Abstract
σS (RpoS), the master regulator of the general stress response in Escherichia coli, is a model system for regulated proteolysis in bacteria. σS turnover requires ClpXP and the response regulator RssB, whose phosphorylated form exhibits high affinity for σS. Here, we demonstrate that recognition by the RssB/ClpXP system involves two distinct regions in σS. Region 2.5 of σS (a long α-helix) is sufficient for binding of phosphorylated RssB. However, this interaction alone is not sufficient to trigger proteolysis. A second region located in the N-terminal part of σS, which is exposed only upon RssB–σS interaction, serves as a binding site for the ClpX chaperone. Binding of the ClpX hexameric ring to σS-derived reporter proteins carrying the ClpX-binding site (but not the RssB-binding site) is also not sufficient to commit the protein to degradation. Our data indicate that RssB plays a second role in the initiation of σS proteolysis that goes beyond targeting of σS to ClpX, and suggest a model for the sequence of events in the initiation of σS proteolysis.
Keywords: protease/proteolysis/rpoS/σ factor/stress response
Introduction
Protein degradation is crucial for clearing cells of denatured, aggregated and/or incomplete polypeptides. In addition, it is increasingly recognized that proteolysis plays an important physiological role in global regulation. A number of key master regulators of stress responses, cell cycle control or developmental processes are conditionally unstable (for a recent overview see Jenal and Hengge-Aronis, 2003). A prominent example is the σS subunit of RNA polymerase in Escherichia coli, which functions as the master regulator of the general stress response. σS is synthesized and at the same time efficiently degraded in rapidly growing cells, which therefore contain little σS. However, σS proteolysis is inhibited in response to a number of different stress conditions, resulting in a rapid and strong increase in the cellular σS level (recently summarized in Hengge-Aronis, 2002b).
The proteases involved in regulated proteolysis are often complex multisubunit proteases such as ClpXP, ClpAP or Lon (Wickner et al., 1999). These complex molecular machines contain chaperone subunits or domains that are responsible for specific recognition, ATP hydrolysis-dependent unfolding and threading of the substrate into the proteolytic cavity (Horwich et al., 1999; Singh et al., 2000; Reid et al., 2001). Some proteolysis substrates carry recognition sites at their C-termini (Levchenko et al., 1995; Keiler et al., 1996; Flynn et al., 2001; Ishii and Amano, 2001), wherease others are recognized via N-terminally located determinants (Gonciarz-Swatek et al., 1999; Gonzalez et al., 2000; Hoskins et al., 2000a,b).
Some substrate proteins require additional specific recognition or targeting factors that increase specificity of recognition or even make recognition possible as the chaperone/protease system would otherwise not attack the substrate. In addition, specific recognition factors provide unique opportunities for regulation of proteolysis, since their substrate binding can be controlled, i.e. by modification. Thus, ClpXP-dependent degradation of σS requires the specific recognition factor RssB, which binds and targets σS to ClpXP, without being degraded itself (Muffler et al., 1996; Pratt and Silhavy, 1996; Becker et al., 1999; Klauck et al., 2001; Zhou et al., 2001). Here, recognition is tightly regulated, since RssB is a two-component response regulator, whose affinity for σS is dependent upon phosphorylation of its receiver domain (Becker et al., 1999; Klauck et al., 2001; Zhou et al., 2001). This allows the RssB/ClpXP system to integrate information from converging stress signal transduction pathways (Hengge-Aronis, 2002a).
An internal ’turnover element’ in σS, and in particular a specific amino acid (K173), was shown to be essential for interaction with phosphorylated RssB and therefore for σS proteolysis (Becker et al., 1999). According to secondary structure prediction and the recently published crystal structures of bacterial RNA polymerase holoenzymes (Murakami et al., 2002; Vassylyev et al., 2002), K173 is located at the beginning of an α-helix that consists of region 2.5 and, together with the two following α-helices, constitutes domain 3 of σS. K173 is highly likely to be surface exposed in free σS as well as in the holoenzyme, consistent with its role in recognition by RssB (Becker et al., 1999) as well as in specific promoter recognition (Becker and Hengge-Aronis, 2001).
These features make σS a highly interesting model substrate for conditional proteolytic recognition. What is the extension of the RssB recognition site in σS that includes K173 as a crucial amino acid? Is RssB binding sufficient to trigger σS proteolysis? Is σS also specifically bound by ClpX and, if so, where is the ClpX-binding site? Is RssB binding a prerequisite for interaction of σS with ClpX? In the present study, we demonstrate that σS contains distinct binding sites for interaction with RssB and ClpX, and we present a model for the sequence of events in the initiation of σS degradation, which explains how the cell achieves tight regulation of σS proteolysis at the molecular level.
Results
Region 2.5 in σS is sufficient for RssB binding, but interaction with RssB is not sufficient to commit σS to proteolysis
Lys173 in σS, which is essential for σS proteolysis in vivo and for RssB binding in vitro (Becker et al., 1999), is part of the surface-exposed α-helix that comprises region 2.5 (α2.5). In order to test whether α2.5 is sufficient for interaction with RssB, we constructed a gene fusion that directs the expression of a hybrid protein containing α2.5 (H170–L189 in σS) linked to the N-terminus of β-galactosidase (α2.5::LacZ). As a control, a similar construct with a K173E exchange was used (α2.5K173E::LacZ; glutamate is the amino acid present at the corresponding position of the vegetative σ factor σ70). When these hybrid proteins were overproduced from a tac promoter plasmid in an otherwise wild-type laboratory strain (MC4100), we observed that α2.5::LacZ, but not α2.5K173E::LacZ or LacZ alone, resulted in increased σS levels (data not shown), suggesting that α2.5 may interfere with σS proteolysis, probably by sequestering RssB.
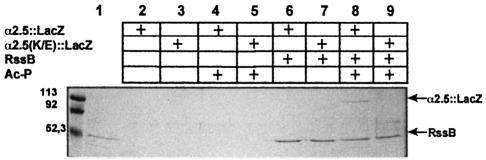
Interaction with RssB was therefore tested in vitro by affinity chromatography (‘pull-down’) experiments (Becker et al., 1999; Klauck et al., 2001). Purified RssB (carrying an S-TRX tag) as well as cellular extracts from cells overexpressing α2.5::LacZ or α2.5K173E::LacZ were used (Figure 1). α2.5::LacZ, but not α2.5K173E::LacZ, co-eluted specifically with phosphorylated RssB, indicating specific binding via K173 in α2.5. This result indicates that α2.5 is sufficient for specific interaction with RssB. The immunoblot data mentioned above indicate that this interaction also takes place in vivo.
Fig. 1. A hybrid protein consisting of the α-helical region 2.5 of σS and β-galactosidase (α2.5::LacZ) binds to phosphorylated RssB. Direct interaction between RssB (carrying an S-TRX-His6 tag, present in lanes 1 and 6–9) and either His6-RpoS (lane 1) or crude cell extracts of strains overexpressing α2.5::LacZ or α2.5K173E::LacZ (lanes 2–9) was assayed by affinity chromatography on S-protein–agarose (as detailed in Materials and methods). Proteins adsorbed to S-protein–agarose were washed and eluted with sodium citrate (pH 2), separated by SDS–PAGE and visualized by immunoblotting. Numbers at the left indicate molecular masses (kDa) of size standard proteins.
