Abstract
GATA-6 is expressed in presumptive cardiac mesoderm before gastrulation, but its role in heart development has been unclear. Here we show that Xenopus and zebrafish embryos, injected with antisense morpholino oligonucleotides designed specifically to knock-down translation of GATA-6 protein, are severely compromised for heart development. Injected embryos express greatly reduced levels of contractile machinery genes and, at the same stage, of regulatory genes such as bone morphogenetic protein-4 (BMP-4) and the Nkx2 family. In contrast, initial BMP and Nkx2 expression is normal, suggesting a maintenance role for GATA-6. Endoderm is critical for heart formation in several vertebrates including Xenopus, and separate perturbation of GATA-6 expression in the deep anterior endoderm and in the overlying heart mesoderm shows that GATA-6 is required in both for cardiogenesis. The GATA-6 requirement in cardiac mesoderm was confirmed in zebrafish, an organism in which endoderm is thought not to be necessary for heart formation. We therefore conclude that proper maturation of cardiac mesoderm requires GATA-6, which functions to maintain BMP-4 and Nkx2 expression.
Keywords: heart/morpholinos/transcription factors/Xenopus/zebrafish
Introduction
The heart is the first organ to develop in vertebrates. It arises from two paired cardiac primordia in the anterior lateral mesoderm that migrate to the ventral midline and fuse to form a linear heart tube. In Xenopus, specification of cardiac tissue depends on two important sources of signals: the Spemann organizer and the deep anterior endoderm, which lie between and beneath the presumptive heart precursors, respectively (Sater and Jacobsen, 1990; Nascone and Mercola, 1995). Interestingly, the heart primordia form and differentiate normally in zebrafish mutant embryos unable to make endoderm, suggesting either a different induction pathway or receipt of the requisite signal from elsewhere (Alexander and Stainier, 1999).
Members of the GATA family are amongst the first genes to be expressed in the mesoderm and endoderm during the period of heart specification (reviewed in Charron and Nemer, 1999; Patient and McGhee, 2002). The GATA family in vertebrates comprises six members, which can be divided into two subfamilies based on sequence similarity and expression profiles. GATA-1, -2 and -3 are involved in haematopoiesis and ectodermal patterning (Zon et al., 1991; Read et al., 1998), whilst GATA-4, -5 and -6 are expressed in cardiac tissue and endodermal derivatives (reviewed in Molkentin, 2000). GATA-4 has been shown to be essential for cardiomyocyte differentiation of the embryonal carcinoma cell line, P19 (Grepin et al., 1995, 1997), but GATA-4-null mice form differentiated myocardium, although fusion at the ventral midline does not occur and cardia bifida is seen (Kuo et al., 1997; Molkentin et al., 1997). It has been suggested that increased levels of GATA-6 may compensate for the loss of GATA-4 (Narita et al., 1996; Kuo et al., 1997; Molkentin et al., 1997), a suggestion supported in chick and rat (Jiang et al., 1998; Charron et al., 1999). Loss of GATA-5 in mice has little effect on myocardium, whereas, in zebrafish, loss of cardiomyocytes and cardia bifida are seen (Reiter et al., 1999; Molkentin et al., 2000). Little information on the role of GATA-6 during cardiogenesis has emerged from GATA-6-null mice, which die prior to heart induction due to defects in extraembryonic endoderm (Morrisey et al., 1998; Koutsourakis et al., 1999). However, the analysis of chimeras for contributions of null cells to the heart, and of embryoid bodies for contraction, have both suggested that GATA-6 may have no cell-autonomous effect on the differentiation of the myocardium. In Xenopus, GATA-6 is expressed early during gastrulation in a broad domain in the endoderm and mesoderm, including the presumptive heart (Jiang and Evans, 1996; Gove et al., 1997; R.Ciau-Uitz and R.Patient., unpublished). Expression in the heart starts to decrease at around stage 27 as this tissue is beginning to express contractile machinery genes. Elevated levels of GATA-6 delay the onset of the maturation of cardiac precursors (Gove et al., 1997). Thus, GATA-4, -5 and -6 have all been implicated in cardiogenesis, but their specific roles have not yet been elucidated.
Members of this subfamily of GATA factors were thought to be aligned so that the resulting proteins begin with the amino acid sequence MYQ/P (Jiang and Evans, 1996). However, we have demonstrated that human, mouse, Xenopus and zebrafish GATA-6 proteins possess, in addition to the MYQ initiation, an upstream start codon that results in a polypeptide longer by 111 amino acids initiating with the amino acid sequence MALT or MDL (Brewer et al., 1999; T.Peterkin, C.Gove and R.Patient, unpublished; J.Broadbent, N.Holder and R.Patient, unpublished). These long isoforms have greater transcriptional transactivation potential, and studies in mouse indicate that the long isoform may be the only one present in the heart (Brewer et al., 1999, 2002; T.Peterkin, C.Gove and R.Patient, unpublished).
The early lethality of GATA-6-null mutations in the mouse, and the capacity of Xenopus and zebrafish embryos to survive, and enable the analysis of, interference with heart formation, led us to investigate the function of GATA-6 in cardiogenesis in these organisms. To knock-down the function of GATA-6, we designed antisense morpholino oligonucleotides (MOs) specifically to inhibit translation of each GATA-6 isoform. We show for the first time that GATA-6 is required for differentiation of the cardiac lineage during embryogenesis. The requirement appears to be in the maturation of the cardiac phenotype rather than in its initial induction. The long GATA-6 isoform is the dominant player since an MO specific for the shorter isoform has substantially less effect. We also demonstrate with large dorsal marginal zone (DMZ) explant conjugates from Xenopus embryos that the GATA-6 requirement is in the mesoderm and also in the adjacent deep anterior endoderm. The importance of GATA-6 in heart mesoderm during cardiogenesis was confirmed in zebrafish, where heart development is thought not to depend on the endoderm.
Results
Translation of GATA-6 RNA is inhibited by morpholinos
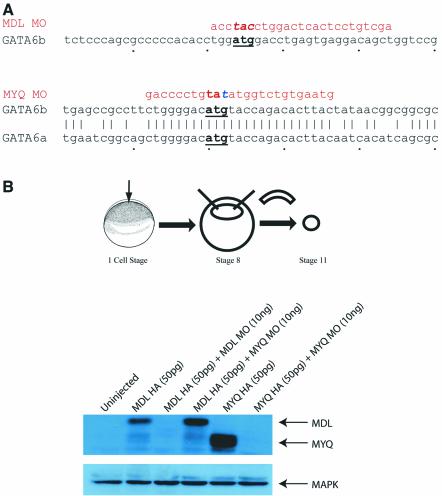
To study loss of function of GATA-6 in Xenopus embryos, we designed antisense MOs to block translation and these were tested in Xenopus animal caps. We designed two MOs, in order to block translation of both long (MDL) and short (MYQ) isoforms. In the absence of sequence information for one of the pseudo-alleles of the longer MDL isoform, both MOs were designed to span the ATG to maximize the chances of conservation (Figure 1A). We tested MO activity by western blot of extracts from animal caps injected with haemagglutinin (HA)-tagged mRNAs coding for both long and short versions of GATA-6 in either the presence or absence of MYQ or MDL morpholinos. Figure 1B demonstrates that both MOs specifically inhibit translation of the appropriate HA-tagged mRNA. In several of the subsequent experiments, both MOs were tested and in every case the activity of the longer MO (MDL) was qualitatively similar to the short version but significantly more efficient (data not shown). We therefore report the activities of the longer version alone. The specificity of this MO for GATA-6 as opposed to GATA-4 or -5 has been demonstrated in animal cap assays (B.Afouda and R.Patient, unpublished).
