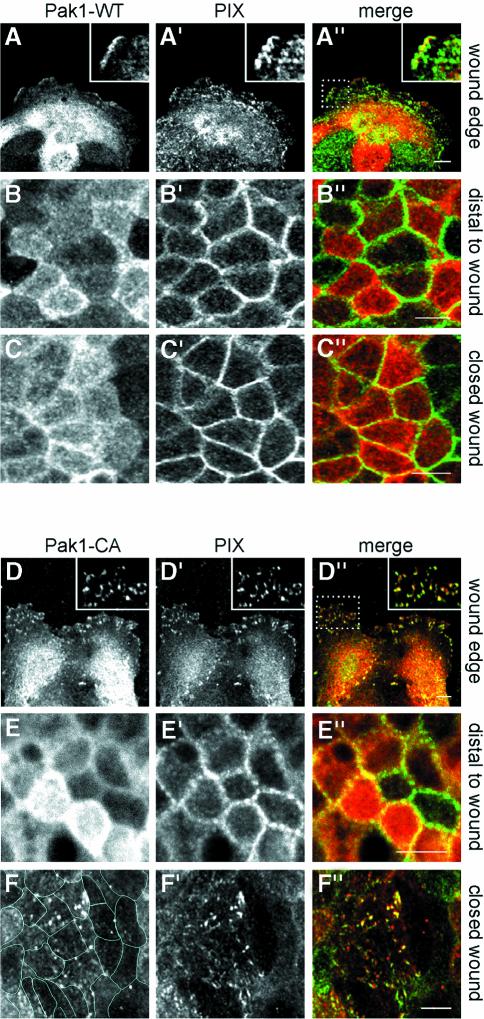
A large proportion of endogenous PIX (β-PIX) was found in the cytoplasm of MDCK subconfluent cells (Figure 5A′) in line with a previous report (Koh et al., 2001). At the wound edge in Pak1-WT-expressing (Figure 5A–A′′) and control cells (data not shown), PIX was detected at focal contacts to a significant extent, and co-localization with Pak1-WT was restricted to cell ruffles. Distal (>20 rows of cells) to the wound (Figure 5B–B′′) and in closed wounds of Pak1-WT-expressing (Figure 5C–C′′) and control cells (data not shown), a strikingly different localization was found in that PIX was highly concentrated at areas of cell–cell contact. Serial sectioning analyses revealed that PIX co-localized with β-catenin (Supplementary figure 3). However, in contrast to β-catenin staining, which is uniform along lateral membranes of MDCK cells, PIX labeling was discontinuous and suggestive of PIX localizing to distinct contact points between membranes (Supplementary figure 3). Hence, in Pak1-WT-expressing cells, PIX, like Pak1, translocates from cell–substratum to cell–cell contacts upon reaching confluency. This translocation of PIX occurred gradually and full redistribution was observed ∼3 days after initial cell–cell contact. This time course correlated closely with the establishment of contact inhibition. In contrast, Pak1-CA (Figure 5D–D′′) or Pak1-KD (data not shown) showed extensive co-localization with PIX in focal contacts in cells at the wound edge. Moreover, within closed wounds of Pak1-CA- (Figure 5F–F′′) or Pak1-KD-expressing cells (data not shown), PIX was largely absent from the cell–cell contacts and still co-localized to a significant extent with mutant Pak1 in focal contact-like structures. In addition, PIX was found in abundance throughout the cytoplasm (Supplementary figure 3). This effect was specific for the wounded area as PIX localization in Pak1-CA- (Figure 5E–E′′) and Pak1-KD-expressing cells (data not shown) distal to the wound was virtually indistinguishable from Pak1-WT-expressing (Figure 5B–B′′) or control cells.