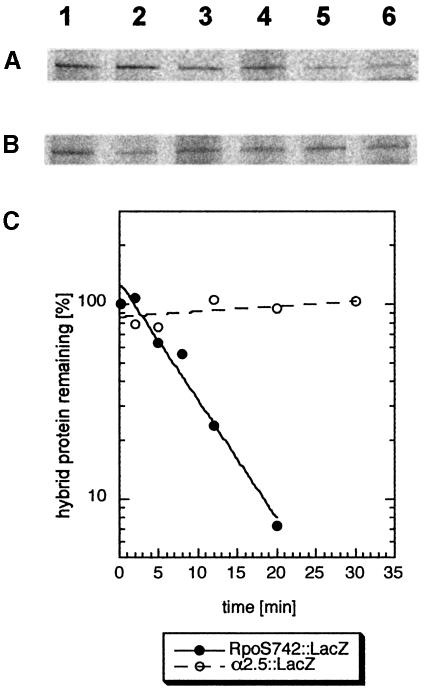
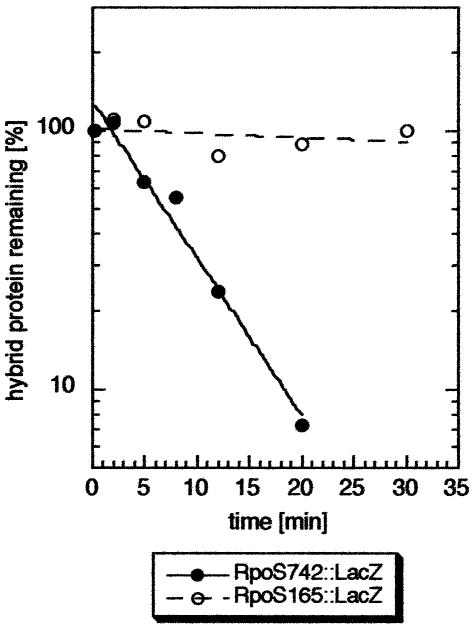
From this result arose the question of whether binding to RssB is sufficient to trigger degradation. In pulse–chase labeling experiments followed by immunoprecipitation (Figure 2), we observed that α2.5::LacZ is stable in vivo. In contrast, the hybrid protein RpoS742::LacZ (which carries the N-terminal 247 amino acids which include α2.5 of σS) is degraded with a similar half-life (3.8 min; Figure 2C) to σS itself (between 1.5 and 4 min, depending on the growth conditions; Lange and Hengge-Aronis, 1994; Becker et al., 1999; Pruteanu and Hengge-Aronis, 2002).
Fig. 2. Despite RssB binding, region α2.5 is not sufficient to trigger proteolysis. Cells expressing RpoS742::LacZ (A) or α2.5::LacZ (B) from pRH800 derivatives were grown in minimal medium (M9/0.1% glucose) to an OD578 of 0.6 and pulse-labeled with [35S]methionine. The pulse time was 2 min; chase times varied between 2 and 30 min. For immunoprecipitation, a polyclonal serum against β-galactosidase was used. Quantification of the data shown in (A) and (B) is given in (C), with closed symbols for RpoS742::LacZ and open symbols for α2.5::LacZ.
We conclude that region 2.5 in σS, i.e. the α2.5 helix, is sufficient for binding to the proteolytic recognition factor RssB. This interaction, however, is not sufficient to commit a protein to degradation. Thus, σS must contain at least one additional determinant that is essential for its proteolysis by the RssB/ClpXP system.
Analysis of σS(Δ7–35): identification of an N-terminal element essential for σS proteolysis
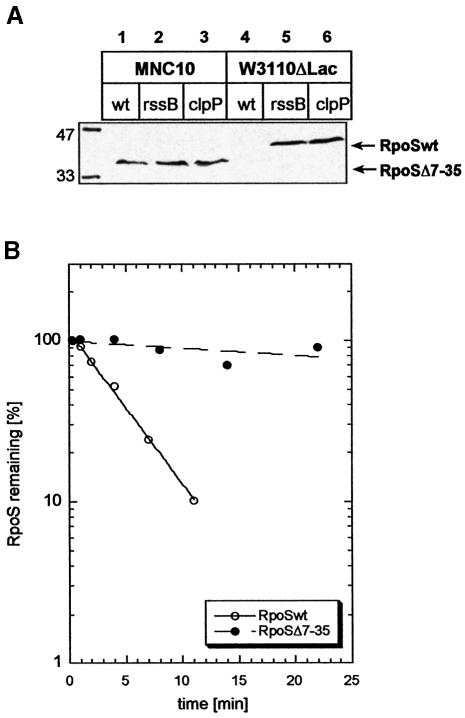
In the course of studies on IS element transposition (M.Noirclerc-Savoye and D.Schneider, in preparation), it was observed that the W3110-related E.coli K-12 strain 431 (Ghosal and Saedler, 1977; Raabe et al., 1988) exhibited relatively high levels of a σS protein of somewhat smaller size. Sequencing revealed a deletion of nucleotides 21–104 in the rpoS open reading frame (ORF), which results in an in-frame deletion of codons 7–35 [the resultant gene product was termed σS(Δ7–35)]. High log phase levels of σS(Δ7–35) were not increased further in rssB or clpP mutant backgrounds (Figure 3A). Moreover, pulse–chase labeling (Figure 3B) demonstrated that σS(Δ7–35) has a largely increased half-life (∼75 min), which is in pronounced contrast to the rapid proteolysis of wild-type σS, which in W3110 is very similar to the half-life in MC4100 (∼3 min). These data suggested that there is a determinant close to the N-terminus of σS that is also important for σS degradation.
Fig. 3. σS(Δ7–35) is present at increased cellular levels that are not altered by mutations affecting RssB or ClpP. (A) Cells of strain MNC10 (lanes 1–3), which carries the rpoS(Δ7–35) mutation, or W3110Δlac (lanes 4–6), which expresses wild-type σS, and rssB or clpP mutations as indicated were grown in LB. During mid-log phase (at an OD578 between 0.7 and 0.9), σS levels were assayed by immunoblot analysis. Numbers at the left indicate molecular masses (kDa) of size standard proteins. (B) Using the same conditions as those described in the legend to Figure 2, degradation of σS and σS(Δ7–35) was assayed using strains W3110Δlac (open circles) and MNC10 (closed circles), respectively.
Complementation experiments indicated that σS(Δ7–35) is as active in the initiation of transcription as wild-type σS. With the corresponding rpoS alleles subcloned onto pBAD18, basal expression levels of σS and σS(Δ7–35) (which in the absence of inducer are comparable with expression levels observed with a chromosomal rpoS gene in a wild-type strain) not only complemented defective glycogen and catalase production in a rpoS::Tn10 background, but resulted in expression levels of the σS-dependent genes osmY and csiD, which where proportionate to σS and σS(Δ7–35) protein levels (data not shown). Moreover, RssB levels as determined by immunoblot analysis were similar in strains expressing either σS or σS(Δ7–35) (data not shown). This indicates that the two variants of σS have similar specific activity in transcription initiation. This also rules out an indirect effect of the Δ7–35 mutation on σS proteolysis due to potentially reduced levels of RssB, whose expression is itself σS dependent (Ruiz et al., 2001; Pruteanu and Hengge-Aronis, 2002) and limiting for the cellular rate of σS degradation (Pruteanu and Hengge-Aronis, 2002).
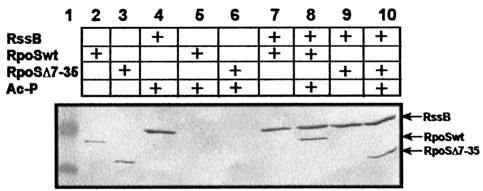
In ‘pull-down’ experiments with purified σS(Δ7–35) (Figure 4), we found that interaction with RssB was not affected by the Δ7–35 deletion in σS. This demonstrates that the N-terminal region affected does not contribute to RssB binding, and represents a second line of evidence indicating that RssB binding alone is not sufficient for σS proteolysis. We conclude that σS contains a second element involved in proteolysis, which is located close to the N-terminus and plays a role that is distinct from σS interaction with RssB.
Fig. 4. σS(Δ7–35) binds to phosphorylated RssB in vitro. Equimolar amounts (0.3 nmol) of S-TRX-His6-RssB and His6-σS or His6-σS(Δ7–35) were incubated at room temperature with or without 50 mM acetyl phosphate (Ac-P), and subject to affinity chromatography on S-protein agarose, separated by SDS–PAGE and visualized using Penta-His antibodies. Size standard proteins (49 and 32.5 kDa) are shown in lane 1. In lanes 2 and 3, His6-σS and His6-σS(Δ7–35), respectively, were directly loaded on the gel.