Fig. 1. MOs inhibit translation of long and short isoforms of Xenopus GATA-6. (A) MO sequences and the ATG regions to which they bind. The MYQ MO was changed in one nucleotide, denoted in blue, to reduce the chances of MO hairpins forming. (B) Western blot of Xenopus animal caps injected with HA-tagged mRNAs and MOs.
Depletion of GATA-6 results in heartless embryos
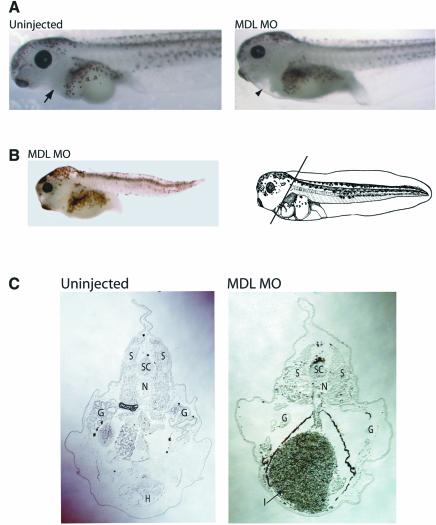
To test whether GATA-6 is required during cardiogenesis, targeted injections of MDL MO into Xenopus embryos were carried out and heart formation was monitored. Four-cell embryos were injected in the position of the future dorsal lateral marginal zone (DLMZ), the presumptive heart tissue (see Figure 3A, top), and embryos were monitored at stage 43, when beating hearts are easily visible under the microscope. An initial titration experiment showed that 5 ng of MO injected into each blastomere was optimal: 1 ng showed little or no phenotype, 3–5 ng gave reproducible heart phenotypes without gross effects on the overall morphology of the embryos, and 10–20 ng resulted in embryos with multiple, serious defects, large amounts of necrotic tissue and a high number of fatalities. Only 2% of embryos depleted of long GATA-6 had visible hearts, as seen in uninjected embryos (Figure 2A, arrow). A small proportion (<5%) of the remaining embryos lacked any tissue in the region where the heart should be (Figure 2A, arrowhead); however, most seemed to have gut tissue extending forward into the cardiac region (Figure 2B and C). Sections through the heart region of uninjected embryos at low power clearly show the heart (H) with its internal trabeculations (Figure 2C). In contrast, sections through embryos injected with MDL MO show no signs of heart formation, and instead have enlarged gut tissue (I) in its place (Figure 2C, arrow). Furthermore, whereas the intestines in uninjected embryos were coiled and had lumens, the injected embryos had uncoiled intestines without lumens (data not shown). Thus it is currently unknown whether the enlarged intestinal volume was due to more gut tissue or its disorganization. The spinal cord (SC), notochord (N) and somites (S) were present and appeared normal in the injected embryos, whereas the gills (G) were reduced in size (Figure 2C). We conclude from these observations that long GATA-6 is crucial for heart development, and that its depletion results in a dramatic loss of heart tissue. This heartless phenotype is not reflective of a generalized effect on organogenesis during development as defects predominate in tissues expressing GATA-6, namely the heart and the gut.
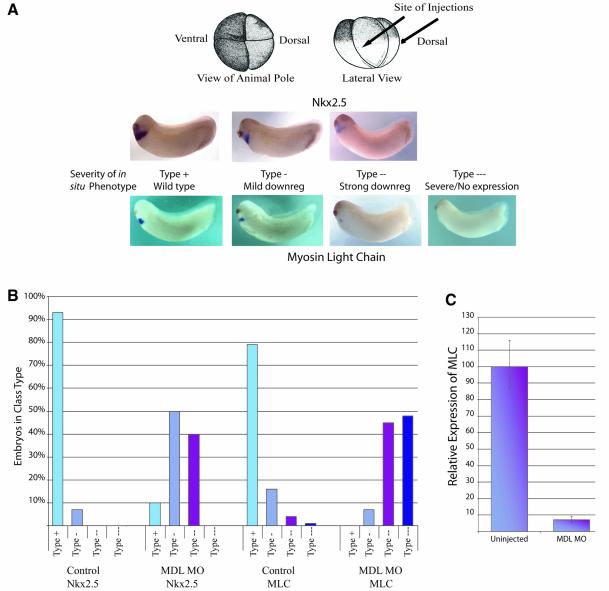
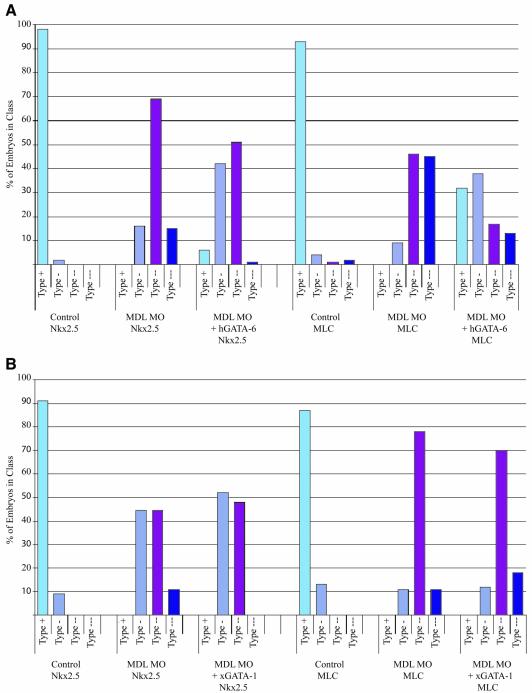
Fig. 3. Expression of Nkx2.5 and MLC2 is downregulated in Xenopus embryos depleted of GATA-6. (A) Whole-mount in situ hybridization for Nkx2.5 and MLC of stage 27/28 embryos injected with MDL MO into the DLMZ at the 4-cell stage. Embryos were classified into four classes: wild-type (type +), mild downregulation (type -), strong downregulation (type - -) and severe downregulation/no expression (type - - -) of the cardiac markers. All phenotypes were observed, with one exception: Nkx2.5 was never completely absent from the injected embryos. (B) Graphical representation of the proportion of embryos in each class. (C) Quantitative real-time RT–PCR of MLC expression in MO-injected embryos. Expression relative to uninjected embryos was determined in four experiments, which were averaged, and the error bars represent standard deviations. MLC expression in uninjected embryos was arbitrarily set at 100%.
Fig. 2. Depletion of GATA-6 results in heartless Xenopus embryos. MDL MO-injected embryos at stage 43+. (A) Uninjected embryos possess beating hearts (arrow) anterior to the gut. Some injected embryos have a tissue-free region (arrowhead) where the heart should be, but in others this region is occupied by the intestines (B). The heart can be seen clearly in the uninjected embryos, but not in MDL MO-injected embryos (C). The intestines, which are normally present in more posterior sections, are enlarged. SC = spinal cord, S = somites, N = notochord, G = gills, H = heart, I = intestines.
Cardiac gene expression is markedly reduced in GATA-6-depleted embryos
To explore further the requirement for GATA-6 during cardiogenesis, we analysed the effect of its reduction on expression of a cardiac differentiation marker, myosin light chain 2 (MLC2), and a heart regulatory gene, Nkx2.5, by in situ hybridization. Figure 3A shows the area targeted by the injections (DLMZ) at the 4-cell stage; the embryos were then examined at stage 27/28 when many of the cardiac muscle-specific genes are beginning to be expressed. The embryos were classified into four classes: those that showed wild-type expression (type +), a mild downregulation (type -), a strong downregulation (type - -) and no visible expression, designated severe (type - - -) (Figure 3A). Figure 3B shows the percentage of embryos in each class. As expected, the profiles of uninjected embryos for both MLC and Nkx2.5 had the vast majority of embryos with wild-type expression (Figure 3B, ‘Control’ histograms). When MDL MO was injected, an increase in the mild and strong phenotypes was seen, compared with uninjected controls (Figure 3B). Forty percent of MDL MO-injected embryos fell into the strong category for Nkx2.5, while only 10% showed normal expression. Furthermore, for MLC, 45% of MDL MO-injected embryos showed the strong phenotype, 48% were in the severe category and 0% of embryos were normal. Therefore, it is clear that when GATA-6 expression is downregulated during Xenopus development, expression of heart differentiation and regulatory genes is affected, with MLC more affected than Nkx2.5.