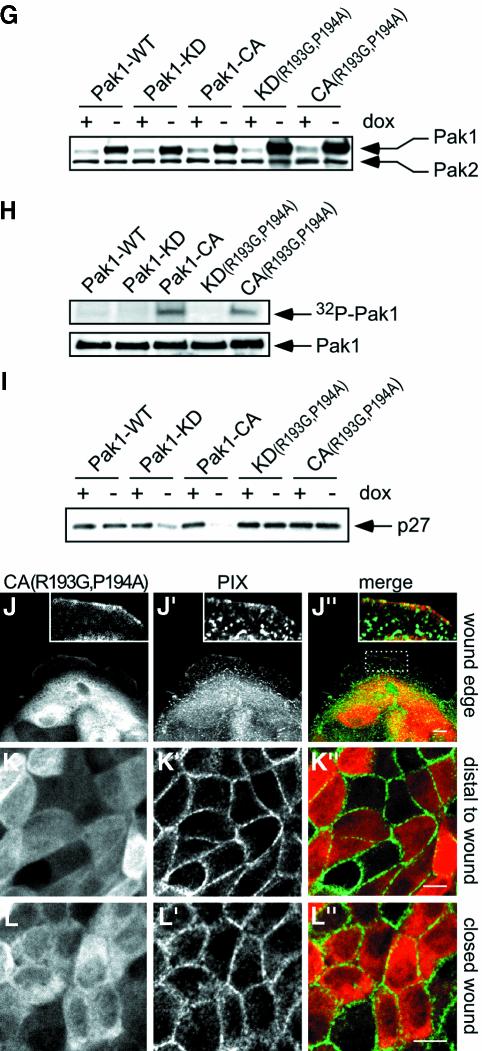
Fig. 5. Pak1 regulates PIX localization during epithelial wound closure. Pak1 and PIX localization in MDCK cells at wound edges (A–A′′ and D–D′′) and within closed wounds (C–C′′ and F–F′′). In addition, cells >200 µm (∼20 cells) distal to the wound edge were analyzed (B–B′′ and E–E′′). Images represent staining for Pak1 (A–F), PIX (A′–F′) and Pak1 (red) and PIX (green) merged (A′′–F′′). Inserts in (A′′) and (D′′) show co-localization of Pak1 and PIX and represent enlargements of areas enclosed by rectangles. (A–C) Pak1-WT-expressing cells. At wound edges, Pak1-WT accumulates in cell ruffles (A), and shows limited co-localization with PIX (A′). In cells distal to wound edges and in closed wounds, Pak1-WT partly accumulates at sites of cell–cell contacts (B and C) and co-localizes with PIX (B′ and C′), which is concentrated at areas of cell–cell contact. (D–F) Pak1-CA-expressing cells. Pak1-CA (D) and PIX (D′) co-localize extensively in focal contacts in cells at the wound edge (D–D′′, and insert in D′′). In cells distal to the wound edge, Pak1-CA (E) localizes to areas of cell–cell contact and diffusely within the cytoplasm, while PIX (E′) is concentrated at cell–cell contacts. In closed wounds, Pak1-CA (F) and PIX (F′) co-localize in focal contact-like structures while PIX staining at cell–cell contacts is largely absent. Lines in (F) outline individual cells. (G–I) Cell lysates were prepared from cells grown for 5 days with or without dox. (G) Pak expression was analyzed by probing western blots with an antibody, which recognizes Pak1 and Pak2 in MDCK cells. In the absence of dox, Pak1-WT, Pak1-KD and Pak1-CA overexpression levels are 3- to 4-fold, whereas Pak1-KD(R193G,P194A) and Pak-CA(R193G,P194A) are overexpressed ∼8-fold. (H) Kinase activity of Pak1 mutants was determined by autophosphorylation (top panel) of Pak immunoprecipitates (bottom panel) as described in Materials and methods. (I) Western blot showing levels of p27kip1. (J–L) Pak1-CA(R193G,P194A)-expressing cells. In these cells, the localization of Pak1 (J–L) and PIX (J′–L′) and their merged distribution (J′′–L′′) is virtually identical to those observed with cells expressing Pak1-WT. Pak1-expressing cells comprised 70–80% of all cells. Images are representative for all Pak1-expressing cells at the indicated sites. Scale bars, 10 µm.
Our results suggest that Pak1-CA- and Pak1-KD-expressing cells are unable to redistribute PIX from Pak–PIX–paxillin-containing focal contacts to areas of cell–cell contact upon wound closure. To test whether the phenotype of cells expressing Pak1 mutants was directly dependent on the Pak–PIX interaction, we generated MDCK cells expressing Pak1-CA or Pak1-KD harboring a double point mutation (R193G,P194A) that prevents PIX binding (Manser et al., 1998). Although these mutants were expressed at levels exceeding those of other Pak1 forms (Figure 5G), they were unable to interact with PIX, as confirmed by co-immunoprecipitation experiments (data not shown). In contrast to their PIX-binding counterparts, association of these mutants with the detergent-insoluble fraction was not increased as compared with controls (data not shown). While not affecting the kinase activity (Figure 5H), introduction of the (R193G,P194A) double point mutation into Pak1-CA or Pak1-KD resulted in a complete reversal of the mutant Pak1 phenotype. The phenotypes of MDCK cells expressing Pak1-CA(R193G,P194A) or Pak1-KD(R193G,P194A) were indistinguishable from that of Pak1-WT-expressing cells or cells expressing Pak1-WT(R193G,P194A). When plated at sparse densities, Pak1-CA(R193G,P194A) and Pak1-KD(R193G,P194A) expressing MDCK cells grew in small clusters identical to cells expressing Pak1-WT or controls (data not shown). Moreover, the R193G,P194A mutation in the Pak1-CA or Pak1-KD backgrounds restored contact inhibition of cell proliferation, as evidenced by BrdU incorporation experiments (data not shown) and normalization of p27kip1 levels (Figure 5I). Accordingly, wound healing of MDCK cells expres sing Pak1-CA(R193G,P194A) (Figure 5J–L) or Pak1-KD(R193G,P194A) (data not shown) was normal. Finally, the localization of PIX in cells expressing Pak1-CA(R193G,P194A) (Figure 5J′,K′ and L′) or Pak1-KD(R193G,P194A) (Supplementary figure 4) was identical to that of Pak1-WT-expressing or control cells, both at the wound edge (Figure 5J′) and in cells distant from the wound (Figure 5K′) as well as in the closed wound (Figure 5L′). These results demonstrate that the effects of Pak1-CA and Pak1-KD on epithelial wound healing are completely dependent on interaction with PIX.