The N-terminal region of σS contains an element that interacts with ClpX
In order to study the role in proteolysis of the N-terminal region of σS in more detail, a fusion protein was constructed that contains the N-terminal 55 amino acids of σS linked to β-galactosidase (RpoS165::LacZ). This length of the σS moiety was chosen because secondary structure prediction as well as the recently published RNA polymerase holoenzyme structures (Murakami et al., 2002; Vassylyev et al., 2002) indicated that this part of σS is unstructured, whereas a regular pattern of α-helices begins downstream of L55.
As expected, and unlike α2.5::LacZ, RpoS165::LacZ did not interact with RssB as tested by ‘pull-down’ affinity chromatography (data not shown). RpoS165::LacZ is also not degraded in vivo (Figure 5). However, just like α2.5::LacZ, overproduction of RpoS165::LacZ resulted in increased cellular σS levels (data not shown), suggesting that the N-terminal 55 amino acids of σS may also inhibit σS degradation in trans. Therefore, interference with σS degradation was directly monitored in pulse–chase experiments (Figure 6). In contrast to overproduction of wild-type β-galactosidase (LacZ), overproduction of RpoS165::LacZ completely inhibited the turnover of σS over the time tested. This finding suggested that RpoS165::LacZ binds and sequesters some factor essential for σS proteolysis. Such sequestering should be suppressed by overproduction of this factor. As the interacting factor is not RssB (see above), an obvious candidate was ClpX. We therefore introduced a second plasmid, from which ClpX could be overproduced, into the strains used for the interference experiment. ClpX overproduction indeed suppressed inhibition of σS proteolysis by RpoS165:: LacZ (Figure 6). The vector alone had no effect (Figure 6), and also ClpX overproduction did not affect σS degradation in a control strain that overexpressed wild-type LacZ instead of the hybrid protein (data not shown). These in vivo suppression data provide genetic evidence that a region within the N-terminal 55 amino acids of σS can interact with ClpX.
Fig. 5. RpoS165::LacZ is not degraded in vivo. Cells expressing RpoS742::LacZ (closed symbols) or RpoS165::LacZ (open symbols) from pRH800 derivatives were grown in M9/0.1% glucose to an OD578 of 0.6 and pulse-labeled with [35S]methionine. Further treatment was as described in the legend to Figure 2.
Fig. 6. RpoS165::LacZ interferes with σS proteolysis, and this interference can be suppressed by ClpX overexpression. MC4100Δ(ara-leu) derivatives were used that carried combinations of pRH800 and pBAD33 derivatives. From the pRH800 series, either wild-type β-galactosidase (LacZ) or RpoS165::LacZ were expressed (using IPTG as an inducer); from pBAD33, ClpX was overproduced (with arabinose as an inducer). Cells were grown in M9/0.4% glycerol. After induction with 0.2 mM IPTG and 0.001% arabinose (for one generation), cells were harvested at an OD578 of 0.6 and pulse-labeled with [35S]methionine for 1 min. Immunoprecipitation of σS and further treatment were as described in the legend to Figure 2.
Interaction between RpoS165::LacZ and ClpX could also be demonstrated in vitro by co-immunoprecipitation experiments using purified ClpX and cellular extracts as a source of the hybrid proteins (Figure 7; co-immunoprecipitation was used, because in pull-down experiments as described above, the ClpX chaperone alone tended to exhibit too much unspecific interaction). Complexes were isolated using an antibody against His-tagged ClpX (this His tag does not interfere with ClpX function; for details, see Materials and methods). The interaction between RpoS165::LacZ and ClpX is specific for the σS-derived part of the hybrid protein, since it is lost as a consequence of the Δ7–35 deletion in RpoS165::LacZ. Moreover, interaction takes place only in the presence of ATP-γ-S, i.e. requires the formation of the native hexameric ClpX ring structure. From these in vivo and in vitro data, we conclude: (i) that the N-terminal 55 amino acids of σS contain a determinant that is recognized specifically by the hexameric ClpX chaperone ring; and (ii) that this recognition is not sufficient to commit at least the RpoS165::LacZ hybrid protein to proteolysis.
Fig. 7. RpoS165::LacZ binds to ClpX in vitro. Equimolar amounts of crude cell extracts of strains overexpressing either RpoS165::LacZ or RpoS165Δ7–35::LacZ were incubated with 0.3 nmol ClpX in the presence or absence of ATP-γ-S and subject to co-immunoprecipitation, SDS–PAGE and protein staining as described in Materials and methods.
Sequence of events in the initiation of σS proteolysis and the role of RssB
The finding that RpoS165::LacZ is bound by ClpX in vivo (Figure 6) and in vitro (Figure 7), but is not degraded in vivo (Figure 5), is surprising. It means that there is some requirement beyond ClpX binding for the initiation of degradation, which is fulfilled by the larger hybrid protein RpoS742::LacZ, but not by RpoS165::LacZ. Either a third proteolytic determinant is required, at least part of which should be located between D56 and K173 in σS, or, RssB, which binds to RpoS742::LacZ, but not to RpoS165:: LacZ, could play an active role in the initiation of proteolysis that goes beyond transfer of σS to ClpX.
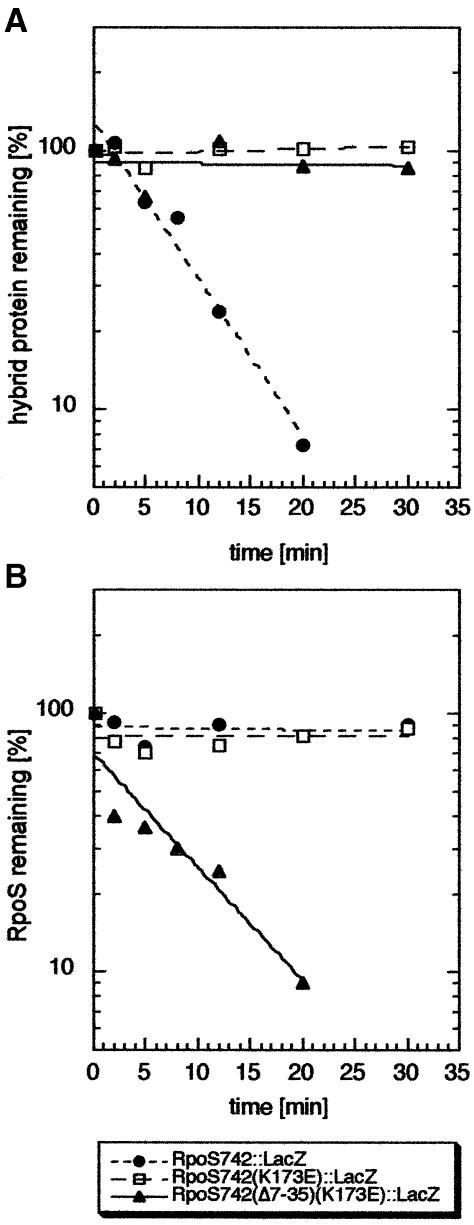
In order to distinguish between these two possibilities, we compared the long hybrid proteins RpoS742::LacZ, RpoS742K173E::LacZ and RpoS742(Δ7–35+K173E)::LacZ. All contain the σS region, in which a putative third proteolytic determinant should be located. Only RpoS742::LacZ, however, exhibited degradation in vivo (Figure 8A). Then, interference with σS proteolysis was assayed by pulse–chase experiments (Figure 8B). As expected, overproduction of RpoS742::LacZ, which contains binding sites for RssB as well as for ClpX, inhibits σS degradation completely. However, RpoS742K173E::LacZ also interferes with σS turnover (Figure 8B). The latter interference is due to interaction with ClpX since it was not observed with RpoS742(Δ7–35+K173E)::LacZ (Figure 8B). This means that even RpoS742K173E::LacZ, which cannot bind to RssB, can interact with ClpX. However, this ClpX interaction remains as unproductive for proteolysis as the ClpX interaction of the shorter RpoS165::LacZ hybrid protein (see above), indicating that the missing component in the latter is not a third proteolytic determinant somewhere between D56 and K173. These data suggest that with these hybrid proteins, RssB is required for proteolysis, but its role is not to make the ClpX-binding site accessible. Rather, it may play a second active, not yet characterized role, e.g. in the initiation of denaturation or entry of σS and/or the σS-derived reporter proteins into the proteolytic cavity.