To confirm these conclusions based on visual inspection of embryos subjected to whole-mount in situ hybridization, we measured MLC expression in MO-injected embryos by real time RT–PCR (Figure 3C). Expression levels were calculated relative to uninjected controls and normalized to expression of the house keeping gene, ornithine decarboxylase (ODC). Figure 3C shows an average of four experiments and was representative of each experiment. Embryos injected with MDL MO showed a decrease in MLC compared with the controls of >10-fold. These data provide quantitative confirmation of the importance of long GATA-6 in myocardial development.
Loss of GATA-4 in mice, or GATA-5 in zebrafish, has been reported to result in cardia bifida, thought to be a failure of the bilateral heart precursors to migrate to the ventral midline and fuse (Kuo et al., 1997; Molkentin et al., 1997; Reiter et al., 1999). Cardia bifida was evident in the majority (∼70%) of MDL MO-injected embryos with residual expression of Nkx2.5 and MLC (Figure 4A). Thus, as seen for GATA-4 in mice and GATA-5 in zebrafish, downregulation of GATA-6 in Xenopus embryos can give rise to cardia bifida. However, although these could have resulted from a failure of migration to the midline, for both the GATA-6 downregulation reported here and the zebrafish faust (GATA-5) mutant, the numbers of cardiac marker-expressing cells were dramatically decreased, even in embryos with residual expression (Figure 4A, arrows). Therefore, we cannot rule out that the absence of such cells in the midline is due to downregulation of expression rather than a failure of migration. Such a conclusion is consistent with the absence of an obvious decrease in the thickness of the mesodermal layer at the midline in injected embryos (Figure 4A).
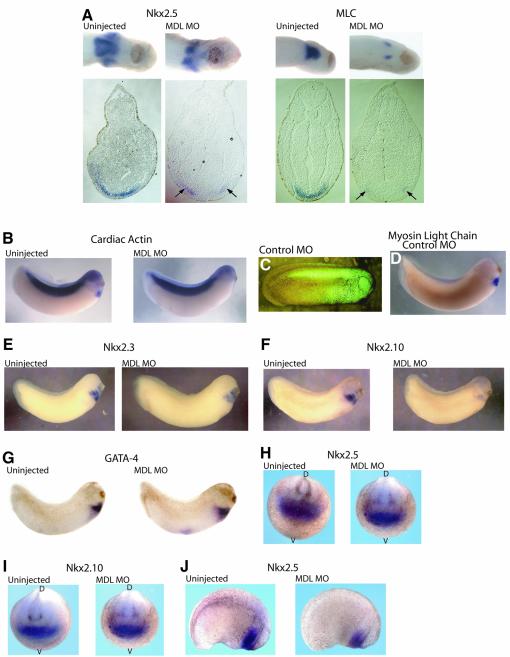
Fig. 4. Depletion of GATA-6 results in Xenopus embryos exhibiting cardia bifida and a downregulation of several cardiac genes. (A) Upper panels: ventral views of the heart regions of stage 28 embryos, either uninjected or injected with MDL MO, stained for expression of Nkx2.5 and MLC by whole-mount in situ hybridization. Lower panels: sections showing two separated populations of cardiomyocytes (arrows). Lateral views of stage 28 embryos: CA expression in the heart is absent (B) although somite expression is unaffected, even though this tissue would have received the MO, as revealed by injection of a control fluorescent MO (C) which itself does not affect MLC expression (D). Expression of Nkx2.3 and Nkx2.10 is downregulated in MDL MO-injected embryos (E and F). GATA-4 expression levels are unchanged by injection of MDL MO (G). (H) Nkx2.5 is expressed normally at stage 16. (I) At stage 16, Nkx2.10 expression remains unchanged on injection of MDL MO (H and I, anterior view; D = dorsal, V = ventral). (J) A slight decrease of Nkx2.5 transcripts is seen at stage 22.
To explore the specificity of the MDL MO further, we examined the expression of cardiac actin (CA), which is expressed early in somites, a region where GATA-6 is absent, and later in cardiac tissue (Mohun et al., 1984). Downregulation of GATA-6 by injection of MO into the heart area resulted in the reduction of CA in the cardiac tissue but not in somites (Figure 4B). We know from co-injecting a standard control fluorescent MO that the somites of these embryos will have received the MO (Figure 4C); therefore, the MDL MO specifically inhibits CA expression in the heart, where GATA-6 is expressed. Embryos injected with the fluorescent MO alone were indistinguishable from uninjected embryos at late stages, and all possessed beating hearts (data not shown). Fluorescent MO-injected embryos were also collected at earlier stages and examined by in situ hybridization for heart markers. Expression of MLC (Figure 4D), Nkx2.5 and CA (data not shown) all remained unchanged. To test the specificity further, we injected MOs designed to reduce translation of another member of the GATA family, GATA-2 (M.Walmsley and R.Patient, unpublished). GATA-2 has not been implicated in cardiogenesis and embryos injected with GATA-2 MOs (18/18) possessed beating hearts similar to uninjected embryos. Furthermore, MLC expression levels were normal in these embryos (23/25). We therefore conclude that the inhibition of myocardial development seen upon injection of the MDL MO is a specific consequence of reduction in GATA-6 expression.
GATA-6 is required for maintenance, but not the initial induction, of Nkx2 expression
To determine where in the cardiogenic regulatory network GATA-6 might be acting, we examined the expression of several other heart markers. Nkx2.5 is a member of a large Nkx2 family, two other members of which are expressed in a similar pattern to Nkx2.5. These are Nkx2.3 and Nkx2.10 (the latter originally named 2.9; Newman and Krieg, 1998). Currently little is known about Nkx2.10, but studies suggest that Nkx2.5 and Nkx2.3 have a redundant nature (Fu et al., 1998). We therefore investigated the effect of downregulation of GATA-6 on these other members of the Nkx2 family (Figure 4E and F). The loss of long GATA-6 substantially reduced expression not only of Nkx2.5, but also Nkx2.3 and Nkx2.10. Within the GATA family, GATA-4 has been strongly implicated in cardiogenesis (Grepin et al., 1995, 1997). In particular, there is a large body of evidence implicating GATA-4 in the expression of several genes associated with maturation of cardiomyocytes, as seen here. We therefore examined the effect of GATA-6 downregulation on GATA-4 expression, and no effect was seen (Figure 4G). We conclude that the effects of GATA-6 downregulation on cardiomyocyte development could be mediated in part by the Nkx2 family but not by GATA-4. The presence of normal numbers of GATA-4-expressing cells indicates that at these stages depletion of GATA-6 is interfering with the maturation of cardiomyocyte precursors rather than their survival.
Thus far we have concentrated on a time point (stage 27/28) around the onset of contractile gene expression. To determine at what stage of cardiogenesis GATA-6 acts, we investigated the expression of the Nkx2 genes at earlier times. At stage 16, soon after the onset of their expression in the cardiac primordia, embryos depleted of GATA-6 have similar levels of Nkx2.3, 2.5 and 2.10 expression to uninjected embryos (Figure 4H and I). However, by stage 22, downregulation of Nkx2.5 at least has begun (Figure 4J), and by stage 27/28 all three are affected. These data suggest that GATA-6 is not required for the initiation of Nkx2 family expression but for their maintenance.