The Pak1 AID perturbs contact inhibition and PIX redistribution during wound healing
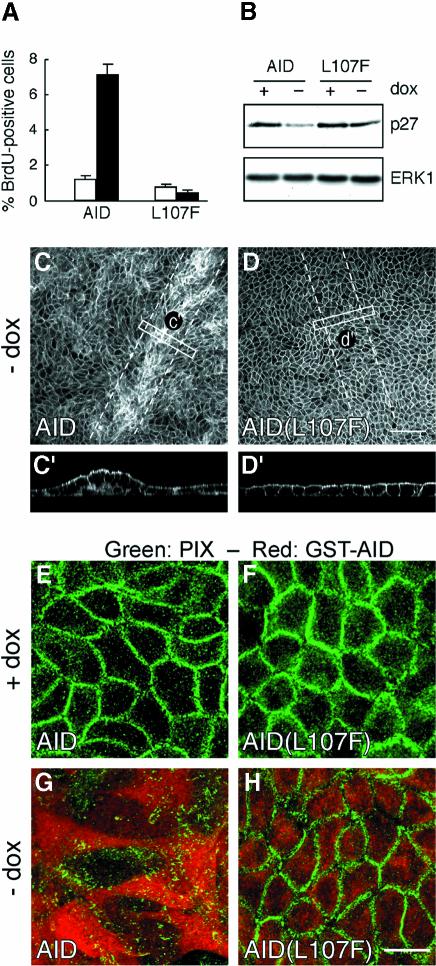
Based on the highly similar effects of the Pak1-CA and Pak1-KD mutants, we hypothesized that the observed phenotypes might result from interference with the function of endogenous Pak1. To test this hypothesis, we generated MDCK cells lines expressing either the regulatory region of Pak1 consisting of residues 1–246, Pak1(1–246), or the autoinhibitory domain (AID) comprised of amino acids 83–149 under the control of the tetracycline-controllable transactivator. As a control for the AID, we used an AID construct harboring a L107F point mutation, which abolishes autoinhibition and leads to constitutive activation when introduced into full-length Pak (Zhao et al., 2000a). In spite of the fact that Pak1(1–246) additionally contains the motifs for binding PIX and the SH2–SH3 adapter Nck as well as the CRIB domain, the data obtained with this fragment (data not shown) were virtually identical to those observed following expression of the AID alone, which in turn closely mimicked the results of the experiments with the kinase mutants described above. Hence, expression of the AID, but not AID(L107F), induced cell scattering of MDCK cell islets (data not shown) and interfered with contact inhibition of cell proliferation, as evidenced by increased BrdU incorporation (Figure 6A) and decreased p27kip1 levels in confluent cell monolayers (Figure 6B). Moreover, wound healing in AID-expressing cells was perturbed and linked to a retention of PIX in focal contacts in areas of cell monolayers, whereas wound healing and PIX localization in AID(L107F)-expressing cells was indistinguishable from that of control cells (Figure 6C–H). Cell polarity was not affected by expression of the AID (data not shown).
Fig. 6. Pak1 AID perturbs PIX redistribution and contact inhibition during wound healing. MDCK cells expressing GST-tagged Pak1 AID or the inactive Pak1 AID(L107F) were grown for 5 days with or without dox. (A) Cell proliferation analyzed by BrdU incorporation. (B) Western blot showing p27 levels. (C and D) Five-day-old monolayers of filter grown MDCK cells were scrape wounded as indicated, and induced for 48 h, resulting in cell multilayering in closed wounds of AID- (C) but not in AID(L107F)-expressing (D) cells. (E–H) The localization of PIX and AID (E and G) or AID(L107F) (F and H) in closed wounds was determined by immunostaining with monoclonal anti-PIX (green) and polyclonal anti-GST (red) antibodies, respectively. Expression of AID (G), but not AID(L107F) (H) prevents the relocalization of PIX to cell–cell contacts after wound closure. AID- and AID(L107F)-expressing cells comprised 80–90% of all cells. Images are representative for all AID/AID(L107F)-expressing cells. Scale bar in (C and D), 100 µm; (E–H), 10 µm.