Fig. 8. RpoS742K173E::LacZ, which is defective for RssB binding, still interacts with ClpX but is not degraded in vivo. MC4100 derivatives expressing RpoS742::LacZ, RpoS742K173E::LacZ or RpoS742Δ7-35+K173E::LacZ from pRH800 derivatives were grown in M9/0.1% glucose. For direct measurement of hybrid protein degradation (A), pulse-labeling was performed at an OD578 of 0.6, and a polyclonal antibody against β-galactosidase was used for immunoprecipitation. In order to test for interference with σS proteolysis by hybrid protein overproduction (B), 0.2 mM IPTG was added at an OD578 of 0.3, and cells were harvested, pulse-labeled and further treated as in (A), with the exception that the polyclonal antibody against σS was used for immunoprecipitation.
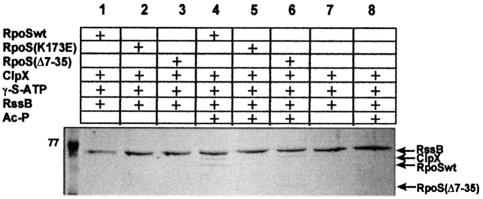
Earlier gel filtration data (Zhou et al., 2001) had indicated that, in contrast to the behavior observed here for RpoS::LacZ hybrid proteins, wild-type σS is not bound by ClpX. We could confirm this also in the experimental system used in the present study (co-immunoprecipitation, data not shown). Thus, the ClpX-binding site close to the N-terminus appears to be cryptic in full-size σS, but accessible in the various RpoS::LacZ hybrid proteins. Additional pull-down experiments, in which ternary complex formation between RssB, several variants of σS and ClpX was assayed, confirmed this conclusion (Figure 9) and even provided a mechanistic explanation. With wild-type σS, phosphorylated RssB (i.e. acetyl phosphate has to be present) and hexameric ClpX6 (due to the presence of ATP-γ-S), ternary complex formation can be observed (Figure 9, lane 4); in the absence of any σS variant, ClpX does not interact with RssB (whether or not it is phosphorylated; Figure 9, lanes 7 and 8); this is also confirmed by the finding that in the presence of σS(K173E), which cannot bind to RssB, ClpX is also not found in a complex with RssB (Figure 9, lane 5). Together with the observation (mentioned above) that native σS and ClpX do not interact, this means that binding to RssB is a prerequisite for interaction of σS with ClpX. This indicates that interaction with RssB triggers a change of conformation in σS that exposes the binding site for ClpX, which in the absence of RssB would be cryptic. The finding that the RpoS742(K173E)::LacZ hybrid protein (lacking the C-terminal 25% of the σS sequence), which cannot bind to RssB, can interact with ClpX via its N-terminal site (Figure 8B) suggests that occlusion of the N-terminal binding site for ClpX is due to interaction with a region in the C-terminal part of σS.
Fig. 9. Ternary complex formation between RssB, different variants of σS and ClpX. Interaction of these proteins was assayed in vitro by affinity chromatography as described in the legend to Figure 1 and Materials and methods (with the exception that ClpX-His6 was also included). 77, molecular mass (in kDa) of a size standard protein.
Finally, we also observed ternary complex formation for phosphorylated RssB, ClpX and σS(Δ7–35) (Figure 9, lane 6). This indicates that at least in σS(Δ7–35), additional ClpX-binding sites become accessible upon interaction with RssB. These sites could be just hydrophobic patches that become surface exposed upon the RssB-triggered change of conformation of σS(Δ7–35). Alternatively, this change of conformation could expose a secondary specific ClpX interaction site, which may serve as a backup for recognition. The experiments with single amino acid mutations in the N-terminal site (see below) suggest such a secondary site in the C-terminal region of σS.
Identification of relevant amino acids in the N-terminal element for ClpX recognition
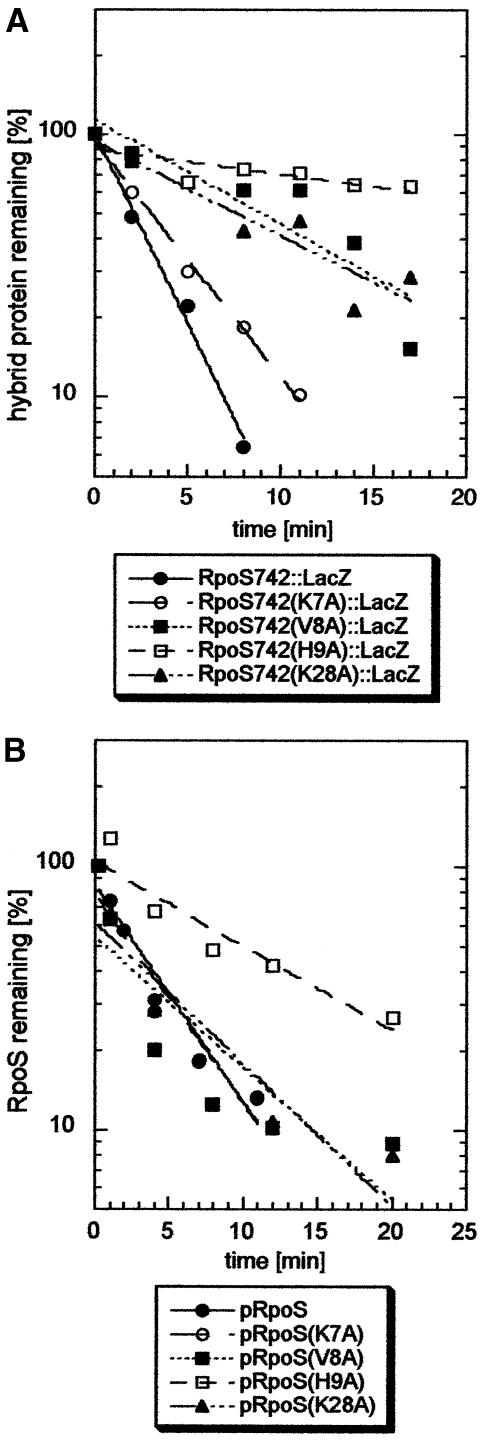
While this manuscript was under revision, K7 in σS was suggested to play a role in recognition by ClpX, because a lysine occurs in a comparable position close to the N-terminus in several other ClpXP substrates (e.g. Dps) and a K5D replacement in a Dps2–12::Arc fusion protein eliminated its proteolysis (Flynn et al., 2003). In parallel, we had changed this K7 and the adjacent amino acids V8 and H9 as well as K28 to alanine (K28 provides the only positive charge in a long stretch of amino acids in σS, which in the closely related σ70 only contains negative charges). The effects of these point mutations on proteolysis were studied in otherwise wild-type σS as well as in the RpoS742::LacZ hybrid protein (Figure 10). The H9A exchange strongly affected proteolysis of both proteins. In contrast, and unlike predicted by Flynn et al. (2003), replacing K7 produced almost no effect. Interestingly, the defects observed for each mutation were more pronounced in the RpoS742::LacZ hybrid proteins (Figure 10A) than in the full-size σS proteins (Figure 10B). This indicates that the C-terminal region in σS may provide for residual recognition by ClpX, if the N-terminal binding site fails to operate adequately.
Fig. 10. Single amino acid exchanges in the N-terminal region affect proteolysis of the RpoS742::LacZ hybrid protein and of σS. In (A), derivatives of strain MC4100 expressing (from pRH800) the RpoS742::LacZ fusion protein (closed circles) or derivatives thereof with the K7A (open circles), V8A (closed squares), H9A (open squares) or K28A mutations (triangles) were grown in minimal medium and treated with tetracycline as described in Materials and methods. In (B), derivatives of the rpoS mutant RH90 expressing σS from pRH800 or derivatives thereof carrying the same mutations as above (with the same designations in the figure) were assayed in a similar way (with the exception that chloramphicol was used for terminating protein biosynthesis).