GATA-6 is required for maintenance, but not the initial induction, of BMP-4 expression
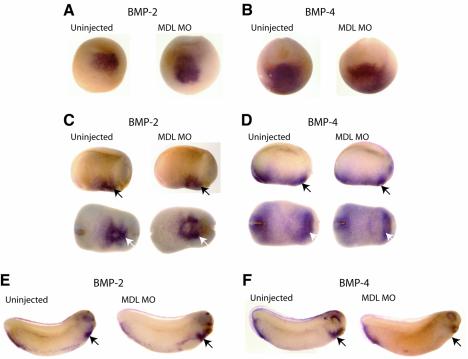
The phenotype described in this study due to loss of GATA-6 is similar to the phenotype seen when bone morphogenetic protein (BMP) signalling is blocked (Shi et al., 2000; Walters et al., 2001). Embryos injected with truncated, dominant-negative forms of BMP receptors, such as tBR or tAlk3, showed a reduction of contractile machinery and Nkx2.5 expression at stages 21–35, whilst levels of GATA-4, -5 and -6 remained unchanged. Moreover, analysis of Nkx2.5 expression earlier in development (stages 14–20) revealed that initiation occurred normally. In addition, cardia bifida was also reported. Furthermore, very recently it has been demonstrated that the BMP-4 promoter contains functional GATA sites and that both GATA-4 and GATA-6 are able to transactivate this promoter in vitro (Nemer and Nemer, 2003). To determine whether GATA-6 is a likely activator of BMPs in vivo, BMP expression was studied in MDL MO-injected embryos (Figure 5). BMP-2 and -4 have been implicated in heart development and are expressed in a similar domain to Nkx2.5 (Suzuki et al., 1993; Clement et al., 1995; Schultheiss et al., 1997; Andree et al., 1998). Expression of BMP-2 was not reduced in the injected embryos when analysed at stages 16 (49/54 embryos), 22 (49/50) or 28 (23/29) (Figure 5A, C and E, respectively). Cardia bifida was seen, but the overall level of BMP-2 expression was unaltered (Figure 5C, white arrows). BMP-4 levels also remained unaltered in injected embryos at stages 16 (39/44) and 22 (53/54); however, by stage 28, a clear reduction in transcripts in the heart was seen in 53/55 embryos (Figure 5B, D and F, arrows). Thus, GATA-6 is required for the maintenance of BMP-4 as well as Nkx2 expression in the heart region.
Fig. 5. GATA-6 is not essential for BMP initiation, but is required for BMP-4 maintenance. (A, C and E) Embryos injected with MDL MO were analysed for BMP-2 expression. At stage 16 (A), stage 22 (C) and stage 28 (E), BMP-2 expression remained unchanged in the morphants in the heart (arrows). (B, D and F) BMP-4 was also monitored in MDL-depleted embryos. At stage 16 (B) and stage 22 (D), BMP-4 expression was unaltered in the heart (arrows). However, by stage 28, a decrease in transcripts was observed in the heart (F, arrows) in the injected embryos. (C and D) Upper panel lateral view, lower panel ventral view.
Morpholino-mediated depletion of GATA-6 in Xenopus embryos can be rescued specifically by co-injection of human GATA-6 RNA
Confidence in the specificity of the effects on cardiogenesis of the MO-mediated depletion of GATA-6 reported here has thus far been based on the minimal effects of two control MOs (the fluorescent control MO and GATA-2 MOs) and, to some extent, the much reduced effect of the MO against the short isoform of GATA-6. In addition, both tissue and gene specificities have been demonstrated. However, non-specific effects of MOs on heart development have been reported (Gerber et al., 2002), so we carried out rescue experiments as an additional check. To effect a rescue, we co-injected the MDL MO with human GATA-6 mRNA, whose translation should be inhibited much less efficiently by the MO. Initially titrations of human GATA-6 RNA with a constant level of MDL MO were performed, and the expression of Nkx2.5 and MLC was used as an indicator of rescue. Embryo phenotypes were assigned as in Figure 3A. An increase of rescued embryos was seen in conjunction with an increase in human GATA-6 RNA, which then plateaued and eventually decreased as very high levels of mRNA were injected. The failure to effect complete rescue may be due in part to an inhibitory effect of GATA-6 overexpression on cardiac maturation (Gove et al., 1997). Figure 6A shows a graphical representation of the rescue at the optimal level of human GATA-6 RNA (50–75 pg per blastomere). As seen previously, the profile of phenotypes in MDL MO-injected embryos compared with uninjected embryos showed increases in the strong and severe classes. When human GATA-6 RNA was co-injected with MDL MOs, a decrease in these groups with concomitant increase in less severe groups (wild-type and mild) was seen compared with embryos injected with MDL MO alone. Thus, human GATA-6 RNA can partially rescue the phenotypes induced by MO-mediated depletion of GATA-6. Importantly, in a separate experiment, this rescue phenomenon was significantly less effective on co-injection of Xenopus GATA-1 RNA (Figure 6B). Nkx2.5 and MLC phenotype distributions consequent upon injection of MDL MO were altered much less dramatically by co-injection with Xenopus GATA-1 RNA (42 and 57 embryos, respectively). Thus, the partial restoration of the expression of cardiac markers was specific to GATA-6, with GATA-1 being able to substitute only poorly in the assay, further validating the specificity of the MO-mediated depletion of GATA-6.
Fig. 6. GATA-6 MO effects on Xenopus embryos can be partially rescued by co-injection of human GATA-6 RNA but not by Xenopus GATA-1 RNA. Embryos were injected with MDL MO alone or co-injected with human GATA-6 mRNA (A) or Xenopus GATA-1 mRNA (B), and the expression of Nkx2.5 and MLC was studied by whole-mount in situ hybridization at stage 28. Embryos were classified into classes as in Figure 3A.
GATA-6 is required in the mesoderm and endoderm for cardiogenesis in Xenopus embryos
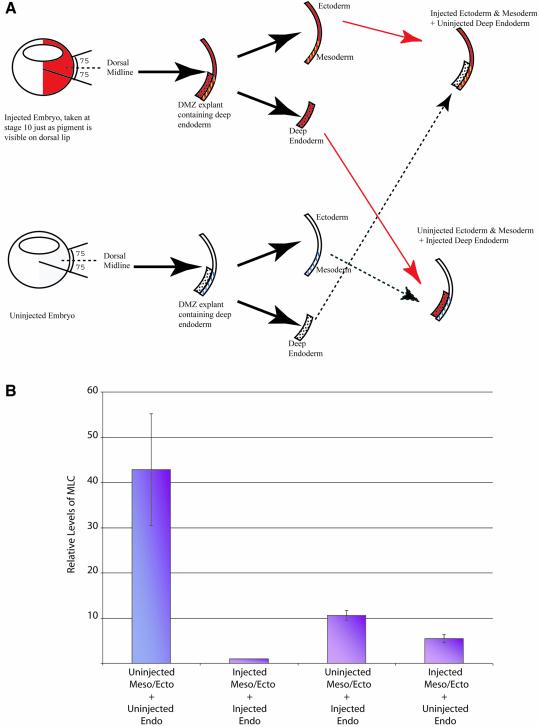
Endoderm is involved in heart specification and GATA-6 is expressed in both the mesoderm and the endoderm at this time (Jiang and Evans, 1996; Gove et al., 1997; Morrisey et al., 1998; Koutsourakis et al., 1999). We therefore wished to determine if the cardiac phenotype resulting from the depletion of GATA-6 was due to its loss in endoderm or in mesoderm. Embryos were injected with the MDL MO into the DLMZ at the 4-cell stage and, at stage 10, just as the pigment forms on the dorsal lip, but prior to involution, we dissected out DMZs as described by Nascone and Mercola (1995). Deep endodermal tissue, removed from uninjected DMZs, was then transplanted adjacent to the remaining mesodermal tissue from injected DMZs (Figure 7A). Injected endoderm was also placed adjacent to uninjected mesoderm. Uninjected endoderm and mesoderm were reconstituted as a control for the procedure, as was injected endoderm and mesoderm. The efficiency of endoderm removal was determined by monitoring heart formation with or without endoderm removal. Beating heart tissue was only detected in one DMZ without endoderm (n = 16), whereas 21/25 DMZ explants containing endoderm had beating cardiac tissue. The DMZ conjugates were left to develop until stage 28/29 equivalent and analysed for MLC expression by real-time RT–PCR. When the mean relative expression levels were plotted, as expected, a large decrease (P < 0.05) in MLC expression was seen in MDL MO-injected DMZ conjugates compared with uninjected DMZ conjugates (Figure 7B). However, there was also a significant decrease (P = 0.06) in MLC expression in uninjected mesoderm conjugated with MDL MO-injected endoderm, compared with uninjected DMZ conjugates, indicating that GATA-6 is required in the endoderm for heart formation. Furthermore, injected mesoderm conjugated with uninjected endoderm had a reduced level of MLC compared with uninjected DMZ conjugates (P < 0.05). Thus, a role for GATA-6 during heart development in both the mesoderm and endoderm is indicated.
