Abstract
Suicide gene therapy systems such as the herpes simplex thymidine kinase/ganciclovir system (TK/GCV) may kill cancer cells by apoptosis through as yet undefined mechanisms. Here we show that TK/GCV treatment induces p53 accumulation and increases cell surface expression of CD95 and tumor necrosis factor receptor, which is likely to involve p53-mediated translocation of CD95 to the cell surface. TK/GCV-induced apoptosis involves CD95-L-independent CD95 aggregation leading to the formation of a Fas-associated death domain protein (FADD) and caspase-8-containing, death-inducing signaling complex. Dominant negative FADD, the caspase-8 inhibitor zIETD-fmk [Z-Ile-Glu(OMe)-Thr-Asp(OMe)-fluoromethylketone], and zVAD-fmk (Z-Val-Ala-Asp-fluoromethylketone) partially abrogate TK/GCV-induced apoptosis. In addition to apoptosis induction, TK/GCV treatment strongly sensitizes for CD95-L-, TNF-, and TNF-related, apoptosis-inducing, ligand (TRAIL)-induced cell death in constitutively resistant cells. These findings may be used to increase the efficacy of TK/GCV and other suicide gene therapy systems for the treatment of cancer.
As gene therapy of cancer such as suicide gene therapy has entered the clinic, the factors limiting its efficacy have attracted increasing attention. Thus, target specificity and low in vivo transfection efficacy are subjects of intense research efforts. Surprisingly, very little is known about the mechanisms by which suicide gene approaches actually kill cancer cells. Previously, the herpes simplex virus thymidine kinase/ganciclovir (TK/GCV) system has been shown to induce apoptosis in target cells (1).
According to current concepts, TK phosphorylates the nontoxic prodrug ganciclovir, which then becomes phosphorylated by endogenous kinases to GCV-triphosphate, causing chain termination and single-strand breaks upon incorporation into DNA (2). The mechanisms linking TK/GCV-induced DNA damage to apoptosis, however, are largely unknown. Execution of cell death in most forms of apoptosis has been found to be mediated by activation of the caspase cascade. Caspase activation may be a consequence of death receptor triggering, mitochondrial activation, or other not yet defined initiation events. Induction of CD95 ligand (CD95-L) and CD95 ligand/receptor interaction has been found to contribute to apoptosis induced by some anticancer agents (3).
Therefore, we investigated the mechanisms of apoptosis in the TK/GCV system, which is paradigmatic for suicide gene therapy of cancer. We found that TK/GCV induces accumulation of p53 and increases cell surface expression of death receptors likely involving p53-mediated translocation of CD95 associated with CD95-L-independent formation of a death-inducing signaling complex (DISC), eventually leading to apoptosis involving the Fas-associated death domain protein (FADD) and caspases.
MATERIALS AND METHODS
Cells.
The human neuroblastoma cell line SH-EP was cultured in RPMI 1640 medium (Life Technologies, Eggenstein, Germany) supplemented with 10% heat-inactivated FCS (Biochrom, Berlin), 10 mM of Hepes, pH 7.3 (Biochrom), 100 units/ml of penicillin, 100 μg/ml of streptomycin (both from Life Technologies), and 2 mM of l-glutamine (Biochrom).
Generation of TK Retroviral Vector.
The retroviral vector tgLs(+)HyTK, which contains a hygromycin phosphotransferase–TK fusion gene driven by the long terminal repeat promoter (4), was employed.
Transduction and Transfection of Cells.
The TK gene was transduced as described (5). Bulk cultures (SH-EP pHyTK) were expanded and subcloned. In addition, SH-EP cells were transfected with the control vector pLXSN (6) (SH-EP pLXSN), by using calcium phosphate precipitation according to instructions of the manufacturer (Stratagene). pcDNA3 containing FADD, with the death effector domain deleted [clone NFD4 (7)], was electroporated into SH-EP pHyTK, and transfectants were subcloned (SH-EP pHyTK/FADD). As a control, a SHEP pHyTK subclone was electroporated with pcDNA3 lacking an insert (SH-EP pHyTK/pcDNA).
Measurement of Apoptosis.
DNA fragmentation was measured by flow cytometric analysis of propidium iodide-stained nuclei as described (8). The percentage of specific apoptosis was calculated as follows: [experimental apoptosis (%) − spontaneous apoptosis in medium (%)]/[100% − spontaneous apoptosis in medium (%)] × 100.
Immunoblot Analysis.
Immunoblot analysis was performed as described (9) with minor modifications. Membranes were incubated with primary and secondary antibodies for 1 h each and developed with enhanced chemiluminescence (Amersham Pharmacia). The following mouse mAbs were used: anti-FLICE C15 [1:10 dilution of hybridoma supernatant (10)], anti-FADD (1:1,000; Transduction Laboratories, Lexington, KY), anti-CPP32 (1:1,000; Transduction Laboratories), anti-p53 (1:1,000; Transduction Laboratories), anti-CD95 (1:500; Transduction Laboratories), anti-β-Actin (1:5,000; Sigma), and anti-α-tubulin (1:3,000; Calbiochem). Rabbit polyclonal antibodies were employed for the detection of TK (1:300) and poly(ADP-ribose) polymerase (PARP) (1:5,000; Boehringer Mannheim). Goat anti-mouse and anti-rabbit IgGs were used as secondary antibodies (1:5,000; Santa Cruz Biotechnology).