Discussion
σS has distinct binding sites for the recognition factor RssB and the chaperone ClpX
A single amino acid in σS (K173) has been known to be essential for σS degradation in vivo and for binding of the proteolytic recognition factor RssB in vitro, indicating that K173 is an essential part of the RssB-binding site in σS (Becker et al., 1999). Here, we demonstrate that the RssB-binding site is a single α-helix, i.e. region 2.5 of σS (α2.5). When N-terminally attached to β-galactosidase, i.e. a protein which in its wild-type form is not bound by RssB nor degraded in vivo, α2.5 allows binding to RssB in vivo (resulting in RssB sequestration and therefore inhibition of σS degradation in trans) and in vitro (Figure 1). Besides this binding site for RssB, σS has a second site that is essential for degradation. This determinant is located close to the N-terminus of σS (within the first 55 amino acids of σS), and is not involved in RssB binding, but is a site of interaction with hexameric ClpX6 rings (Figures 3, 5, 7 and 8). Amino acids in this N-terminal recognition site for ClpX, which are important for proteolysis, are above all His9 and, to some minor extent, also Val8 and Lys28 (Figure 10). At first glance, our finding that σS(Δ7–35) is defective for proteolysis seems at variance with a recent report (Rajkumari and Gowrishankar, 2002) that claimed that a σS variant with the first 50 amino acids deleted (and replaced by a different sequence) exhibited normal proteolysis control. Unfortunately, this study did not measure proteolysis directly, e.g. by pulse–chase experiments, and used plasmid constructs throughout in such a way that known alterations of plasmid copy numbers in response to starvation and other stress conditions may equally explain apparently ‘normal’ regulation of σS levels.
The location of the ClpX-binding site close to the N-terminus of σS is consistent with other natural ClpXP substrates, which possess degradation determinants close to either the N- or C-terminus (Keiler et al., 1996; Gonciarz-Swatek et al., 1999; Gonzalez et al., 2000; Hoskins et al., 2000a,b; Flynn et al., 2001, 2003). A few other ClpXP substrates also have two separate determinants that contribute to degradation (Gonciarz-Swatek et al., 1999; Ryan et al., 2002). To our knowledge, however, σS is the only known ClpXP substrate where two distinct recognition sites are each essential for proteolysis and have been shown to interact with two separate but cooperating proteolysis-promoting factors. With CtrA, a global cell cycle regulator in Caulobacter, the situation may be similar, but a specific targeting factor besides ClpX has yet to be identified (Ryan et al., 2002).
Finally, our observation that point mutations in the N-terminal ClpX-binding site all have a more pronounced effect on proteolysis of RpoS::LacZ hybrid proteins (which lack the wild-type C-terminus) than of full-size σS suggests that the C-terminal part of σS may also contribute to recognition by ClpX. However, such a C-terminal site seems to be a minor ‘back-up’ site only that is not able to trigger ClpXP-mediated proteolysis on its own, since a deletion of the N-terminal ClpX recognition site (Δ7–35) strongly increases σS half-life (to ∼75 min; Figure 3).
Neither binding of RssB alone nor of ClpX alone is sufficient to commit σS or a σS-derived reporter protein to degradation
We have shown here that α2.5 alone allows specific binding to RssB but that this interaction is not sufficient to trigger proteolysis of a reporter protein, i.e. β-galactosidase (Figure 2). This finding as well as the corresponding observation that σS(Δ7–35) can interact with RssB but is not degraded (Figures 3B and 5) indicates that the presence of a ClpX-binding site, which we have localized close to the N-terminus of σS, is a prerequisite for RssB/ClpXP-dependent degradation. Thus, RssB is not a factor that extends the substrate spectrum for ClpXP by providing substrate binding to a protein, which by itself would not bind to ClpX. Interaction with ClpX of a specific site in the substrate protein remains a necessity, most probably for the initiation of substrate unfolding and threading of the unfolded substrate into the proteolytic cavity formed by the ClpP complex.
There is an interesting difference between the various RpoS::LacZ hybrid proteins and native full-size σS with respect to accessibility of the ClpX-binding site. In the fusion proteins, this binding site is exposed, which allows interaction with ClpX without RssB binding (Figures 7, 8 and 9). Wild-type σS, however, does not interact with ClpX alone. Only in the presence of phosphorylated RssB is a complex formed (Figure 9). Together with the observation that RssB and ClpX do not form a binary complex (Figure 9), this means that (i) σS is the ‘bridging’ component in the RssB–σS–ClpX complex; and (ii) the N-terminal ClpX-binding site is cryptic in native σS, probably due to shielding by sequences or a domain not present even in the longest fusion protein (RpoS742::LacZ, which contains the N-terminal 75% of the σS sequence), i.e. domain 4 of σS. In terms of molecular structure, this can easily be envisaged, since a σ70-like σ factor consists of an unstructured N-terminal region followed by three domains that are connected to each other by flexible linkers (Campbell et al., 2002; Murakami et al., 2002; Vassylyev et al., 2002). It also indicates that such an intramolecular interaction cannot only prevent a σ factor from binding to DNA in the absence of RNAP core (by occluding regions 2.4/2.5 and 4.2) (Dombroski et al., 1993), but can also prevent it from being constitutively degraded (by occluding a recognition site in region 1). This intramolecular interaction makes RssB intervention a necessity for σS proteolysis, which establishes complex control over σS turnover due to regulation of the cellular level and phosphorylation state of RssB (Pruteanu and Hengge-Aronis, 2002). Sequestration of the RpoS742::LacZ hybrid protein by the ClpX complex may also explain why even strong overproduction of this fusion protein, which contains complete domains 2 and 3 of σS and therefore in principle should be able to interact with RNAP core, is not toxic for the cells.
The experiments with RpoS::LacZ hybrid proteins of various lengths also revealed that interaction with ClpX is not necessarily sufficient to commit these proteins to degradation, i.e. does not result in ‘automatic’ unfolding and threading into the ClpP complex of all substrates (although it seems sufficient for some substrates). σS and σS-derived hybrid proteins may have to overcome yet another hurdle before they definitely become degraded. What is this hypothetical hurdle mechanistically? RpoS742::LacZ is degraded by RssB/ClpXP, whereas RpoS742K173E::LacZ, in which the RssB-binding site is mutated, is stable. Both hybrid proteins, however, bind to ClpX. Thus, successful degradation after ClpX binding correlates with the ability to interact with RssB, suggesting that RssB plays a second role that goes beyond initial interaction with σS and its transfer to ClpX.
It seems likely that RssB is a σS-specific proteolysis promoting factor (Zhou and Gottesman, 1998). This suggests that σS possesses some specific proteolysis-inhibiting feature not present in other ClpXP substrates that would have to be overcome by a second activity of RssB. In principle, this second action of RssB could affect σS, ClpX or ClpP. An interesting point to take into account may be that chaperones that cooperate with a processive protease obviously have to switch between two modes of substrate binding, i.e. (i) initial binding to a specific site of the substrate; and (ii) sequence-unspecific binding that allows sliding of an elongated substrate relative to the chaperone/enzyme. The switch between the first and second mode of binding should involve a specific binding site clearance event, which bears conceptual similarity to promoter clearance by RNA polymerase. If initial specific binding is relatively strong, clearance may become rare and perhaps even energy dependent. The putative RssB dephosphorylation, i.e. hydrolysis of a high energy acyl phosphate bond, may not only result in RssB release from the RssB–σS–ClpXP complex, but may also be instrumental in this transition to the ‘elongation mode’ of the ClpXP machine with σS as a substrate. Future experiments with σS variants with different affinities for ClpX will have to test this hypothesis.