Fig. 7. GATA-6 is required in the mesoderm and endoderm for heart formation in Xenopus embryos. Embryos were injected into the DLMZ at the 4-cell stage with MDL MO. At stage 10, DMZs were dissected, the endoderm was removed and replaced with endoderm from an uninjected DMZ; injected endoderm and uninjected mesoderm were also juxtapposed (A); uninjected endoderm and mesoderm, and injected endoderm and mesoderm were reconstitiuted as controls. Conjugates were collected at stage 28/29 equivalent and MLC levels measured by real-time RT–PCR (B). Relative levels were calculated for four experiments (n = 40) and the mean values plotted, with standard errors. For each experiment, readings were normalized to ODC and compared with injected DMZ conjugates, which were arbitrarily designated as 1.
GATA-6 is required for cardiogenesis in zebrafish embryos
The role of GATA-6 in development of the myocardium was also determined in zebrafish embryos. By studying this organism, we sought to confirm the requirement for GATA-6 in the cardiac mesoderm, as opposed to the underlying endoderm, because in zebrafish, beating myocardium can form in normal proportions even in Casanova mutant embryos, which have little or no endoderm (Alexander and Stainier, 1999). An MO was designed to span the initiation codon for the long form of zebrafish GATA-6 (Figure 8A). To check the activity of the MO in blocking translation from GATA-6 RNA, injections into the animal pole of Xenopus embryos were carried out, as described for the Xenopus MOs (Figure 8B). Translation of GATA-6 RNA (zfMDL HA) was prevented by co-injection of 5 ng of the zfMDL MO. We conclude that the amount of MO used in zebrafish embryo injections (5 ng, see below) is sufficient to completely ablate GATA-6 production.
Fig. 8. MO-induced depletion of GATA-6 in zebrafish embryos inhibits maturation of cardiac precursors. (A) The zfMDL MO binds to 25 nucleotides of GATA-6 sequence around the ATG. (B) Western blot showing that the translation of zfGATA6 (MDL) in Xenopus animal caps is inhibited by zfMDL MO. (C) Downregulation of zfGATA6 results in reduced numbers of cells expressing cardiac genes and cardia bifida at 26 h.p.f. (dorsal views). See Supplementary table 1 for numbers. (D) zfSCL MO has no effect on Vmhc expression (dorsal views). Reduced and patchy GATA-1 expression (dorsal view) in embryos injected with zfSCL MO indicates that the MO was functioning. (E) Nkx2.5 expression initiates normally in zfMDL MO-injected embryos but, by 10 somites, expression is slightly reduced in 10/13 embryos. At 18 somites, 13/19 injected embryos showed a stronger reduction. (F and G) GATA-4 and -5 expression is unaffected by zfMDL MO injection.
A titration experiment was carried out with zfMDL MO in zebrafish embryos to determine the optimal amount required to give a reproducible, specific phenotype (see Xenopus titration). Large numbers of zebrafish embryos were then injected with zfMDL MO (5 ng) and myocardial development was examined at 26 h post-fertilization (h.p.f.) using in situ hybridization. Expression of both generalized cardiac markers, such as cardiac myosin light chain 2 (Cmlc2), the transcription factor, Nkx2.5, and the ventricle-specific marker, ventricular myosin heavy chain (Vmhc), were downregulated in the vast majority of embryos (Figure 8C; Supplementary table 1 available at The EMBO Journal Online). In addition, a range of degrees of cardia bifida were seen. Even in non-bifidic embryos, cardiac primordia failed to adopt a left of centre final position in the embryo, reflecting a failure to respond to left–right asymmetry signalling. Thus, overall, downregulation of GATA-6 expression in zebrafish embryos leads to a substantial reduction and malfunctioning of myocardial cells, as seen in Xenopus embryos.
To control for the specificity of the zfMDL MO, two experiments were carried out. First, 5 ng of the flourescent control MO (flCONMO) were injected and 100% of the embryos were normal (data not shown). Secondly, 5 ng of a MO designed to block translation of the blood gene, SCL/Tal-1, were injected and Vmhc expression was monitored (Figure 8D). Sixteen out of 17 injected embryos displayed normal Vmhc expression compared with 1/28 embryos injected with zfMDL MO. In most of these embryos, however, the heart, although non-bifidic, was centrally located, for reasons unknown. As a control for the activity of zfSCL MO, expression of the blood gene GATA-1 was monitored and a typically reduced and patchy pattern was observed (Figure 8D, and L.Patterson, M.Gering and R.Patient, unpublished). We therefore conclude that the reduction of heart marker gene expression seen in zebrafish embryos in response to injection of the GATA-6 MO is specific.
In order to determine when in zebrafish myocardial development GATA-6 is required, we monitored the expression of Nkx2.5 at earlier times (Figure 8E). At seven somites, soon after the first signs of myocardial differentiation, the expression of Nkx2.5 was normal. However, by 10 somites, a reduction in the level of expression was beginning to become apparent, and by 18 somites the reduction was clear (arrows). We therefore conclude from the zebrafish experiments, as for the Xenopus experiments, that GATA-6 is not required for the initial activation of Nkx2.5 expression, but for its maintenance. The expression of two other transcription factors thought to be important early in myocardial development, namely GATA-4 and 5, was also unaffected at 10 somites (Figure 8F and G). We therefore conclude that, as for Xenopus embryos, GATA-6 is required for the maintenance of Nkx2.5 expression and the initiation of contractile machinery gene expression. Further confirmation of the phenotype comes from the continued expression of GATA-4 and -5 at a time when Nkx2.5 downregulation is evident, consistent with a failure of maturation rather than loss of the cardiac precursors. The fact that these events can take place in the absence of endoderm in zebrafish embryos supports the conclusion from explant experiments in Xenopus that GATA-6 is required in the myocardial precursors themselves.