Inhibition with zVAD-fmk and zIETD-fmk.
Cells (2 × 104 per well) were treated with 10 μM of GCV (Hoffmann–La Roche). Broad-range tripeptide caspase inhibitor zVAD-fmk (50 μM; Bachem) was used, with an additional 30 μM of zVAD-fmk added after 36 h. zIETD-fmk (100 μM; Calbiochem) was employed with a medium change containing the same zIETD-fmk concentration after 24 h. Specific apoptosis was determined after 72 h.
Assessment of FADD Dependency by a Dominant Negative FADD Mutation.
Randomly chosen SH-EP pHyTK/FADD clones were treated in individual experiments with 2 μM of staurosporine (Sigma) for 12 h, 1 μg/ml of anti-APO-1/5 ng/ml of protein A/2 μg/ml of cycloheximide for 24 h, or 10 μg/ml of GCV for 72 h. Apoptosis was measured and compared with identically treated SH-EP pHyTK subclones and SH-EP pHyTK/pcDNA. Clonogenicity was determined after treating cells with 10 μM of GCV for 9 days by replating 500 cells per well in 24-well plates. Colonies were counted after 4 days.
Reverse Transcription–PCR.
Total RNA was extracted by using the RNeasy kit (Qiagen). Reverse transcription–PCR of CD95-L, TNF-α, and TNF-related apoptosis-inducing ligand (TRAIL) was performed with primers described previously (3, 11). Primer for the TRAIL receptor DR5 were 5′-GAGAGCGGCCCCACAACAAAAGA-3′ and 5′-CCTGGGTGGGCTGCAAGATACTCA-3′. β-Actin primer was used for controls (MWG-Biotech, Ebersberg, Germany). The Gene Amplification RNA-PCR kit (Perkin-Elmer) was used according to the manufacturer’s instructions with the following modifications: annealing temperature and cycle number for CD95-L were 57°C and 40; for TNF-α, 58°C and 34; for DR5, 56C° and 27; and for β-actin, 58C° and 28; respectively. PCR products were analyzed by using 1.5–2.0% agarose gels stained with ethidium bromide.
Blockage of Death Receptors and Death Ligands.
SH-EP pHyTK11 cells were treated in individual experiments with 0.1 μg/ml of anti-APO-1 (12) with or without 25 μg/ml of the blocking F(ab′)2 anti-APO-1 (13), 1 ng/ml of recombinant human TNF-α (Calbiochem) in the presence or absence of 25 μg/ml of anti-TNF-α (Sigma), and 10 μM of GCV with or without F(ab′)2 anti-APO-1, and anti-TNF-α, respectively.
Measurement of TK/GCV-Mediated Sensitization to Death Ligands.
Cells were treated with 10 μM of GCV for 24 h followed by incubation without GCV for an additional 24 h in the presence or absence of either 0.1 μg anti-APO-1 with 5 ng/ml of protein A, 100 ng/ml of TNF-α, 1:30 dilution of TRAIL hybridoma supernatant, or 0.001 μM staurosporine. Apoptosis was measured and compared with cells treated for 24 h (12 h for staurosporine) with the same inducers in identical concentrations but without prior GCV treatment.
Assessment of CD95 Aggregation by Limiting Antibody Conditions.
Cells were treated with 10 μM of GCV for 36 h. CD95 crosslinking and immunoprecipitation was performed as described (14) with the following modifications: CD95 was immunoprecipitated by using anti-APO-1 antibody at a limiting concentration of 0.5 μg/ml and at excess concentration of 2.0 μg/ml. After precipitation with protein A-Sepharose, the precipitate was resolved by SDS/PAGE and electroblotted to a nitrocellulose membrane (Amersham). CD95, FADD, and caspase-8 were detected by immunoblot, using the appropriate primary and secondary antibodies as detailed above.
Determination of Death Receptor Expression.
CD95 and TNF receptor 1 (TNF-R1) expression was detected by flow cytometry as described previously (9), using mouse anti-APO-1 and anti-TNF-R1 mAbs (Boehringer Ingelheim) followed by goat anti-mouse IgG-phycoerythrin (Immunotech, Luminy, France).
Golgi Blockade.
This was performed by blocking the Golgi with 1 μg/ml of brefeldin A (Sigma) for 24 h.
RESULTS
Kinetic and Dose Response of TK/GCV-Induced Apoptosis.
SH-EP pHyTK bulk cultures as well as the selected clone SH-EP pHyTk11 were equally killed effectively by GCV, depending on time and GCV dose, with minimal unspecific toxicity, as shown by SH-EP pLXSN cells, which did not contain TK (data not shown). Therefore, both SH-EP pHyTK bulk cultures and SH-EP pHyTK11 were used interchangeably in subsequent experiments, in which GCV concentrations of 2 and 10 μM were employed.
TK/GCV-Induced Apoptosis Involves Activation of Caspases.