Pak1 kinase mutants inhibit recruitment of endogenous activated Pak to lateral membranes
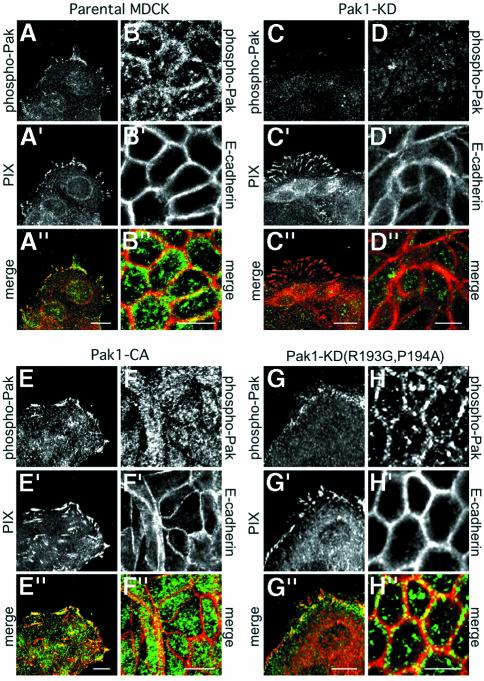
The results obtained with AID-expressing cells demonstrate that inhibition of endogenous Pak function perturbs contact inhibition but do not reveal the subcellular localization where activated Pak is required to regulate cell growth during wound healing. To address this issue we examined the localization of activated Pak during wound healing using an antibody specific for phospho-Pak1 (S199/204) and phospho-Pak2 (S192/197) as detailed in Materials and methods. In untransfected control cells, phospho-Pak redistributed from focal complexes (Figure 7A–A′′) to membranes engaged in cell–cell contact (Figure 7B–B′′) during wound closure. An identical response was observed in cells overexpressing Pak1-WT, indicating that transfected Pak1-WT behaves similarly to endogenous Pak1 and does not interfere with the function of endogenous Pak1 (data not shown). In contrast, Pak1-KD-expressing cells were largely devoid of phospho-Pak labeling at focal contacts (Figure 7C–C′′) as well as at lateral membranes (Figure 7D–D′′). Strikingly, while Pak1-CA-expressing cells exhibited strong phospho-Pak staining in focal contact-like structures (Figure 7E–E′′), no, or very sparse, labeling for endogenous or transfected phospho-Pak1 was found at areas of cell–cell contact in closed wounds (Figure 7F–F′′). Likewise, no phospho-Pak staining was observed at lateral membranes in cells expressing the Pak1 AID (data not shown). In contrast, cells expressing Pak1 kinase mutants defective in PIX binding exhibited translocation of phospho-Pak from focal complexes (Figure 7G–G′′) to lateral membranes (Figure 7H–H′′) during wound healing as observed for untransfected or Pak1-WT-expressing cells. This result supports the conclusion that the Pak1 kinase mutants must form a complex with PIX to inhibit endogenous Pak. Therefore, loss of contact inhibition in not only Pak1-KD- but also Pak1-CA-expressing cells is associated with perturbed recruitment of activated Pak to membranes engaged in cell–cell contact by a PIX-dependent mechanism. Collectively, our data support a critical role for endogenous activated Pak and PIX in transducing contact inhibition signals during closure of epithelial wounds.
Fig. 7. Pak1 kinase mutants perturb recruitment of endogenous Pak to lateral membranes. The localization of activated, phosphorylated Pak (phospho-Pak) was analyzed in cells at wound edges and within closed wounds. Staining of untransfected, T23 MDCK cells reveals that endogenous phospho-Pak is present in focal complexes in cells at wound edges (A) where it co-localizes with PIX (A′ and A′′). After wound closure, endogenous phospho-Pak relocalizes to areas of cell–cell contacts (B), which are delineated by staining with E-cadherin (B′ and B′′). Cells expressing Pak1-KD (∼80% of all cells) exhibit a virtual absence of phospho-Pak staining at wound edges (C–C′′) as well as at cell–cell contacts (D–D′′). In Pak1-CA-expressing cells (∼85% of all cells), phospho-Pak staining is increased at focal contacts (E–E′′), but is absent from cell–cell contacts (F–F′′). Localization of phospho-Pak in cells expressing Pak1-KD(P193G,R194A) is virtually similar to untransfected control cells, both at wound edges (G–G′′) and at cell–cell contacts in closed wounds (H–H′′). Images are representative for all Pak1-expressing cells. Scale bars, 10 µm.
Discussion
We have investigated the role of Pak1, which is widely expressed in epithelial organs, including the kidney (UNIGENE, NCBI), in wound healing in MDCK cells. Contrary to control or Pak1-WT-expressing cells, we demonstrate that cells expressing Pak1-CA or Pak1-KD exhibit cell multilayering upon closure of a wound. This phenotype is not observed in cells separated from the wound edge; it requires a free surface. This conclusion is corroborated by experiments where Pak1 expression is induced in low-density cultures and the cells grown to confluency. Under these conditions, we find that contact inhibition of cell proliferation is substantially attenuated in Pak1-CA- or Pak1-KD-expressing cells. Our data further indicate that cells expressing Pak1 kinase mutants show defects in contact inhibition of cell migration, as increased motility in these cells does not translate into faster wound healing. We suggest that cells expressing Pak1-CA or Pak1-KD are unable to sense neighboring cells and use this cue for orienting cell migration away from neighboring cells as well as for arresting cell proliferation and cell motility upon reaching confluency. Indeed, precedence for a role of Pak in cell–cell recognition exists in Drosophila, where Pak regulates photoreceptor axon guidance (Hing et al., 1999). Interestingly, not only inhibition of Pak function but also overexpression of membrane-bound Pak resulted in severely disrupted photoreceptor axon projection patterns onto the optic lobe (Hing et al., 1999). Precedence for a role for Pak1 in eliciting cell multilayering has also recently been provided by the demonstration of hyperplasia of the ductal epithelium of the lactating mammary gland in transgenic mice expressing Pak1(T423E) under the control of a tissue-specific promoter (Wang et al., 2002).