Discussion
GATA-6 is required for cardiomyocyte maintenance and maturation
The role of GATA-6 in cardiogenesis to date has been unclear. Although gain-of-function experiments suggested a role for this GATA factor in heart formation (Jiang and Evans, 1996; Gove et al., 1997; Charron et al., 1999), some loss-of-function data suggested that it may not be required at all except in the absence of GATA-4 and -5 (Jiang et al., 1998; Morrisey et al., 1998; Koutsourakis et al., 1999). However, antisense studies demonstrated a requirement for GATA-6 for specific gene expression during the differentiation of rat neonatal cardiomyocytes in culture (Charron et al., 1999). In this study, we demonstrate for the first time a crucial requirement for GATA-6 in the maturation and maintenance of cardiomyocytes during embryonic development. We have employed a loss-of-function model using antisense MOs to study the function of GATA-6. The depletion of GATA-6 in Xenopus and zebrafish embryos causes a dramatic loss of heart tissue. We found that maturation of the myocardium was inhibited, as indicated by reduced expression of contractile machinery genes and the Nkx2 family, but with continued expression of GATA-4 and -5.
A clue to the position of GATA-6 in the cardiogenic pathway emerged from the Nkx2 data. Downregulation of expression of this gene family may not be surprising since the murine Nkx2.5 enhancer has been shown to contain GATA sites, which are essential for Nkx2.5 transcriptional activity (Lien et al., 1999). In addition, the GATA-6 gene has been shown to be Nkx dependent (Davis et al., 2000; Molkentin et al., 2000); therefore, these genes may be involved in mutually regulatory loops. Our data suggest that GATA-6 is not essential for the initiation of Nkx2.3, 2.5 or 2.10 expression, but rather their maintenance. This begins to place GATA-6 in the genetic regulatory network leading to cardiomyocyte formation (Cripps and Olson, 2002; http://www.nottingham.ac.uk/genetics/staff/rogerpatient/networks/heart.html, model 1).
The effect of loss of GATA-6 on heart development is progressive, so that as the cascade of events in cardiac progenitors proceeds, the perturbation becomes greater. Expression of dominant-negative Nkx2.3 or Nkx2.5 in prospective cardiac tissue causes a downregulation in cardiac differentiation markers, such as MLC, CA and TnIc (Fu et al., 1998; Grow and Krieg, 1998). Moreover, when both constructs were co-injected, the defective phenotype frequency was increased, and rescue was achieved by co-injecting either wild-type Nkx2.3 or Nkx2.5 mRNA, suggesting functional redundancy. Therefore, the downregulation of all three cardiac Nkx members in Xenopus embryos by depletion of GATA-6 could account for the perturbation of CA and MLC expression. GATA-4 is thought to drive cardiac differentiation and directly interact and synergize with Nkx2.5 to activate CA and atrial natriuretic factor (Molkentin, 2000). Although we have shown that GATA-4 expression is not downregulated in GATA-6-depleted embryos, the loss of Nkx2.5 will prevent this cooperative synergy.
How can we explain the contribution of GATA-6-null cells to myocardium in chimeric mouse embryos and the formation of beating tissue in embryoid bodies comprised entirely of GATA-6-null cells (Morrisey et al., 1998; Koutsourakis et al., 1999)? In neither case have marker studies or quantitation been carried out, leaving open the possibility that the cells are compromised in some way. Nevertheless, these observations raise the possibility that the requirement for GATA-6 may be at least in part non-cell autonomous. Together with the data presented here, these observations suggest that GATA-6 is required for the production of an essential secreted factor, which in the chimeras would have been provided by the surrounding wild-type cells. In the embryoid bodies derived entirely from null cells, the secreted factor or a functionally equivalent molecule would have had to be present in the culture media, which included serum. This interpretation would suggest that, as long as the secreted factor is present, GATA-6-null cells are able to compensate to some extent for the loss of GATA-6, possibly by the activity of another GATA factor, such as GATA-4.
How can these loss-of-function data be reconciled with our earlier gain-of-function data, which showed a superficially similar phenotype, namely a block to differentiation of cardiomyocytes (Gove et al., 1997)? In that study, we showed that normal numbers of Nkx2.5-expressing cells were present at stage 28 but that contractile gene expression was blocked. We have since found that the Nkx2.5-expressing cells are, however, not expressing GATA-4, which could account for their failure to differentiate (T.Peterkin and R.Patient, unpublished results). We therefore conclude that Nkx2.5 and GATA-4 have opposite sensitivities to the presence of normal or elevated levels of GATA-6, with Nkx2.5 being sensitive to its absence and GATA-4 to its excess. Since there is evidence that both GATA-4 and Nkx2 are important in cardiomyocyte differentiation, elevation or reduction of GATA-6 results in a block to differentiation.
Requirement for GATA-6 in the mesoderm and endoderm for cardiogenesis
Endoderm is a source of heart-inducing signals (reviewed in Mohun and Sparrow, 1997). In Xenopus, this is the deep anterior endoderm: DMZ explants with these cells removed exhibit a reduced frequency of beating cardiac tissue in comparison with those left with intact endoderm (Nascone and Mercola, 1995). The timing of this requirement was found to be before stage 10.5. Conservation of this method of heart induction does not extend to all vertebrates, however, because zebrafish mutants thought to lack endoderm are able to undergo cardiogenesis (Alexander and Stainier, 1999). In our experiments, targeting injections to the heart mesoderm nevertheless also delivered MO to the adjacent anterior endoderm (Dale and Slack, 1987; Moody, 1987). GATA-6 is expressed in the mesoderm and the endoderm, GATA-6–/– mice die due to endodermal defects, and GATA-6 is able to activate the HNF4 promoter, an endodermal gene, in non-endodermal cells (Morrisey et al., 1998; Koutsourakis et al., 1999). We also have evidence that forced expression of the long isoform of GATA-6 in Xenopus ectoderm can activate the endodermal genes, Sox17α and HNF1β (B.Afouda, T.Peterkin and R.Patient, unpublished). Thus, a role for GATA-6 in endoderm formation is indicated and therefore the heartless phenotype resulting from perturbation of GATA-6 expression could have been due to its loss in the mesoderm or in the endoderm. The DMZ conjugate experiment provides evidence that in fact GATA-6 is required in both of these tissues for efficient cardiogenesis, with its requirement in cardiac mesoderm substantiated by the zebrafish data. The essential role of GATA-6 in the endoderm, however, does not appear to be in its initial formation since apparently copious amounts of gut tissue were formed in MO-injected embryos. We conclude that the essential role of GATA-6 in the endoderm may be related to the production of a cardiogenic signal.
GATA-6 and BMP signalling
Cardiomyocyte maturation requires BMP signalling (Schultheiss et al., 1997; Shi et al., 2000; Walters et al., 2001). This is a member of the transforming growth factor-β (TGF-β) family, and signalling from this family is mediated by Smad proteins, which themselves bind DNA weakly, but are usually recruited to target genes by cofactors (reviewed in Attisano and Wrana, 2000). It has been noted previously that the effect of BMP inhibition on heart development has some similarities to that caused by loss of GATA function (Walters et al., 2001). Loss of GATA function has been reported to result in cardia bifida (Kuo et al., 1997; Molkentin et al., 1997; Jiang et al., 1998; Reiter et al., 1999), and this is evident in our GATA-6-depleted embryos. Interference with BMP signalling also results in this phenomenon (Shi et al., 2000; Walters et al., 2001). Furthermore, inhibition of BMP caused a downregulation of cardiomyocyte differentiation markers troponin c (TnC) and MLC, and of Nkx2.5 (Shi et al., 2000; Walters et al., 2001). However, at earlier stages of development, Nkx2.5 levels were unchanged. Thus, BMP signalling is not required for the initial expression of Nkx2.5 in cardiac precursors, but it is necessary for its maintenance and the correct maturation of cardiomyocytes. These results are reminiscent of our GATA-6 knock-down experiments which suggested a role for GATA-6 in BMP signalling. Direct measurement of BMP-2 and -4 expression showed that at least part of this role is in the maintenance of BMP-4 expression in the cardiac region. In contrast, it has been shown that GATA-4, -5 and -6 expression is not affected when BMP signal transduction is blocked in Xenopus embryos (Walters et al., 2001). We therefore conclude that GATA-6 has an essential function upstream of BMP signalling during cardiac development (http://www.nottingham.ac.uk/genetics/staff/rogerpatient/networks/heart.html, model 2). Clearly BMP-4 is a strong candidate for the essential secreted factor predicted by the GATA-6-null chimeric mouse and embryoid body studies discussed above.