Conclusions: roles of RssB and ClpX and sequence of events in the initiation of σS proteolysis
In summary, we would like to propose a model that includes the following sequence of events in the initiation of σS degradation: (i) initially, σS is bound by RssB-P, which results in a change of conformation of σS that exposes the ClpX-binding site close to the N-terminus of σS; (ii) this leads to the formation of the RssB–σS–ClpX6 complex, which is joined by ClpP14; (iii) the initiation of σS degradation then requires a second activity of RssB (perhaps associated with RssB dephosphorylation), that may be essential for the initiation of σS denaturation and/or for disruption of the initial specific σS–ClpX interaction, i.e. the initiation of threading of σS into the ClpP proteolytic cavity. In principle, the interaction with the ClpP complex may turn the hexameric ClpX ring into a processive enzyme, but in the case of strongly bound σS there may be a need for assistance by RssB. The third step, which again requires RssB, may serve as a proofreading mechanism in the regulation of the initiation of σS proteolysis.
In conclusion, no other known prokaryotic proteolysis substrate is subject to such a complex multistep recognition process as described here for σS. In contrast to σS, however, other proteolysis model substrates are constitutively degraded. By rendering even more than one of these recognition steps dependent on the modification state of a specific recognition factor, the cells achieve precise regulation of σS recognition and therefore σS proteolysis by multiple signals.
Materials and methods
Bacterial strains and growth conditions
With the exception of strain MNC10 (identical to strain 431, which is a derivative of W3110) (Ghosal and Saedler, 1977; Raabe et al., 1988), all bacterial strains used in this study are derivatives of MC4100 (Silhavy et al, 1984). RH90 is a rpoS359::Tn10 derivative of MC4100 (Lange and Hengge-Aronis, 1991). The strains carrying chromosomal osmY::lacZ and csiD::lacZ fusions have been described elsewhere (Becker and Hengge-Aronis, 2001; Germer et al., 2001). Cultures were grown at 37°C under aeration in Luria–Bertani (LB) medium or minimal medium M9 (Miller, 1972) supplemented with glucose or glycerol as a carbon source. Antibiotics were added as recommended (Miller, 1972). Growth was monitored by measuring the optical density at 578 nm (OD578).
Cloning of rpoS::lacZ fusions
The multiple cloning site (MCS) of pJL28 (Lucht et al., 1994) (with a point mutation to eliminate the HindIII restriction site in the MCS) and the following promoterless lacZ gene (starting with the eighth codon of wild-type lacZ) was cloned into the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible vector pRH800 (Lange and Hengge-Aronis, 1994) (an EcoRI–HindIII-digested PCR fragment obtained with pJL28 as a template and the primers 5′-GATCGAATTCCCGGGGATCCGTC GACCTGCAGCCTAGCTTGCGATCCC-3′ and 5′-GATCAAGCTT ATTATTATTTTTGACACCAGACCAACTGG-3′ were used, with underlined nucleotides indicating the EcoRI and HindIII restriction site, respectively; the second primer introduced two additional stop codons). This pRH800 derivate (pAS1) and PCR-amplified rpoS fragments of different lengths were digested with EcoRI and BamHI and ligated, which resulted in the following plasmids: pα2.5::LacZ (containing codons 170–190 of the rpoS coding sequence immediately downstream of the initiator methionine and serine codons as present in wild-type rpoS); pα2.5K173E::LacZ (identical to pα2.5::LacZ except for a point mutation that leads to K173E); pRpoS165::LacZ (containing codons 1–55 of the rpoS coding sequence fused in-frame to the eighth codon of lacZ), pRpoS165Δ7–35::LacZ (identical to pRpoS165::lacZ except for the presence of the Δ7–35 deletion in the rpoS part) and pRpoS742::LacZ (containing codons 1–247 of the rpoS coding sequence fused in-frame to the eighth codon of lacZ). For PCRs, the following primer pairs were used (with EcoRI and BamHI restriction sites, respectively, underlined in the primer sequence, and mutational exchanges in bold): pα2.5::LacZ, 5′-GAGAATTCTGGAGCCACCTTATGAGCCACATCGTAAAGGAGC TGAACGTTTAC-3′ and 5′-GATCGGATCCTCCAGCTTATGGGAC AACTC-3′; pα2.5Δ7–35::LacZ, 5′-GAGAATTCTGGAGCCACCTTA TGAGCCACATCGTAGAGGAGCTGAACGTTTAC-3′ and the same downstream primer as for pα2.5::LacZ; 5′-GAGAATTCTGGAG CCACCTTATGAGTCAGAATACGC-3′ as the upstream primer combined with 5′-GAGATCGGATCCTCCAACACACCTGTGTGGCTC-3′ (pRpoS165::LacZ and pRpoS165Δ7–35::lacZ) or 5′-GAGATCGGATC CTCTTCCGGACCGTTCTC-3′ (pRpoS742::LacZ).
Site-directed mutagenesis in the 5′-terminal region of rpoS
Mutations in the N-terminal region of σS were introduced by a four-primer two-step PCR procedure described previosly (Becker and Hengge-Aronis, 2001). As ‘internal’ primers, 5′-GAATACGCTGGCAGTTCA TGATTTAAATG-3′, 5′-GAACTGCCAGCGTATTCTGAC-3′ (K7A); 5′-ACGCTGAAAGCACATGATTTAAATG-3′, 5′-CATTTAAATCAT GTGCTTTCAGC-3′(V8A); 5′-CTGAAAGTTGCAGATTTAAATGA AGATGC-3′, 5′-CATTTAAATCTGCAACTTTCAGC-3′ (H9A); and 5′-TGACGAAGCAGCCTTAGTAG-3′, 5′-CTAAGGCTGCTTCGTC AAAAACC-3′ (K28A) were used. As external primers, 5′-GCGCC GACATCATAACGGTTC-3′ and 5′-CGCAACTCTCTACTGTTTCT CCATACCC-3′ served as upstream primers for lacZ fusions and rpoS mutants, respectively, and 5′-GCAAAATAAACTTCTTCTTCGG-3′ as a downstream primer. The EcoRI–EagI-digested final PCR fragments were cloned into pRpoS742::LacZ or pRpoS18 (Becker et al., 1999), respectively, treated with the same restriction enzymes. For better expression levels, all pRpoS18-mutants were subcloned later into pRH800 (using EcoRI and HindIII).
Cloning of the clpX gene
For purification of ClpX protein, the clpX coding region was cloned into the pBAD33 vector (Guzman et al., 1995). A KpnI–HindIII-digested PCR fragment obtained with MC4100 chromosomal DNA as a template and the primers 5′-GAGATCGGTACCAGGAGGAATTCACCATGACAG ATAAACGCAAAGATGGCC-3′ and 5′-ACTAAAGCTTTCATTAA TGATGATGATGATGGTGTTCACCAGATGCCTGTTGCGCTTCC-3′ were used (with underlined nucleotides indicating additions to or deviations from the wild-type sequence introduced in order to create KpnI and HindIII restriction sites, respectively, a ribosomal-binding site in the first as well as a His6 tag and an additional stop codon in the second primer). The gene product of the plasmid complemented a chromosomal clpX1::kan insertion (cellular levels of σS were assayed by immunoblot analysis).
Preparation of crude cell extracts
Overnight cultures of MC4100 derivatives carrying plasmids encoding various RpoS::LacZ hybrid proteins (see above) were grown overnight in LB with 100 µg/ml ampicillin, diluted 100-fold and grown at 30°C to an OD578 of 0.5–0.6. IPTG (1 mM) was then added. After 5 h, cells were harvested, resuspended in French press buffer (50 mM Tris–HCl pH 7.5, 50 mM KCl, 5 mM MgCl2, 0.1 mM EDTA) and disrupted in a French pressure cell. The lysate was cleared by centrifugation (30 min at 16 000 r.p.m.). Hybrid protein concentrations were determined by using the SDS–gel electrophoresis calibration kit (Amersham).