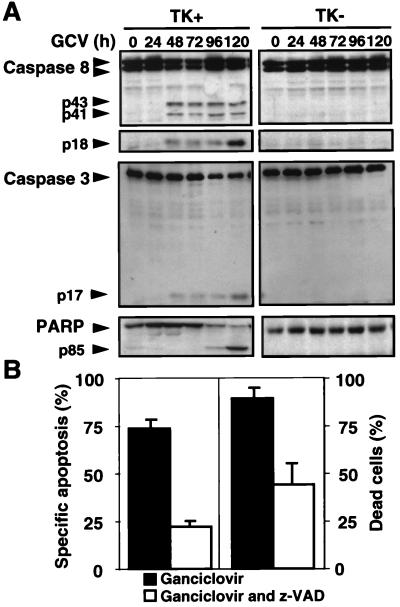
To delineate the effector pathways of TK/GCV-induced apoptosis, we first examined the role of caspases, central effectors of apoptosis, to which various known, death-inducing signaling pathways converge. In TK-containing cells but not in cells lacking TK, the caspase cascade became activated after GCV treatment (Fig. 1A). Caspase-8 and the downstream caspase-3 (CPP32) were cleaved starting 48 h after administration of GCV, coinciding with the onset of apoptosis. Consecutively, PARP was almost completely cleaved at 96–120 h. Similar results were obtained with bulk cultures. To determine whether caspase activation is necessary for TK/GCV-mediated apoptosis, we blocked the caspase pathway by the broad-range caspase inhibitor zVAD-fmk. As shown in Fig. 1B, both DNA fragmentation, as measured by enumerating hypodiploid nuclei, and cell membrane permeability, as determined by trypan blue uptake, were inhibited markedly, indicating the importance of caspase activation for TK/GCV-induced apoptosis and cell death. However, inhibition was not complete and decreased at 96 h (data not shown), suggesting that either caspase-independent cell death mechanisms were utilized at later time points or that blockage of caspases by zVAD-fmk was incomplete. Similar results were obtained with SH-EP pHyTK bulk cultures (data not shown).
Figure 1.
TK/GCV-induced apoptosis involves activation of caspases and is attenuated by caspase inhibitors. (A) Activation of caspases by TK/GCV. HSV-TK cells (1 × 107) containing (TK+) SH-EP pHyTK11 cells and SH-EP pLSXN cells that lack TK (TK−) were treated with 10 μM of GCV for the times indicated. Protein was extracted and separated by SDS/PAGE. Caspase-8, caspase-3, and PARP were detected by immunoblot. Caspase-8 was seen as a double-band corresponding to the isoforms caspase-8/a and 8/b (14). p43 and p41 are cleavage intermediates of caspase-8 leading to the active subunit p18. The cleavage products of CPP32 and PARP are p17 and p85, respectively. Cleavage products were seen only in TK+ but not in TK− cells. (B) TK/GCV-mediated cell death is attenuated by the caspase inhibitor zVAD-fmk. Cells were treated with 10 μM of GCV alone or in the presence of 80 μM of zVAD-fmk. Apoptosis, as determined by flow cytometry (Left) and counting of trypan blue-positive cells (Right), was measured after 72 h. Results are means of triplicates, and similar results were obtained in three separate experiments.
TK/GCV Induces Expression of TRAIL but Not of CD95-L and TNF.
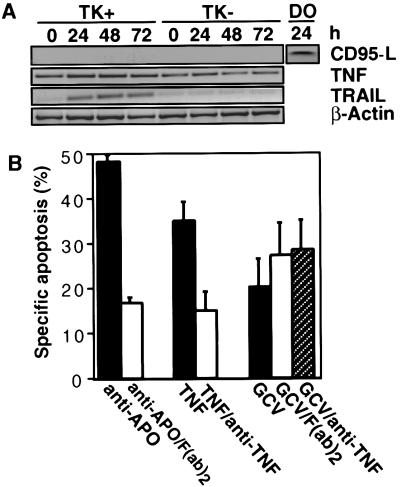
We then searched for triggers activating the caspase cascade. Some cytotoxic drugs have been shown to increase expression of CD95-L and CD95, which may contribute to killing under certain conditions (3, 9). Therefore, it was conceivable that TK/GCV-mediated apoptosis may proceed through such an autocrine loop. We hence determined the induction of death-inducing ligands by TK/GCV. Although TRAIL mRNA was induced, TNF mRNA did not increase above the constitutive mRNA level, and no constitutive or induced CD95-L mRNA was detectable (Fig. 2A). Low-level expression of CD95-L protein was detected by Western blot (data not shown).
Figure 2.
TK/GCV-induced apoptosis is not mediated by induction of CD95-L and TNF but may involve TRAIL induction. (A) TK/GCV induces expression of TRAIL but not of CD95-L and TNF. 1.5 × 107 SH-EP pHy TK11 cells containing 1.5 × 107 SH-EP pLSXN cells that lack TK (TK−) were treated with 10 μM GCV for the times indicated. mRNA was extracted, and reverse transcription–PCR for CD95-L, TNF, TRAIL, and β-actin was performed. As a positive control for CD95-L induction, SH-EP pHyTK11 cells were treated with 0.5 μg/ml of doxorubicin (DO). (B) TK/GCV-mediated apoptosis is not blocked by antibodies against CD95 and TNF. SH-EP pHyTK11 cells (2 × 104) were treated for 48 h with 0.1 μg/ml of anti-APO-1 with or without 25 μg/ml of the blocking F(ab′)2 anti-APO-1, 1 ng/ml of rhTNF-α in the presence or absence of 25 μg/ml of anti-TNF-α, and 10 μM of GCV alone and with F(ab′)2 anti-APO-1 and anti-TNF-α, respectively.
TK/GCV-Mediated Apoptosis Is Not Blocked by Antibodies Against CD95 and TNF.