Although the mouse studies suggest no cell-autonomous role for GATA-6 in cardiomyocyte development, those studies were not quantitative (Morrisey et al., 1998; Koutsourakis et al., 1999). Furthermore, even though the GATA-6 MO phenotype in Xenopus and zebrafish could be entirely explained by loss of BMP-4 expression, an additional role as a cofactor for Smad effector pro teins cannot be excluded (http://www.nottingham.ac.uk/genetics/staff/rogerpatient/networks/heart.html, model 3). Precedent for GATA factors acting in this way comes from activated T-lymphocytes where GATA-3 and Smad3 physically and functionally interact to regulate transcription of GATA target genes (Blokzijl et al., 2002). Furthermore, Smad-binding sites have been found in the Nkx2.5 promoter adjacent to GATA sites, both of which are required for expression of Nkx2.5 (Lien et al., 1999, 2002; Liberatore et al., 2002). A role for GATA-6 as an effector of BMP signalling could explain why we see downregulation of Nkx2.5 before BMP-4 expression starts to fall in GATA-6 morphants. In this view, the requirement for GATA-6 in maintaining BMP-4 expression itself could reflect an essential role in autoregulation (Jones et al., 1992; Neave et al., 1997). Thus, GATA-6 may be acting as both a mediator and a driver of BMP signalling as the myocardium matures.
Materials and methods
RNA isolation and real-time RT–PCR
Embryos or DMZs were harvested and snap-frozen in batches of five or 10 in a minimal volume of buffer and stored at –70°C. RNA was extracted and cDNA was synthesized as described (Weber et al., 2000). We performed Taqman assays using the comparative CT method. Abgene thermostart mastermix, with Rox and dUTP, was used according to the manufacturer’s instructions in a 25 µl volume, containing 10 pmol of each forward and reverse primer and 5 pmol of probe. Samples were loaded onto a 96-well plate in triplicate. Uninjected samples were used as the calibrators for whole embryos, whilst for the DMZ experiments injected samples were used as the calibrators. PCRs were performed in an Abi Prism 7700 Sequence Detector; after an initial 50°C for 2 min, 95°C for 10 min, PCR conditions were 40 cycles of 95°C for 0.15 min and 60°C for 1 min. MLC (forward primer) GAGGGCAAAGGACCCATTAAC, MLC (reverse primer) CTTCTGGGTCCGTTCCATTAAG, MLC probe CACAGTCTTCCTGTCGTTGTTTGGCGA, ODC (forward primer) CTGCCGCCTCAGTGTGAA, ODC (reverse primer) GCAGCCAC TGCCAACATG, ODC probe CTGCAGCCTCGACATTCAATGG ATTTC.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We would like to thank Andrew Johnson, Maz O’Reilly, Maggie Walmsley and the reviewers for helpful comments on the manuscript, Matt Loose for computing support, Tim Mohun, Les Dale, Mike Jones and Didier Stainier for probes, John Brookfield for assistance with statistical calculations, and Paul Roach for assistance with real-time RT–PCR. This work was supported by the British Heart Foundation and Nottingham University.
References
- Alexander J. and Stainier,D.Y.R. (1999) A molecular pathway leading to endoderm formation in zebrafish. Curr. Biol., 9, 1147–1157. [DOI] [PubMed] [Google Scholar]
- Andree B., Duprez,D., Vorbusch,B., Arnold,H.H. and Brand,T. (1998) BMP-2 induces ectopic expression of cardiac lineage markers and interferes with somite formation in chicken embryos. Mech. Dev., 70, 119–131. [DOI] [PubMed] [Google Scholar]
- Attisano L. and Wrana,J.L. (2000) Smads as transcriptional co-modulators. Curr. Opin. Cell Biol., 12, 235–243. [DOI] [PubMed] [Google Scholar]
- Blokzijl A., ten Dijke,P. and Ibanez,C.F. (2002) Physical and functional interaction between GATA-3 and Smad3 allows TGF-β regulation of GATA target genes. Curr. Biol., 12, 35–45. [DOI] [PubMed] [Google Scholar]
- Brewer A. et al. (1999) The human and mouse GATA-6 genes utilize two promoters and two initiation codons. J. Biol. Chem., 274, 38004–38016. [DOI] [PubMed] [Google Scholar]
- Brewer A., Nemer,G., Gove,C., Rawlins,F., Nemer,M., Patient,R. and Pizzey,J. (2002) Widespread expression of an extended peptide sequence of GATA-6 during murine embryogenesis and non-equivalence of RNA and protein expression domains. Gene Expr. Patterns, 2, 123–131. [DOI] [PubMed] [Google Scholar]
- Charron F. and Nemer,M. (1999) GATA transcription factors and cardiac development. Semin. Cell Dev. Biol., 10, 85–91. [DOI] [PubMed] [Google Scholar]
- Charron F., Paradis,P., Bronchain,O., Nemer,G. and Nemer,M. (1999) Cooperative interaction between GATA-4 and GATA-6 regulates myocardial gene expression. Mol. Cell. Biol., 19, 4355–4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement J.H., Fettes,P., Knochel,S., Lef,J. and Knochel,W. (1995) Bone morphogenetic protein 2 in the early development of Xenopus laevis. Mech. Dev., 52, 357–370. [DOI] [PubMed] [Google Scholar]
- Cripps R.M. and Olson,E.N. (2002) Control of cardiac development by an evolutionarily conserved transcriptional network. Dev. Biol., 246, 14–28. [DOI] [PubMed] [Google Scholar]
- Dale L. and Slack,J. (1987) Fate map for the 32-cell stage of Xenopus laevis. Development, 99, 527–551. [DOI] [PubMed] [Google Scholar]
- Davis D.L., Wessels,A. and Burch,J.B. (2000) An Nkx-dependent enhancer regulates cGATA-6 gene expression during early stages of heart development. Dev. Biol., 217, 310–322. [DOI] [PubMed] [Google Scholar]
- Fu Y., Yan,W., Mohun,T.J. and Evans,S.M. (1998) Vertebrate tinman homologues XNkx2-3 and XNkx2-5 are required for heart formation in a functionally redundant manner. Development, 125, 4439–4449. [DOI] [PubMed] [Google Scholar]
- Gerber W.V., Vokes,S.A., Zearfoss,N.R. and Krieg,P.A. (2002) A role for the RNA-binding protein, hermes, in the regulation of heart development. Dev. Biol., 247, 116–126. [DOI] [PubMed] [Google Scholar]
- Gove C., Walmsley,M., Nijjar,S., Bertwistle,D., Guille,M., Partington,G., Bomford,A. and Patient,R. (1997) Over-expression of GATA-6 in Xenopus embryos blocks differentiation of heart precursors. EMBO J., 16, 355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grepin C., Robitaille,L., Antakly,T. and Nemer,M. (1995) Inhibition of transcription factor GATA-4 expression blocks in vitro cardiac muscle differentiation. Mol. Cell. Biol., 15, 4095–4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grepin C., Nemer,G. and Nemer,M. (1997) Enhanced cardiogenesis in embryonic stem cells overexpressing the GATA-4 transcription factor. Development, 124, 2387–2395. [DOI] [PubMed] [Google Scholar]