Protein purification
RssB (carrying a S-TRX-His6 tag) and His6-σS were purified as previously described (Becker et al., 1999). For the purification of ClpX, the strain RH166 [MC4100 Δ(ara-leu)7697] carrying pClpX (see above) was grown overnight in LB medium with 30 µg/ml chloramphenicol, diluted 100-fold and grown again at 37°C to an OD578 of 0.5–0.6. Arabinose (0.005%) was then added. After 5 h, cells were harvested. All the following purification steps were carried out at 4°C. Cells were resuspended in French press buffer (50 mM NaH2PO4, 300 mM NaCl, 5 mM MgCl2, 10 mM imidazole pH 8.0) and disrupted in a French pressure cell. The lysate was cleared by centrifugation (30 min at 16 000 r.p.m.) and loaded onto an Ni-NTA–agarose column (Qiagen) previously equilibrated in French press buffer. The column was washed with wash buffer (50 mM NaH2PO4, 300 mM NaCl, 5 mM MgCl2, 20 mM imidazole pH 8.0) until the flow-through exhibited an absorption at 280 nm (A280) of <0.02. The ClpX-His6 protein was eluted with wash buffer containing 250 mM imidazole. The protein solution was dialyzed overnight against storage buffer [50 mM Tris pH 7.5, 250 mM NaCl, 10 mM MgCl2, 50% glycerol, 0.1 mM EDTA, 1 mM dithiothreitol (DTT)] (Nguyen and Burgess, 1996).
SDS–PAGE and immunoblot analysis
Sample preparation for SDS–PAGE and immunoblot analysis were performed as described (Lange and Hengge-Aronis, 1994). A 12–15 µg aliquot of total cellular protein was applied per lane. A polyclonal serum against σS, a goat anti-rabbit IgG–alkaline phosphatase conjugate (Sigma) and a chromogenic substrate (BCIP/NBT; Boehringer Mannheim) were used for visualization of σS bands.
Assays for protein degradation in vivo
Pulse labeling of cells with l-[35S]methionine and immunoprecipitation of σS or RpoS::LacZ hybrid proteins was described previously (Lange and Hengge-Aronis, 1994). Cells were grown in either M9/0.1% glucose (for pAS1 derivates) or M9/0.4% glycerol (for pBAD33 derivates). For assaying interference with σS degradation, exponentially growing cells were induced with 0.2 mM IPTG (pAS1 derivates) or 0.001% arabinose (pBAD33 derivates) at an OD578 of 0.3. Cells were harvested at an OD578 of 0.6. For the determination of half-lives of σS and RpoS::LacZ hybrid proteins, the pulse time was 1 and 2 min, respectively. As a σS-deficient control, strain RH90 was used (labeled in exponential phase, samples harvested at an OD578 between 0.5 and 0.7). For immunoprecipitation, polyclonal sera against σS and β-galactosidase were used. Protein bands were quantified on a FLA2000G phospho/fluoroimager (Fuji Photo Film Co., Japan). The intensity of the bands representing σS and the hybrid proteins was calculated relative to the intensity of bands representing stable proteins that cross-reacted with the antisera used.
As a non-radioactive alternative way to assay protein degradation in vivo, 50 µg/ml tetracycline or 100 µg/ml chloramphenicol were added to cells grown as described above at an OD578 of 0.6. Samples were taken as indicated in the figure legends, and the protein of interest was visualized by SDS–PAGE and immunoblotting.
Protein–protein interaction assays
For affinity chromatography (‘pull-down’) assays, 50 µl of S-protein–agarose (Novagen) was equilibrated in binding buffer (20 mM Tris–HCl pH 7.5, 150 mM NaCl, 5 mM MgCl2, 5 mM DTT). Equimolar amounts (0.3 nmol) of S-TRX-His6-RssB, His6-σS, ClpX-His6 or crude cell extracts of strains overexpressing RpoS::LacZ hybrid proteins (corresponding to ∼0.75 nmol hybrid protein), with or without 50 mM acetyl phosphate (Sigma), were incubated for 20 min at room temperature and treated as described (Becker et al., 1999). SDS–gels were either stained as described by Fairbank et al. (1971), or proteins were subject to immunoblot analysis by using polyclonal sera against σS, RssB and β-galactosidase, or a Penta-His antibody (Qiagen).
For co-immunoprecipitation experiments, reaction assays included 50 µl of interaction buffer (35 mM Tris–HCl pH 7.5, 25 mM NaCl, 150 mM KCl, 12 mM MgCl2, 10% glycerol, 0.1 mM EDTA and 1 mM DTT), equimolar amounts of crude cell extracts of strains overexpressing various RpoS::LacZ hybrid proteins (∼0.1 nmol) and 0.3 nmol ClpX-His6. Samples were incubated for 20 min at 37°C in the presence or absence of ATP-γ-S (inverting the test tube every few minutes) and then added to 55 µl of inactivated Staphylococcus aureus Cowan I cells (previously incubated with Penta-His antibody in excess for 1 h at room temperature). After incubation for 1 h at room temperature (inverting the test tube every few minutes), the cells were pelleted and the supernatant was removed. The cells were washed three times with 750 µl of interaction buffer also containing 0.1% NP-40 and once with 10 mM Tris–HCl pH 8.0. The pellet was resolved in 20 µl of SDS–PAGE sample buffer and boiled. After centrifugation, the supernatant was run on a 12% SDS–gel. Gels were Fairbanks stained.
β-galactosidase assay
β-galactosidase activity was assayed by use of o-nitrophenyl-β-d-galactopyranoside (ONPG) as a substrate and is reported as µmol of o-nitrophenol/min/mg of cellular protein (Miller, 1972).
Acknowledgments
Acknowledgements
Financial support was provided by the Deutsche Forschungsgemeinschaft (Gottfried-Wilhelm-Leibniz Program; Priority Program 1132 ‘Proteolysis in Prokaryotes’), the Fonds der Chemischen Industrie and the State of Baden-Württemberg (Landesforschungspreis).