To define further the role of CD95-L and TNF, we blocked CD95 and TNF signaling by antagonistic antibodies. Both F(ab′)2 anti-APO-1 and anti-TNF-α effectively blocked apoptosis induced by the respective ligands but not TK/GCV-mediated apoptosis (Fig. 2B).
TK/GCV Induces p53, Up-Regulates Death Receptors Likely Involving p53-Mediated Translocation of CD95, and Sensitizes to Death-Inducing Ligands.
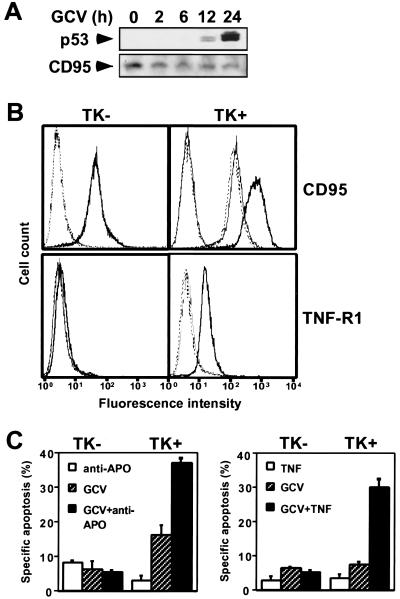
Accumulation of wild-type p53 protein has been found in response to DNA damage. Cytotoxic drugs may up-regulate CD95 expression by increased transcription mediated by p53 (9, 15, 16). Recently, p53-dependent translocation of preformed CD95 from the Golgi apparatus to the cell surface independent of increased transcription has been described (17). We therefore reasoned that TK/GCV may act similarly. TK/GCV, indeed, did induce p53, starting 12 h after TK/GCV, without de novo synthesis of CD95 protein (Fig. 3A) but associated with increased cell surface expression of CD95 and TNF-R1 (Fig. 3B). Disruption of the Golgi by brefeldin A prevented CD95 translocation (Fig. 3B), suggesting that TK/GCV induces p53-mediated translocation of CD95. Given the up-regulation of death receptors, we reasoned that TK/GCV-treated cells may become more susceptible to death induced by their cognate ligands. Apoptosis by anti-APO-1 and TNF-α (Fig. 3C) as well as by TRAIL (data not shown) was enhanced synergistically by TK/GCV, suggesting that TK/GCV sensitizes target cells for apoptosis by death-inducing ligands. TK/GCV also sensitized to low-dose staurosporine, suggesting that, in addition to up-regulation of death receptors, sensitization also occurred downstream of death receptors (data not shown).
Figure 3.
TK/GCV induces p53, up-regulates death receptors likely involving p53-mediated translocation of CD95, and sensitizes to death-inducing ligands. SH-EP pHyTK11 cells (TK+) were compared with SH-EP pLXSN cells (TK−). (A) TK/GCV induces p53 but not CD95. Cells were treated with 10 μM of GCV for the times indicated. Protein was extracted and separated by SDS/PAGE, and p53 and CD95 were detected by immunoblot. (B) TK/GCV up-regulates death receptors on the cell surface likely involving p53-mediated translocation of CD95. SH-EP pHyTK11 (TK+) and SH-EP pLXSN (TK−) cells were treated with 10 μM of GCV for 24–48 h in the absence or presence of brefeldin A, and expression of CD95 and TNF-R1 was determined by flow cytometry. Bold, solid line, stained cells treated with GCV; thin, solid line, stained cells treated with GCV in the presence of brefeldin A; broken line, stained untreated cells; dotted line, unstained cells. Results are means of triplicates, and similar results were obtained in two separate experiments. (C) TK/GCV sensitizes to death-inducing ligands. Cells (2 × 104) were treated with 10 μM of GCV for 24 h followed by incubation without GCV for an additional 48 h in the presence or absence of either 0.1 μg anti-APO-1 with 5 ng/ml of protein A, 100 ng/ml of TNF, or 1:30 TRAIL. Apoptosis was determined as described in Materials and Methods. Results are means of triplicates, and similar results were obtained in two separate experiments.
TK/GCV-Induced Apoptosis Involves Brefeldin A-Inhibitable Aggregation of CD95, Causing DISC Formation.
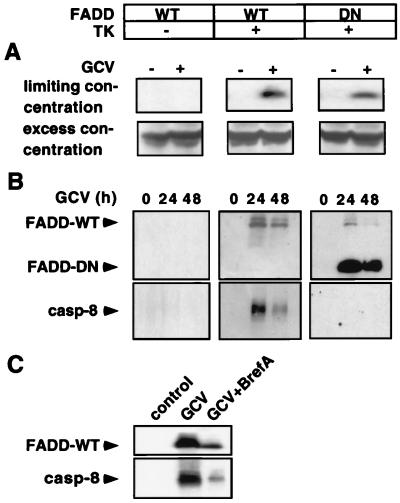
We next wanted to know whether TK/GCV influences the CD95 pathway despite the apparent absence of CD95/CD95-L interaction. Because we found that TK/GCV up-regulates CD95 we asked whether CD95 aggregation and signaling may occur in the absence of CD95/CD95-L interaction. To address this question, we examined CD95 aggregation by immunoprecipitating CD95, using limiting antibody concentrations. Under these conditions CD95 is precipitated significantly only when receptors are crosslinked. Without aggregation of CD95, the few antibody molecules will bind to single receptors only, resulting in unappreciable amounts of precipitated CD95 (18). Indeed, using a limiting anti-CD95 concentration, crosslinked CD95 was detected in TK+ but not in TK− cells upon GCV treatment, whereas with excess antibody, non-cross-linked receptors were detected in TK− cells as well (Fig. 4A). We then wanted to know whether TK/GCV-induced CD95 aggregation may initiate the caspase cascade by formation of a DISC after recruitment of FADD and the receptor-proximal caspase-8 to the CD95 receptor. As shown in Fig. 4B, TK-transfected cells recruited both wild-type (WT) and dominant negative (DN) FADD to CD95 after GCV. Caspase-8 was recruited only in cells containing FADD-WT, but not in those with FADD-DN lacking the death effector domain. These results suggest that CD95 receptor aggregation in the absence of CD95-L may trigger the caspase cascade in TK/GCV therapy. To test whether the observed p53-mediated CD95 translocation has an effect on CD95 signaling, we determined DISC formation after Golgi blockade by using brefeldin A. As shown in Fig. 4C, DISC formation was decreased after Golgi disruption, suggesting that CD95 signaling is facilitated by p53-mediated CD95 translocation after TK/GCV.