Next we show that the phenotype of MDCK cells expressing Pak1-CA and Pak1-KD is mediated by endogenous PIX. We moreover demonstrate that the phenotype of cells expressing Pak1-KD or Pak1-CA is linked to the retention of Pak1 and PIX in focal contacts, and that in these cells activated Pak and PIX fail to localize to areas of cell–cell interaction during wound closure. Previously it was reported that an ECM-dependent redistribution of the Rac GEF Tiam1 from lamellipodia to cell–cell contacts in MDCK cells correlated with motile and adhesive phenotypes, respectively (Sander et al., 1998), but the intracellular signals mediating this redistribution are not known. Our results invoke a role for Pak1 in controlling the release of PIX from focal contacts to cell–cell contacts, in addition to the previously established role for PIX in recruiting Pak to areas of cell–substratum contact (Manser et al., 1998). The question of why activated Pak in some studies (Obermeier et al., 1998; Kiosses et al., 1999; Sells et al., 1999), including this work, are retained in focal contacts but not in others (Manser et al., 1997; Frost et al., 1998; Brown et al., 2002) still remains unsettled. However, the apparent discrepancy may simply reflect cell type-dependent differences in the extent that activated Pak elicits disassembly of focal contacts, which involves PIX-induced conformational changes in GIT (Zhao et al., 2000b). Hence, variations between cell types in the expression and/or activity of PIX and associated proteins may determine the response to activated Pak.
The virtually identical phenotypes elicited by expression of Pak1-CA and Pak1-KD in MDCK cells is elucidated by our finding that similar results are obtained following expression of the Pak1 AID, and that both Pak1 kinase mutants perturb recruitment of endogenous activated Pak and PIX to lateral membranes. The Pak1 kinase mutants therefore interfere with the function of endogenous Pak. This may result from the formation of dimers between Pak1 kinase mutants and endogenous Pak (Lei et al., 2000; Parrini et al., 2002). However, such dimerization is inadequate on its own as the phenotype of the Pak1 kinase mutants is normalized by abrogating PIX binding, which does not interfere with Pak1 dimerization (Parrini et al., 2002). This suggests that the Pak1 kinase mutants target endogenous Pak in complex with PIX, which also dimerizes (Koh et al., 2001). It is possible that Pak1 must undergo an activation–deactivation cycle in order to disengage from focal contacts (Zhao et al., 2000a). We hypothesize that this cycle is perturbed in Pak1-CA- and Pak1-KD-expressing MDCK cells, albeit at different stages, as evidenced by phospho-Pak staining of focal contact-like structures in Pak1-CA- but not Pak1-KD-expressing cells. Accordingly, both kinase mutants (and the AID) affect autophosphorylation (Zenke et al., 1999), which regulates the distribution of Pak1 between cytoplasm and focal complexes (Zhao et al., 2000a).
How might activated Pak1 and PIX transduce contact inhibition signals upon wound closure? In polarized epithelial cells, Rac1 is required for cell migration (Ridley et al., 1995), establishment and maintenance of cell–cell contacts and cell polarity (Van Aelst and Symons, 2002). To accomplish such diverse functions, not only the level of Rac1-GTP but also the temporal and spatial activation of Rac1 within the cell must be tightly controlled for specific activation of effector molecules (del Pozo et al., 2000; Kraynov et al., 2000). GEF molecules like PIX exert pivotal roles in controlling where and when small GTPases are activated (Symons and Settleman, 2000). It is possible that redistribution of Pak1 and PIX from focal complexes to areas of cell–cell interaction is accompanied by an increase in Rac1-GTP levels at these sites. There, Rac1-GTP promotes cell–cell adhesion (Ehrlich et al., 2002), for instance by counteracting the effect of IQGAP, a Rac and Cdc42 effector molecule that is negatively regulated by activated Rac1 and Cdc42 and serves to uncouple E-cadherin-mediated cell–cell adhesion (Kuroda et al., 1998). Hence, in MDCK cells expressing Pak1 kinase mutants or Pak1 AID, where activated Pak1 and PIX cannot redistribute from the wound edge to cell–cell junctions, E-cadherin mediated cell–cell adhesion, as well as growth suppression (St Croix et al., 1998), may be uncoupled resulting in cell multilayering after wound closure. It should be noted, however, that expression of constitutively active Rac1 in MDCK cells on its own does not lead to perturbation of contact inhibition (Van Aelst and Symons, 2002). Thus, the effects observed following expression of Pak1 kinase mutants or the AID are not solely mediated by activation of Rac1.
A second possibility is that the Pak1–PIX complex acts downstream of integrins, which have been implicated in contact inhibition of cell migration (Huttenlocher et al., 1998). MDCK cells express several integrin families at the basal surface and at areas of cell–cell contact (Schoenenberger et al., 1994). These scenarios are not mutually exclusive and may indeed act synergistically (Huttenlocher et al., 1998). Finally, Merlin, the product of the Neurofibromatosis 2 gene, which mediates contact inhibition in its hypophosphorylated form (Morrison et al., 2001), has recently been shown to undergo Rac1-dependent phosphorylation by Pak (Shaw et al., 2001; Kissil et al., 2002; Xiao et al., 2002). Future studies to address these possibilities will further elucidate the role of activated Pak and PIX in wound healing and other processes where contact inhibition, or loss thereof, plays a crucial role, such as embryogenesis and cell transformation.