References
- Becker G. and Hengge-Aronis,R. (2001) What makes an Escherichia coli promoter σS-dependent? Role of the -13/-14 nucleotide promoter positions and region 2.5 of σS. Mol. Microbiol., 39, 1153–1165. [DOI] [PubMed] [Google Scholar]
- Becker G., Klauck,E. and Hengge-Aronis,R. (1999) Regulation of RpoS proteolysis in Escherichia coli: the response regulator RssB is a recognition factor that interacts with the turnover element in RpoS. Proc. Natl Acad. Sci. USA, 96, 6439–6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell E.A., Muzzin,O., Chlenov,M., Sun,J.L., Olson,C.A., Weinman,O., Trester-Zedlitz,M.L. and Darst,S.A. (2002) Structure of the bacterial RNA polymerase promoter specificity σ subunit. Mol. Cell, 9, 527–539. [DOI] [PubMed] [Google Scholar]
- Dombroski A.J., Walter,W.A. and Gross,C.A. (1993) Amino-terminal amino acids modulate σ-factor DNA-binding activity. Genes Dev., 7, 2446–2455. [DOI] [PubMed] [Google Scholar]
- Fairbank G., Steck,T.L. and Wallach,D.F. (1971) Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry, 10, 2606–2617. [DOI] [PubMed] [Google Scholar]
- Flynn J.M., Levchenko,I., Seidel,M., Wickner,S.H., Sauer,R.T. and Baker,T.A. (2001) Overlapping recognition determinants within the ssrA degradation tag allow modulation of proteolysis. Proc. Natl Acad. Sci. USA, 98, 10584–10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn J.M., Neher,S.B., Kim,Y.I., Sauer,R.T. and Baker,T.A. (2003) Proteomic discovery of cellular substrates of the ClpXP protease reveals five classes of ClpX-recognition signals. Mol. Cell., 11, 671–683. [DOI] [PubMed] [Google Scholar]
- Germer J., Becker,G., Metzner,M. and Hengge-Aronis,R. (2001) Role of activator site position and a distal UP-element half-site for σ factor selectivity at a CRP/H-NS activated σS-dependent promoter in Escherichia coli. Mol. Microbiol., 41, 705–716. [DOI] [PubMed] [Google Scholar]
- Ghosal D. and Saedler,H. (1977) Isolation of the Mini insertions IS6 and IS7 of E.coli. Mol. Gen. Genet., 158, 123–128. [Google Scholar]
- Gonciarz-Swatek M., Wawrzynow,A., Um,S.-J., Learn,B.A., McMacken,R., Kelley,W.L., Georgopoulos,C., Sliekers,O. and Zylicz,M. (1999) Recognition, targeting and hydrolysis of the λO replication protein by the ClpP/ClpX protease. J. Biol. Chem., 274, 13999–14005. [DOI] [PubMed] [Google Scholar]
- Gonzalez M., Rasulova,F., Maurizi,M.R. and Woodgate,R. (2000) Subunit-specific degradation of the UmuD/D′ heterodimer by the ClpXP protease: the role of trans recognition in UmuD′ stability. EMBO J., 19, 5251–5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman L.M., Belin,D., Carson,M.J. and Beckwith,J. (1995) Tight regulation, modulation and high-level expression by vectors containing the arabinose pBAD promoter. J. Bacteriol., 177, 4121–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengge-Aronis R. (2002a) Recent insights into the general stress response regulatory network in Escherichia coli. J. Mol. Microbiol. Biotechnol., 4, 341–346. [PubMed] [Google Scholar]
- Hengge-Aronis R. (2002b) Signal transduction and regulatory mechanisms involved in control of the σS subunit of RNA polymerase in Escherichia coli. Microbiol. Mol. Biol. Rev., 66, 373–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwich A.L., Weber-Ban,E.U. and Finlay,D. (1999) Chaperone rings in protein folding and degradation. Proc. Natl Acad. Sci. USA, 96, 11033–11040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins J.R., Kim,S.Y. and Wickner,S. (2000a) Substrate recognition by the ClpA chaperone component of ClpAP protease. J. Biol. Chem., 275, 35361–35367. [DOI] [PubMed] [Google Scholar]
- Hoskins J.R., Singh,S.K., Maurizi,M.R. and Wickner,S. (2000b) Unfolding and internalization of proteins by the ATP-dependent proteases ClpXP and ClpAP. Proc. Natl Acad. Sci. USA, 97, 8892–8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii Y. and Amano,F. (2001) Regulation of SulA cleavage by lon protease by the C-terminal amino acid of SulA, histidine. Biochem. J., 358, 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenal U. and Hengge-Aronis,R. (2003) Regulation by proteolysis in bacterial cells. Curr. Opin. Microbiol., 6, 163–173. [DOI] [PubMed] [Google Scholar]
- Keiler K.C., Waller,P.R. and Sauer,R.T. (1996) Role of a peptide tagging system in degradation of proteins synthesized from damaged messenger RNA. Science, 271, 990–993. [DOI] [PubMed] [Google Scholar]
- Klauck E., Lingnau,M. and Hengge-Aronis,R. (2001) Role of the response regulator RssB in σS recognition and initiation of σS proteolysis in Escherichia coli. Mol. Microbiol., 40, 1381–1390. [DOI] [PubMed] [Google Scholar]
- Lange R. and Hengge-Aronis,R. (1991) Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol. Microbiol., 5, 49–59. [DOI] [PubMed] [Google Scholar]
- Lange R. and Hengge-Aronis,R. (1994) The cellular concentration of the σS subunit of RNA-polymerase in Escherichia coli is controlled at the levels of transcription, translation and protein stability. Genes Dev., 8, 1600–1612. [DOI] [PubMed] [Google Scholar]
- Levchenko I., Luo,L. and Baker,T.A. (1995) Disassembly of the Mu trans posase tetramer by the ClpX chaperone. Genes Dev., 9, 2399–2408. [DOI] [PubMed] [Google Scholar]
- Lucht J.M., Dersch,P., Kempf,B. and Bremer,E. (1994) Interactions of the nucleoid-associated DNA-binding protein H-NS with the regulatory region of the osmotically controlled proU operon on Escherichia coli. J. Biol. Chem., 269, 6578–6586. [PubMed] [Google Scholar]
- Miller J.H. (1972) Experiments in Molecular Genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- Muffler A., Fischer,D., Altuvia,S., Storz,G. and Hengge-Aronis,R. (1996) The response regulator RssB controls stability of the σS subunit of RNA polymerase in Escherichia coli. EMBO J., 15, 1333–1339. [PMC free article] [PubMed] [Google Scholar]
- Murakami K.S., Masuda,S. and Darst,S.A. (2002) Structural basis of transcription initiation: RNA polymerase holoenzyme at 4 Å resolution. Science, 296, 1280–1284. [DOI] [PubMed] [Google Scholar]
- Nguyen L.H. and Burgess,R.R. (1996) Overproduction and purification of σS, the Escherichia coli stationary phase specific σ transcription factor. Protein Expr. Purif., 8, 17–22. [DOI] [PubMed] [Google Scholar]
- Pratt L.A. and Silhavy,T.J. (1996) The response regulator, SprE, controls the stability of RpoS. Proc. Natl Acad. Sci. USA, 93, 2488–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruteanu M. and Hengge-Aronis,R. (2002) The cellular level of the recognition factor RssB is rate-limiting for σS proteolysis: implications for RssB regulation and signal transduction in σS turnover in Escherichia coli. Mol. Microbiol., 45, 1701–1714. [DOI] [PubMed] [Google Scholar]
- Raabe T., Jenny,E. and Meyer,J. (1988) A selection cartridge for rapid detection and analysis of spontaneous mutations including insertions of transposable elements in Enterobacteriaceae. Mol. Gen. Genet., 215, 176–180. [DOI] [PubMed] [Google Scholar]
- Rajkumari K. and Gowrishankar,J. (2002) An N-terminally truncated RpoS (σS) protein in Escherichia coli is active in vivo and exhibits normal environmental regulation even in the absence of rpoS transcriptional and translational control signals. J. Bacteriol., 184, 3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid B.G., Fenton,W.A., Horwich,A.L. and Weber-Ban,E.U. (2001) ClpA mediates directional translocation of substrate proteins into the ClpP protease. Proc. Natl Acad. Sci. USA, 98, 3768–3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz N., Peterson,C.N. and Silhavy,T.J. (2001) RpoS-dependent transcriptional control of sprE: regulatory feedback loop. J. Bacteriol., 183, 5974–5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan K.R., Judd,E.M. and Shapiro,L. (2002) The CtrA response regulator essential for Caulobacter crescentus cell-cycle progression requires a bipartite degradation signal for temporally controlled proteolyis. J. Mol. Biol., 324, 443–455. [DOI] [PubMed] [Google Scholar]
- Silhavy T.J., Berman,M.L. and Enquist,L.W. (1984) Experiments with Gene Fusions. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Singh S.K., Grimaud,R., Hoskins,J.R., Wickner,S. and Maurizi,M.R. (2000) Unfolding and internalization of proteins by the ATP-dependent proteases ClpXP and ClpAP. Proc. Natl Acad. Sci. USA, 97, 8898–8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassylyev D.G., Sekine,S.-I., Laptenko,O.,J.,L., Vassylyeva,M.N., Borukhov,S. and Yokoyama,S. (2002) Crystal structure of a bacterial RNA polymerase holoenzyme at 2.6 Å resolution. Nature, 417, 712–719. [DOI] [PubMed] [Google Scholar]
- Wickner S., Maurizi,M.R. and Gottesman,S. (1999) Posttranslational quality control: folding, refolding and degrading proteins. Science, 286, 1888–1893. [DOI] [PubMed] [Google Scholar]
- Zhou A.N. and Gottesman,S. (1998) Regulation of proteolysis of the stationary-phase σ factor RpoS. J. Bacteriol., 180, 1154–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Gottesman,S., Hoskins,J.R., Maurizi,M.R. and Wickner,S. (2001) The RssB response regulator directly targets σS for degradation by ClpXP. Genes Dev., 15, 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]