Figure 4.
TK/GCV-induced apoptosis involves brefeldin A-inhibitable aggregation of CD95 leading to recruitment of FADD and caspase-8 to CD95. SH-EP pLXSN cells [TK−, containing wild type (WT) FADD] were compared with SH-EP pHyTK11 cells (TK+, containing FADD-WT) and with SH-EP pHyTK11/FADD32 cells (TK+, containing FADD-DN). (A) TK/GCV induces aggregation of CD95 as demonstrated by immunoprecipitation. Cells were treated with 10 μM of GCV for 36 h. CD95 was crosslinked and immunoprecipitated by using anti-APO-1 at the limiting concentration of 0.5 μg/ml and the excess concentration of 2 μg/ml, respectively, and protein A-Sepharose. The precipitate was subjected to immunoblot analysis for CD95. (B) TK/GCV induces DISC formation. Cells (2 × 107) were treated with 10 μM of GCV for the indicated time. Immunoprecipitation was performed by using anti-APO-1 IgG3 mAb (2 μg/ml) and protein A-Sepharose. Immunoblot was performed as described for FADD and caspase-8 protein expression. Similar results were obtained in two separate experiments. (C) TK/GCV-induced DISC formation is inhibited by brefeldin A. Experimental conditions were as for B except that 1 μg/ml of brefeldin A was added to GCV during the incubation period of 24 h.
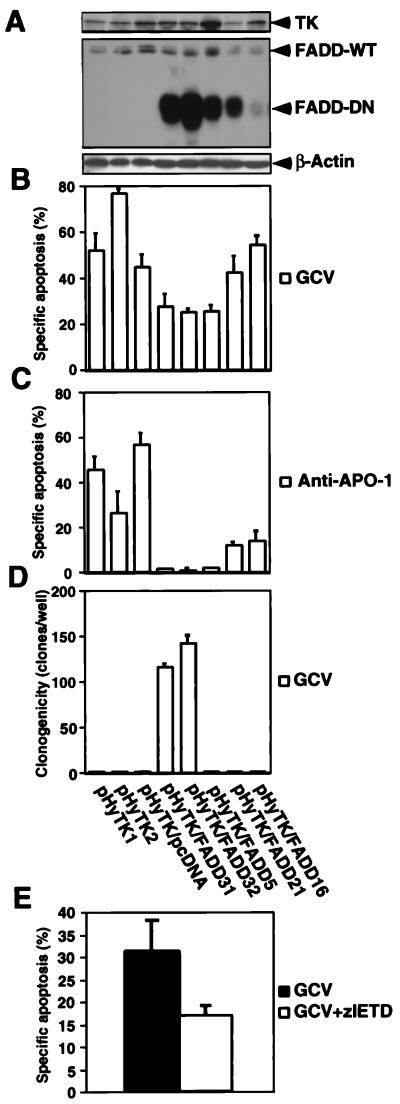
TK/GCV-Mediated Apoptosis Is Decreased by Inhibition of FADD and Caspase-8.
FADD is recruited to death receptors of the TNF receptor family such as CD95, the TNF receptors, and TRAIL-DR5 upon activation and functions as an adapter molecule between these receptors and caspases, e.g., caspase-8 (7, 19). Therefore, we reasoned that a dominant negative FADD mutation (FADD-DN) would interfere with TK/GCV-mediated apoptosis. As shown in Fig. 5A, clones transfected with TK and FADD-DN expressed both genes to varying degrees. TK/GCV-mediated apoptosis was decreased in the FADD mutants depending on the amount of FADD-DN expression (Fig. 5B). As expected, anti-APO-1-triggered apoptosis was decreased markedly in the presence of high levels of FADD-DN (Fig. 5C). Two clones with almost complete ablation of FADD function remained clonogenic after prolonged exposure to GCV (Fig. 5D). To exclude nonspecific effects of FADD-DN we employed the complementary approach of inhibiting the most apical initiator caspase of CD95, TNF-R1, and TRAIL-DR5 signaling, caspase-8. The caspase-8 inhibitor zIETD-fmk decreased TK/GCV-induced apoptosis, supporting a role of death receptor signaling in TK/GCV cytotoxicity (Fig. 5E). All clones and controls underwent apoptosis to the same degree, with 2 μM of the protein C kinase inhibitor staurosporine given for 12 h, showing that the apoptosis machinery downstream of FADD was intact in these cells (data not shown).