Materials and methods
Reagents
Rabbit anti-Pak1, anti-β-catenin and anti-vitronectin pAbs were from Santa Cruz Biotechnology, Inc. Rabbit anti-phospho-Pak1(S199/204)/phospho-Pak2(S192/197) pAb was from Cell Signaling Technology. Rabbit anti-collagen IV and anti-laminin pAbs were from Abcam and Sigma, respectively. Mouse mAbs against paxillin, βPIX and p27kip1 fibronectin were from Becton Dickinson Laboratories. Other reagents used for this work have been detailed previously (Hansen et al., 2000).
Constructs and cell lines
Expression constructs encoding Pak1-WT, Pak1-CA(T423E) and Pak1-KD(K299R) in pTet-Splice have been described previously (Sells et al., 1999). Pak1-CA(R193A,P194A) and Pak1-KD(R193A,P194A) in pTet-Splice were generated by PCR. All Pak expression constructs were verified by sequencing. Pak1-pTet-Splice expression constructs were transfected into MDCK cells harboring the tet-off transactivator as previously described (Hansen et al., 2000). For selection, pcDNA6/V5-His (Invitrogen) was co-transfected at a 1:100 ratio of the Pak1 expression plasmids, and ∼25 clones resistant to blasticidin were isolated for each construct. Generally, three inducible clones were selected and used in parallel for experiments yielding similar results.
Confocal fluorescence microscopy
For F-actin staining, samples were processed as previously described (Hansen et al., 2000). For staining of paxillin, Pak1, PIX and ECM constituents, samples were fixed in 2% paraformaldehyde in PBS for 2 min at room temperature, followed by additional fixation/permeabilization in methanol at –20°C. Cells processed for phospho-Pak labeling were fixed for 5 min in methanol/acetone at –20°C. Labeling with antibodies, as well as image sampling, processing and preparation were carried out as described previously (Hansen et al., 2000). All immunofluoresence experiments were carried out a minimum of three times, yielding essentially identical results.
Cell migration
Boyden chamber assays were performed essentially as described by Sander et al. (1998). Twenty-four hours after induction of Pak expression, 1 × 105 cells in growth medium were seeded in the upper compartment of Transwells (6.5 mm diameter, 8 µm pore size) previously coated overnight at 4°C with 10 µg/ml collagen I. The lower compartment contained growth medium supplemented with 10 ng/ml recombinant human HGF. After 5 h at 37°C, non-migrated cells in the top chamber were removed with a cotton swab, and cells that had migrated to the underside of the filter were fixed and stained with hematoxylin. Cell migration was quantified by counting the total number of cells in 10 systematically sampled microscopic fields.
The rate of cell migration was measured in triplicate in three independent scrape wounding assays. The borders of ∼1 cm wide wounds were marked, and wound closure was recorded at intervals from 6 to 24 h using a phase-contrast microscope (Axiovert 10; Carl Zeiss Inc.) linked to a CCD camera (Hamamatsu).
Pak1 kinase assay
Kinase activity of the Pak1 constructs was determined by incubating anti-Pak1 immunoprecipitates in 25 µl of kinase buffer (20 mM HEPES pH 7.4, 10 mM MgCl2, 100 mM Na3VO4, 10 mM NaF) containing 10 µM [32P]ATP and 100 µM cold ATP for 15 min at room temperature. The reaction was stopped by addition of 10 µl of 4× Laemmli buffer. Proteins were separated on a 4–15% SDS–polyacrylamide gel, and incorporation of 32P was determined by PhosphorImager analysis (Molecular Dynamics). The data shown are representative of three independent experiments.
BrdU incorporation
Determination of cell proliferation by incorporation of BrdU was performed as previously described (Hansen et al., 2000).
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We are grateful to Drs James E.Casanova (UVA), K.Matlin (UC), and Martin McMahon (UCSF) for helpful suggestions and to Dr Ralph Schwall (Genentech) for recombinant human HGF. This investigation was supported by a fellowship from the California Division of the American Cancer Society (2-7-99) to M.M.P.Z., NIH grants to K.E.M. (R01 AI53194) and J.C. (R01 GM56168), as well as funds from Boston Biomedical Research Institute to S.H.H.