- Grow M.W. and Krieg,P.A. (1998) Tinman function is essential for vertebrate heart development: elimination of cardiac differentiation by dominant inhibitory mutants of the tinman-related genes, XNkx2-3 and XNkx2-5. Dev. Biol., 204, 187–196. [DOI] [PubMed] [Google Scholar]
- Jiang Y. and Evans,T. (1996) The Xenopus GATA-4/5/6 genes are associated with cardiac specification and can regulate cardiac-specific transcription during embryogenesis. Dev. Biol., 174, 258–270. [DOI] [PubMed] [Google Scholar]
- Jiang Y.M., Tarzami,S., Burch,J.B.E. and Evans,T. (1998) Common role for each of the cGATA-4/5/6 genes in the regulation of cardiac morphogenesis. Dev. Genet., 22, 263–277. [DOI] [PubMed] [Google Scholar]
- Jones C.M., Lyons,K.M., Lapan,P.M., Wright,C.V. and Hogan,B.L. (1992) DVR-4 (bone morphogenetic protein) as a posterior-ventralizing factor in Xenopus mesoderm induction. Development, 115, 639–647. [DOI] [PubMed] [Google Scholar]
- Koutsourakis M., Langeveld,A., Patient,R., Beddington,R. and Grosveld,F. (1999) The transcription factor GATA-6 is essential for early extraembryonic development. Development, 126, 723–732. [PubMed] [Google Scholar]
- Kuo C.T., Morrisey,E.E., Anandappa,R., Sigrist,K., Lu,M.M., Parmacek,M.S., Soudais,C. and Leiden,J.M. (1997) GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev., 11, 1048–1060. [DOI] [PubMed] [Google Scholar]
- Liberatore C.M., Searcy-Schrick,R.D., Vincent,E.B. and Yutzey,K.E. (2002) Nkx-2.5 gene induction in mice is mediated by a Smad consensus regulatory region. Dev. Biol., 244, 243–256. [DOI] [PubMed] [Google Scholar]
- Lien C.L., Wu,C., Mercer,B., Webb,R., Richardson,J.A. and Olson,E.N. (1999) Control of early cardiac-specific transcription of Nkx2-5 by a GATA-dependent enhancer. Development, 126, 75–84. [DOI] [PubMed] [Google Scholar]
- Lien C.L., McAnally,J., Richardson,J.A. and Olson,E.N. (2002) Cardiac-specific activity of an Nkx2-5 enhancer requires an evolutionarily conserved Smad binding site. Dev. Biol., 244, 257–266. [DOI] [PubMed] [Google Scholar]
- Mohun T. and Sparrow,D. (1997) Early steps in vertebrate cardiogenesis. Curr. Opin. Genet. Dev., 7, 628–633. [DOI] [PubMed] [Google Scholar]
- Mohun T.J., Brennan,S., Dathan,N., Fairman,S. and Gurdon,J.B. (1984) Cell type-specific activation of actin genes in the early amphibian embryo. Nature, 311, 716–721. [DOI] [PubMed] [Google Scholar]
- Molkentin J.D. (2000) The zinc finger-containing transcription factors GATA-4,-5 and -6—ubiquitously expressed regulators of tissue-specific gene expression. J. Biol. Chem., 275, 38949–38952. [DOI] [PubMed] [Google Scholar]
- Molkentin J.D., Lin,Q., Duncan,S.A. and Olson,E.N. (1997) Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev., 11, 1061–1072. [DOI] [PubMed] [Google Scholar]
- Molkentin J.D., Antos,C., Mercer,B., Taigen,T., Miano,J.M. and Olson,E.N. (2000) Direct activation of a GATA6 cardiac enhancer by Nkx2.5: evidence for a reinforcing regulatory network of Nkx2.5 and GATA transcription factors in the developing heart. Dev. Biol., 217, 301–309. [DOI] [PubMed] [Google Scholar]
- Moody S. (1987) Fates of the blastomeres of the 32-cell stage Xenopus embryo. Dev. Biol., 122, 300–319. [DOI] [PubMed] [Google Scholar]
- Morrisey E.E., Tang,Z., Sigrist,K., Lu,M.M., Jiang,F., Ip,H.S. and Parmacek,M.S. (1998) GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo. Genes Dev., 12, 3579–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita N., Heikenheimo,M., Bielinska,M., White,R.A. and Wilson,D.B. (1996) The gene for transcription factor GATA-6 resides on mouse chromosome-18 and is expressed in myocardium and vascular smooth muscle. Genomics, 36, 345–348. [DOI] [PubMed] [Google Scholar]
- Nascone N. and Mercola,M. (1995) An inductive role for the endoderm in Xenopus cardiogenesis. Development, 121, 515–523. [DOI] [PubMed] [Google Scholar]
- Neave B., Holder,N. and Patient,R.K. (1997). A graded response to BMP-4 spatially coordinates patterning of the mesoderm and ectoderm in the zebrafish. Mech. Dev., 62, 183–196. [DOI] [PubMed] [Google Scholar]
- Nemer G. and Nemer,M. (2003) Transcriptional activation of BMP-4 and regulation of mammalian organogenesis by GATA-4 and -6. Dev. Biol., 254, 131–148. [DOI] [PubMed] [Google Scholar]
- Newman C.S. and Krieg,P.A. (1998) tinman-related genes expressed during heart development in Xenopus. Dev. Genet., 22, 230–238. [DOI] [PubMed] [Google Scholar]
- Patient R.K. and McGhee,J.D. (2002) The GATA family (vertebrates and invertebrates). Curr. Opin. Genet. Dev., 12, 416–422. [DOI] [PubMed] [Google Scholar]
- Read E.M., Rodaway,A.F., Neave,B., Brandon,N., Holder,N., Patient,R.K. and Walmsley,M.E. (1998) Evidence for non-axial A/P patterning in Xenopus and zebrafish pregastrula embryos. Int. J. Dev. Biol., 42, 763–774. [PubMed] [Google Scholar]
- Reiter J., Alexander,J., Rodaway,A., Yelon,D., Patient,R., Holder,N. and Stainier,D. (1999) Gata5 is required for the development of the heart and endoderm in zebrafish. Genes Dev., 13, 2983–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sater A.K. and Jacobsen,A.G. (1990) The restriction of the heart morphogenic field in Xenopus laevis. Dev. Biol., 140, 328–336. [DOI] [PubMed] [Google Scholar]
- Schultheiss T.M., Burch,J.B.E. and Lassar,A.B. (1997) A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes Dev., 11, 451–462. [DOI] [PubMed] [Google Scholar]
- Shi Y., Katsev,S., Cai,C. and Evans,S. (2000) BMP signalling is required for heart formation in vertebrates. Dev. Biol., 224, 226–237. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Nishimatsu,S., Murakami,K. and Ueno,N. (1993) Differential expression of Xenopus BMPs in early embryos and tissues. Zool. Sci., 10, 175–178. [PubMed] [Google Scholar]
- Walters M.J., Wayman,G.A. and Christian,J.L. (2001) Bone morphogenetic protein function is required for terminal differentiation of the heart but not for early expression of cardiac marker genes. Mech. Dev., 100, 263–273. [DOI] [PubMed] [Google Scholar]
- Weber H., Symes,C., Walmsley,M.E., Rodaway,A.R.F. and Patient,R.K. (2002) A role for GATA5 in Xenopus endoderm specification. Development, 127, 4345–4360. [DOI] [PubMed] [Google Scholar]
- Zon L.I., Mather,C., Burgess,S., Bolce,M., Harland,R.M. and Orkin,S.H. (1991) Expression of GATA binding proteins during embryonic development in Xenopus laevis. Proc. Natl Acad. Sci. USA, 88, 10642–10646. [DOI] [PMC free article] [PubMed] [Google Scholar]