Figure 5.
TK/GCV-mediated apoptosis is attenuated by FADD-DN and inhibition of caspase-8. Clones stably transfected with either TK (pHyTK1 and 2) or with TK and an empty pcDNA3 vector (pHyTK1/pcDNA) were compared with clones stably transfected both with TK and FADD-DN (pHyTK/FADD31, 32, 5, 21, and 16). (A) All clones express TK as well as wild-type FADD. Clones bearing FADD-DN express it to varying degrees. Protein expression was detected by immunoblot by using polyclonal anti-TK and monoclonal anti-FADD antibodies. Equal gel loading was controlled by detection of β-actin. (B) TK/GCV-mediated apoptosis is inhibited by FADD-DN. Cells were treated with 10 μM of GCV for 72 h, and apoptosis was assessed. (C) Anti-APO-1-induced apoptosis is inhibited by FADD-DN depending on the extent of expression of the mutated protein. Cells were treated with 1 μg/ml of anti-APO-1, 5 ng/ml of protein A, and 2 μg/ml of cycloheximide for 12 h, and apoptosis was assessed. (D) TK/GCV decreases clonogenicity depending on FADD function. Cells were treated with 10 μM of GCV for 9 days, and 500 cells were replated in six-well plates. After 4 days of culture, the colony number per well was determined. (E) TK/GCV-induced apoptosis is inhibited by the caspase-8 inhibitor zIETD-fmk. Cells were treated for 48 h with 10 μM of GCV in the absence and presence of 50 μM zIETD-fmk. Medium including GCV and zIETD-fmk was changed after 24 h. Apoptosis was determined as described in Materials and Methods. Results are means of triplicates, and similar results were obtained in two separate experiments.
DISCUSSION
TK/GCV strongly induced CD95 receptor expression and CD95 receptor aggregation in the absence of CD95-L induction. CD95-L-independent aggregation of CD95 leading to apoptosis has been described for other forms of cellular stress, e.g., UV light (18). CD95 cell surface expression may be a consequence of p53-dependent transcription (9, 15, 16) or p53-dependent translocation of preformed receptor to the cell surface (17). TK/GCV induced p53 concomitant with up-regulation, but not induction of CD95 protein. Disrupting the Golgi, which has been shown to block p53-mediated CD95 translocation, inhibited CD95 surface expression, suggesting that TK/GCV induces p53-mediated translocation of CD95 to the cell surface. The increased surface expression of CD95 may lead to increased interaction with CD95-L constitutively expressed on the cell surface or, alternatively, may contribute to CD95 self-aggregation by crowding of the receptor. Overexpression of FADD has been shown to trigger apoptosis independent of CD95 (7). However, TK/GCV did not increase FADD expression (data not shown). TK/GCV activates the CD95 as shown by the recruitment of FADD and caspase-8, thus triggering the caspase cascade. Initiation of CD95 signaling is inhibited when CD95 translocation is blocked, showing that TK/GCV-induced CD95 translocation contributes to initiate the CD95-signaling cascade.
In addition to CD95, both BAX and TRAIL-DR5 are transcriptional targets of p53 in response to genotoxic stress (20, 21). However, BAX protein and TRAIL-DR5 mRNA were not up-regulated after TK/GCV (data not shown). We have not ruled out translocation of BAX from the cytosol to the mitochondria or of TRAIL-DR5 from the Golgi to the cell surface in response to TK/GCV. TK/GCV has been shown to arrest tumor cells in late S/G2 phase by p53-dependent induction of p21CIP1/WAF1 (22). Thus, besides mediating apoptosis, TK/GCV-induced p53 accumulation contributes to tumor control by inhibiting proliferation. Finally, p53 is known to induce genes related to the redox state, the formation of reactive oxygen species, and the oxidative degradation of mitochondrial components (23). Therefore, the role of mitochondria in TK/GCV-induced apoptosis warrants further investigation.
Besides CD95 activation, TK/GCV primes the CD95 pathway as shown by sensitization to triggering by the agonistic antibody anti-APO-1. This sensitization may be explained by the up-regulation of the receptor. However, sensitization also may involve downstream elements of the apoptotic pathway (e.g., mitochondrial activation) because staurosporine-induced apoptosis, which acts distal of death receptors, was found to be increased.
It is conceivable that TRAIL-receptor, whose ligand was selectively up-regulated among the death-inducing ligands after TK/GCV, may activate DR4/5 and, thus, trigger apoptosis. Therefore, the role of DR4/5 requires further analysis. In contrast, TNF was not up-regulated, nor did blockage of it decrease apoptosis after TK/GCV, suggesting no role of TNF in this process.
TK/GCV-mediated apoptosis is at least partially FADD-dependent, because only clones strongly expressing FADD-DN were protected from TK/GCV-mediated apoptosis and remained clonogenic after long-term exposure to GCV. This indicates that the FADD-dependent pathway of TK/GCV-mediated apoptosis was nonredundant in these clones with regard to ablation of clonogenic potential, even after prolonged GCV exposure. This implies that death receptor-driven signaling pathways play a role in TK/GCV-mediated apoptosis. Because we have demonstrated activation of CD95, this receptor is likely to be involved in TK/GCV-induced apoptosis. However, this does not exclude a role for other death receptors such as DR5. Other clones with the FADD mutation had lost their clonogenic potential, suggesting either incomplete loss of FADD function or the utilization of FADD-independent cell death pathways by TK-GCV. Recently, several lines of evidence suggested hat FADD, in addition to its role in apoptosis signaling through death receptors, may have a function in proliferative response, e.g., in T cells (24). Proliferation of FADD mutants was decreased slightly during the culture period (data not shown). Because GCV action depends on proliferation (25), the decreased proliferation of the FADD mutants may have contributed to their GCV resistance.