References
- Bagrodia S. and Cerione,R.A. (1999) Pak to the future. Trends Cell Biol., 9, 350–355. [DOI] [PubMed] [Google Scholar]
- Bagrodia S., Taylor,S.J., Jordon,K.A., Van Aelst,L. and Cerione,R.A. (1998) A novel regulator of p21-activated kinases. J. Biol. Chem., 273, 23633–23636. [DOI] [PubMed] [Google Scholar]
- Bagrodia S., Bailey,D., Lenard,Z., Hart,M., Guan,J.L., Premont,R.T., Taylor,S.J. and Cerione,R.A. (1999) A tyrosine-phosphorylated protein that binds to an important regulatory region on the cool family of p21-activated kinase-binding proteins. J. Biol. Chem., 274, 22393–22400. [DOI] [PubMed] [Google Scholar]
- Brown M.C., West,K.A. and Turner,C.E. (2002) Paxillin-dependent paxillin kinase linker and p21-activated kinase localization to focal adhesions involves a multistep activation pathway. Mol. Biol. Cell, 13, 1550–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels R.H., Hall,P.S. and Bokoch,G.M. (1998) Membrane targeting of p21-activated kinase 1 (PAK1) induces neurite outgrowth from PC12 cells. EMBO J., 17, 754–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo M.A., Price,L.S., Alderson,N.B., Ren,X.D. and Schwartz,M.A. (2000) Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J., 19, 2008–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich J., Hansen,M. and Nelson,W. (2002) Spatio-temporal regulation of Rac1 localization and lamellipodia dynamics during epithelial cell–cell adhesion. Dev. Cell, 3, 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S. and Hall,A. (2001) Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCζ. Cell, 106, 489–498. [DOI] [PubMed] [Google Scholar]
- Fagotto F. and Gumbiner,B.M. (1996) Cell contact-dependent signaling. Dev. Biol., 180, 445–454. [DOI] [PubMed] [Google Scholar]
- Fenteany G., Janmey,P.A. and Stossel,T.P. (2000) Signaling pathways and cell mechanics involved in wound closure by epithelial cell sheets. Curr. Biol., 10, 831–838. [DOI] [PubMed] [Google Scholar]
- Frost J.A., Khokhlatchev,A., Stippec,S., White,M.A. and Cobb,M.H. (1998) Differential effects of PAK1-activating mutations reveal activity-dependent and -independent effects on cytoskeletal regulation. J. Biol. Chem., 273, 28191–28198. [DOI] [PubMed] [Google Scholar]
- Hall A. (1998) Rho GTPases and the actin cytoskeleton. Science, 279, 509–514. [DOI] [PubMed] [Google Scholar]
- Hansen S.H., Zegers,M.M., Woodrow,M., Rodriguez-Viciana,P., Chardin,P., Mostov,K.E. and McMahon,M. (2000) Induced expression of Rnd3 is associated with transformation of polarized epithelial cells by the Raf-MEK-extracellular signal-regulated kinase pathway. Mol. Cell. Biol., 20, 9364–9375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hing H., Xiao,J., Harden,N., Lim,L. and Zipursky,S.L. (1999) Pak functions downstream of Dock to regulate photoreceptor axon guidance in Drosophila. Cell, 97, 853–863. [DOI] [PubMed] [Google Scholar]
- Huttenlocher A., Lakonishok,M., Kinder,M., Wu,S., Truong,T., Knudsen,K.A. and Horwitz,A.F. (1998) Integrin and cadherin synergy regulates contact inhibition of migration and motile activity. J. Cell Biol., 141, 515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto A., Martinez-Arias,A. and Martin,P. (2001) Mechanisms of epithelial fusion and repair. Nat. Cell Biol., 3, E117–E123. [DOI] [PubMed] [Google Scholar]
- Kiosses W.B., Daniels,R.H., Otey,C., Bokoch,G.M. and Schwartz,M.A. (1999) A role for p21-activated kinase in endothelial cell migration. J. Cell Biol., 147, 831–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissil J.L., Johnson,K.C., Eckman,M.S. and Jacks,T. (2002) Merlin phosphorylation by p21-activated kinase 2 and effects of phosphorylation on Merlin localization. J. Biol. Chem., 277, 10394–10399. [DOI] [PubMed] [Google Scholar]
- Koh C.G., Manser,E., Zhao,Z.S., Ng,C.P. and Lim,L. (2001) Beta1PIX, the PAK-interacting exchange factor, requires localization via a coiled-coil region to promote microvillus-like structures and membrane ruffles. J. Cell Sci., 114, 4239–4251. [DOI] [PubMed] [Google Scholar]
- Kraynov V.S., Chamberlain,C., Bokoch,G.M., Schwartz,M.A., Slabaugh,S. and Hahn,K.M. (2000) Localized Rac activation dynamics visualized in living cells. Science, 290, 333–337. [DOI] [PubMed] [Google Scholar]
- Ku G.M., Yablonski,D., Manser,E., Lim,L. and Weiss,A. (2001) A PAK1–PIX–PKL complex is activated by the T-cell receptor independent of Nck, Slp-76 and LAT. EMBO J., 20, 457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda S. et al. (1998) Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin- mediated cell-cell adhesion. Science, 281, 832–835. [DOI] [PubMed] [Google Scholar]
- Lei M., Lu,W., Meng,W., Parrini,M.C., Eck,M.J., Mayer,B.J. and Harrison,S.C. (2000) Structure of PAK1 in an autoinhibited conformation reveals a multistage activation switch. Cell, 102, 387–397. [DOI] [PubMed] [Google Scholar]
- Levenberg S., Yarden,A., Kam,Z. and Geiger,B. (1999) p27 is involved in N-cadherin-mediated contact inhibition of cell growth and S-phase entry. Oncogene, 18, 869–876. [DOI] [PubMed] [Google Scholar]