The role of death receptor-driven apoptosis in TK/GCV-mediated cytotoxicity is supported further by the attenuation of apoptosis by the caspase-8 inhibitor zIETD-fmk. This inhibition was not complete, which could imply that apoptosis pathways not driven by death receptors may also be involved. However, the partial inhibition of anti-APO-1-induced apoptosis in zIETD-fmk-treated SH-EP cells (data not shown) suggests incomplete inhibition of caspase-8 by zIETD-fmk. This is in contrast to zVAD-fmk, which almost completely blocked both anti-APO-1 (data not shown) and GCV-induced apoptosis. Therefore, the incomplete inhibition of TK/GCV-mediated apoptosis by zVAD-fmk suggests that caspase-independent cell death pathways are triggered by TK/GCV, at least after prolonged treatment. Recently, it has been shown in some cell lines that the inhibition of caspases causes a switch from caspase-dependent apoptosis to caspase-independent necrosis (26). However, we did not detect the morphological characteristics of necrosis after inhibiting caspases during TK/GCV treatment, suggesting that alternative caspase-independent apoptotic cell death pathways were used. That these pathways became functional after caspases had been blocked suggests that the caspase-dependent pathway is taken as the default route to cell death by TK/GCV in SH-EP cells. In addition, mechanisms of TK/GCV-induced cell death may vary between different cell types, because in B16 melanoma cell lines induction of necrosis rather than apoptosis was found after TK/GCV treatment (22, 27).
The sensitization of TK/GCV-treated cells for CD95, TNF-R1, and TRAIL-receptor-induced apoptosis may be due to up-regulation of the receptors and downstream sensitization of the respective pathways. Death receptor sensitization may have important implications for the bystander effect, the proximal and distal killing of nontransduced cancer cells (1, 28). This effect is crucial for the clinical efficacy of suicide gene cancer therapy, given the low in vivo transfection efficiency of currently used vectors. The immunological component of the bystander effect is exerted by CD4+ and CD8+ T cells, natural killer (NK) cells, and macrophages infiltrating TK/GCV-treated tumors (29–32). These cells kill, in part, by death-inducing ligands: the mononuclear infiltrate has been shown to express TNF (30, 31), cytotoxic T cells can kill both by CD95-L (33) and TRAIL (34), and NK cells use CD95-L (35). We therefore propose that TK/GCV-induced sensitization to these death ligands enhances the bystander effect.
Our findings may provide a starting point to improve the efficacy of suicide gene therapies of cancer by increasing the sensitivity of target and bystander cells through enhancement of apoptosis.
Acknowledgments
We thank Helgard Knauss and Irmgard Küttner for excellent technical assistance, Gudrun Strauss for F(ab)2 anti-APO-1, M. Schwab for SH-EP cells, V. Dixit for FADD clone NFD4, W. C. Summers for TK antibody, and H. Hug for helpful discussions.
ABBREVIATIONS
- CD95-L
CD95 ligand
- DISC
death-inducing signaling complex
- FADD
Fas-associated death domain
- FADD-DN
dominant negative FADD
- GCV
ganciclovir
- PARP
poly(ADP-ribose) polymerase
- TK
thymidine kinase
- TNF
tumor necrosis factor
- TNF-R
TNF receptor
- TRAIL
TNF-related apoptosis-inducing ligand
- zIETD-fmk
Z-Ile-Glu(OMe)-Thr-Asp(OMe)-fluoromethylketone
- zVAD-fmk
Z-Val-Ala-Asp-fluoromethylketone
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Freeman S M, Abboud C N, Whartenby K A, Packman C H, Koeplin D S, Moolten F L, Abraham G N. Cancer Res. 1993;53:5274–5283. [PubMed] [Google Scholar]
- 2.Reid R, Mar E C, Huang E S, Topal M D. J Biol Chem. 1988;263:3898–3904. [PubMed] [Google Scholar]
- 3.Friesen C, Herr I, Krammer P H, Debatin K M. Nat Med. 1996;2:574–577. doi: 10.1038/nm0596-574. [DOI] [PubMed] [Google Scholar]
- 4.Lupton S D, Brunton L L, Kalberg V A, Overell R W. Mol Cell Biol. 1991;11:3374–3378. doi: 10.1128/mcb.11.6.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uckert W, Kammertons T, Haack K, Qin Z, Gebert J, Schendel D J, Blankenstein T. Hum Gene Ther. 1998;9:855–865. doi: 10.1089/hum.1998.9.6-855. [DOI] [PubMed] [Google Scholar]
- 6.Miller A D, Rosman G J. BioTechniques. 1989;7:980–989. [PMC free article] [PubMed] [Google Scholar]
- 7.Chinnaiyan A M, Tepper C G, Seldin M F, O’Rourke K, Kischkel F C, Hellbardt S, Krammer P H, Peter M E, Dixit V M. J Biol Chem. 1996;271:4961–4965. doi: 10.1074/jbc.271.9.4961. [DOI] [PubMed] [Google Scholar]