- Manser E., Huang,H.Y., Loo,T.H., Chen,X.Q., Dong,J.M., Leung,T. and Lim,L. (1997) Expression of constitutively active α-PAK reveals effects of the kinase on actin and focal complexes. Mol. Cell. Biol., 17, 1129–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser E., Loo,T.H., Koh,C.G., Zhao,Z.S., Chen,X.Q., Tan,L., Tan,I., Leung,T. and Lim,L. (1998) PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol. Cell, 1, 183–192. [DOI] [PubMed] [Google Scholar]
- Martin P. (1997) Wound healing—aiming for perfect skin regeneration. Science, 276, 75–81. [DOI] [PubMed] [Google Scholar]
- Morrison H., Sherman,L.S., Legg,J., Banine,F., Isacke,C., Haipek,C.A., Gutmann,D.H., Ponta,H. and Herrlich,P. (2001) The NF2 tumor suppressor gene product, merlin, mediates contact inhibition of growth through interactions with CD44. Genes Dev., 15, 968–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes C.D. and Hall,A. (1999) Rho GTPases control polarity, protrusion and adhesion during cell movement. J. Cell Biol., 144, 1235–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeier A., Ahmed,S., Manser,E., Yen,S.C., Hall,C. and Lim,L. (1998) PAK promotes morphological changes by acting upstream of Rac. EMBO J., 17, 4328–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrini M.C., Lei,M., Harrison,S.C. and Mayer,B.J. (2002) Pak1 kinase homodimers are autoinhibited in trans and dissociated upon activation by Cdc42 and Rac1. Mol. Cell, 9, 73–83. [DOI] [PubMed] [Google Scholar]
- Ridley A.J., Comoglio,P.M. and Hall,A. (1995) Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac and Rho in MDCK cells. Mol. Cell. Biol., 15, 1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander E.E., van Delft,S., ten Klooster,J.P., Reid,T., van der Kammen,R.A., Michiels,F. and Collard,J.G. (1998) Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell–cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J. Cell Biol., 143, 1385–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenenberger C.A., Zuk,A., Zinkl,G.M., Kendall,D. and Matlin,K.S. (1994) Integrin expression and localization in normal MDCK cells and transformed MDCK cells lacking apical polarity. J. Cell Sci., 107, 527–541. [DOI] [PubMed] [Google Scholar]
- Sells M.A., Boyd,J.T. and Chernoff,J. (1999) p21-activated kinase 1 (Pak1) regulates cell motility in mammalian fibroblasts. J. Cell Biol., 145, 837–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R.J. et al. (2001) The Nf2 tumor suppressor, merlin, functions in Rac-dependent signaling. Dev. Cell, 1, 63–72. [DOI] [PubMed] [Google Scholar]
- St Croix B., Sheehan,C., Rak,J.W., Florenes,V.A., Slingerland,J.M. and Kerbel,R.S. (1998) E-Cadherin-dependent growth suppression is mediated by the cyclin-dependent kinase inhibitor p27(KIP1). J. Cell Biol., 142, 557–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons M. and Settleman,J. (2000) Rho family GTPases: more than simple switches. Trends Cell Biol., 10, 415–419. [DOI] [PubMed] [Google Scholar]
- Tang Y., Chen,Z., Ambrose,D., Liu,J., Gibbs,J.B., Chernoff,J. and Field,J. (1997) Kinase-deficient Pak1 mutants inhibit Ras transformation of Rat-1 fibroblasts. Mol. Cell. Biol., 17, 4454–4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner C.E., Brown,M.C., Perrotta,J.A., Riedy,M.C., Nikolopoulos,S.N., McDonald,A.R., Bagrodia,S., Thomas,S. and Leventhal,P.S. (1999) Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: A role in cytoskeletal remodeling. J. Cell Biol., 145, 851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aelst L. and Symons,M. (2002) Role of Rho family GTPases in epithelial morphogenesis. Genes Dev., 16, 1032–1054. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V. and Fuchs,E. (2001) Actin dynamics and cell–cell adhesion in epithelia. Curr. Opin. Cell Biol., 13, 76–84. [DOI] [PubMed] [Google Scholar]
- Wang R.A., Mazumdar,A., Vadlamudi,R.K. and Kumar,R. (2002) p21-activated kinase-1 phosphorylates and transactivates estrogen receptor-α and promotes hyperplasia in mammary epithelium. EMBO J., 21, 5437–5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G.H., Beeser,A., Chernoff,J. and Testa,J.R. (2002) p21-activated kinase links Rac/Cdc42 signaling to merlin. J. Biol. Chem., 277, 883–886. [DOI] [PubMed] [Google Scholar]
- Zenke F.T., King,C.C., Bohl,B.P. and Bokoch,G.M. (1999) Identification of a central phosphorylation site in p21-activated kinase regulating autoinhibition and kinase activity. J. Biol. Chem., 274, 32565–32573. [DOI] [PubMed] [Google Scholar]
- Zhao Z.S., Manser,E. and Lim,L. (2000a) Interaction between PAK and nck: a template for Nck targets and role of PAK autophosphorylation. Mol. Cell. Biol., 20, 3906–3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z.S., Manser,E., Loo,T.H. and Lim,L. (2000b) Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly. Mol. Cell. Biol., 20, 6354–6363. [DOI] [PMC free article] [PubMed] [Google Scholar]