- 8.Nicoletti I, Migliorati G, Pagliacci M C, Grignani F, Riccardi C. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 9.Fulda S, Sieverts H, Friesen C, Herr I, Debatin K M. Cancer Res. 1997;57:3823–3829. [PubMed] [Google Scholar]
- 10.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli K J, Debatin K M, Krammer P H, Peter M E. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herr I, Wilhelm D, Böhler T, Angel P, Debatin K M. Int J Cancer. 1999;80:417–424. doi: 10.1002/(sici)1097-0215(19990129)80:3<417::aid-ijc14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 12.Trauth B C, Klas C, Peters A M, Matzku S, Moller P, Falk W, Debatin K M, Krammer P H. Science. 1989;245:301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- 13.Dhein J, Walczak H, Baumler C, Debatin K M, Krammer P H. Nature (London) 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 14.Scaffidi C, Medema J P, Krammer P H, Peter M E. J Biol Chem. 1997;272:26953–26958. doi: 10.1074/jbc.272.43.26953. [DOI] [PubMed] [Google Scholar]
- 15.Owen-Schaub L B, Zhang W, Cusack J C, Angelo L S, Santee S M, Fujiwara T, Roth J A, Deisseroth A B, Zhang W W, Kruzel E. Mol Cell Biol. 1995;15:3032–3040. doi: 10.1128/mcb.15.6.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Müller M, Wilder S, Bannasch D, Israeli D, Lehlbach K, Li-Weber M, Friedman S L, Galle P R, Stremmel W, Oren M, et al. J Exp Med. 1998;188:2033–2045. doi: 10.1084/jem.188.11.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett M, Macdonald K, Chan S W, Luzio J P, Simari R, Weissberg P. Science. 1998;282:290–293. doi: 10.1126/science.282.5387.290. [DOI] [PubMed] [Google Scholar]
- 18.Rehemtulla A, Hamilton C A, Chinnaiyan A M, Dixit V M. J Biol Chem. 1997;272:25783–25786. doi: 10.1074/jbc.272.41.25783. [DOI] [PubMed] [Google Scholar]
- 19.Walczak H, Degli-Esposti M A, Johnson R S, Smolak P J, Waugh J Y, Boiani N, Timour M S, Gerhart M J, Schooley K A, Smith C A, et al. EMBO J. 1997;16:5386–5397. doi: 10.1093/emboj/16.17.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheikh M S, Burns T F, Huang Y, Wu G S, Amundson S, Brooks K S, Fornace A J, Jr, el-Deiry W S. Cancer Res. 1998;58:1593–1598. [PubMed] [Google Scholar]
- 21.McCurrach M E, Connor T M, Knudson C M, Korsmeyer S J, Lowe S W. Proc Natl Acad Sci USA. 1997;94:2345–2349. doi: 10.1073/pnas.94.6.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halloran P J, Fenton R G. Cancer Res. 1998;58:3855–3865. [PubMed] [Google Scholar]
- 23.Polyak K, Xia Y, Zweier J L, Kinzler K W, Vogelstein B. Nature (London) 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- 24.Zornig M, Hueber A O, Evan G. Curr Biol. 1998;8:467–470. doi: 10.1016/s0960-9822(98)70182-4. [DOI] [PubMed] [Google Scholar]
- 25.Cheng Y C, Huang E S, Lin J C, Mar E C, Pagano J S, Dutschman G E, Grill S P. Proc Natl Acad Sci USA. 1983;80:2767–2770. doi: 10.1073/pnas.80.9.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vercammen D, Brouckaert G, Denecker G, Van de Craen M, Declercq W, Fiers W, Vandenabeele P. J Exp Med. 1998;188:919–930. doi: 10.1084/jem.188.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melcher A, Todryk S, Hardwick N, Ford M, Jacobson M, Vile R G. Nat Med. 1998;4:4581–4587. doi: 10.1038/nm0598-581. [DOI] [PubMed] [Google Scholar]
- 28.Culver K W, Ram Z, Wallbridge S, Ishii H, Oldfield E H, Blaese R M. Science. 1992;256:1550–1552. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- 29.Caruso M, Panis Y, Gagandeep S, Houssin D, Salzmann J L, Klatzmann D. Proc Natl Acad Sci USA. 1993;90:7024–7028. doi: 10.1073/pnas.90.15.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramesh R, Marrogi A J, Munshi A, Abboud C N, Freeman S M. Exp Hematol. 1996;24:829–838. [PubMed] [Google Scholar]
- 31.Vile R G, Castleden S, Marshall J, Camplejohn R, Upton C, Chong H. Int J Cancer. 1997;71:267–274. doi: 10.1002/(sici)1097-0215(19970410)71:2<267::aid-ijc23>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 32.Hall S J, Sanford M A, Atkinson G, Chen S H. Cancer Res. 1998;58:3221–3225. [PubMed] [Google Scholar]
- 33.Lowin B, Hahne M, Mattmann C, Tschopp J. Nature (London) 1994;370:650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- 34.Thomas W D, Hersey P. J Immunol. 1998;161:2195–2200. [PubMed] [Google Scholar]
- 35.Arase H, Arase N, Saito T. J Exp Med. 1995;181:1235–1238. doi: 10.1084/jem.181.3.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]