Abstract
The coordinated migration of neurons is a pivotal step for functional architectural formation of the mammalian brain. To elucidate its molecular mechanism, gene transfer by means of in utero electroporation was applied in the developing murine brain, revealing the crucial roles of Rac1, its activators, STEF/Tiam1, and its downstream molecule, c-Jun N-terminal kinase (JNK), in the cerebral cortex. Functional repression of these molecules resulted in inhibition of radial migration of neurons without affecting their proper differentiation. Interestingly, distinct morphological phenotypes were observed; suppression of Rac1 activity caused loss of the leading process, whereas repression of JNK activity did not, suggesting the complexity of the signaling cascade. In cultured neurons from the intermediate zone, activated JNK was detected along microtubules in the processes. Application of a JNK inhibitor caused irregular morphology and increased stable microtubules in processes, and decreased phosphorylation of microtubule associated protein 1B, raising a possibility of the involvement of JNK in controlling tubulin dynamics in migrating neurons. Our data thus provide important clues for understanding the intracellullar signaling machinery for cortical neuronal migration.
Keywords: cerebral cortex/MAP1B/microtubules/migration/Rac1
Introduction
In radial migration in the cerebral cortex, post-mitotic neurons, generated in the ventricular zone (VZ), move orthogonally through the intermediate zone (IZ) to reach superficial layers of the cortical plate (CP), just beneath the marginal zone (MZ) (Rakic, 1990; Hatten, 2002). Disturbances in these processes may cause brain disorders such as mental retardation and epilepsy (Gleeson, 2001). Although investigations of human and murine genetic mutations have suggested the involvement of several molecules in neuronal migration (e.g. Cdk5, Lis1, Reelin, ApoER2/VLDLR, mDab1) (Gupta et al., 2002; Olson and Walsh, 2002), they do not suffice to explain fully the underlying molecular mechanisms. The reason, in part, may be that a simple genetic approach cannot identify all of the relevant genes that play pivotal roles not only in neuronal migration, but also in early developmental events, because mutations of such genes may cause lethality before development of the cerebral cortex. To overcome this problem, we utilized a recently developed gene transfer method, in utero electroporation (Inoue and Krumlauf, 2001; Saito and Nakatsuji, 2001; Tabata and Nakajima, 2001) (Figure 1A), which enabled us to introduce genes of interest into VZ cells in embryonic cerebral cortices in utero at desired stages from embryonic day 13 (E13) to E17, and to observe resulting phenotypes at subsequent developmental stages.
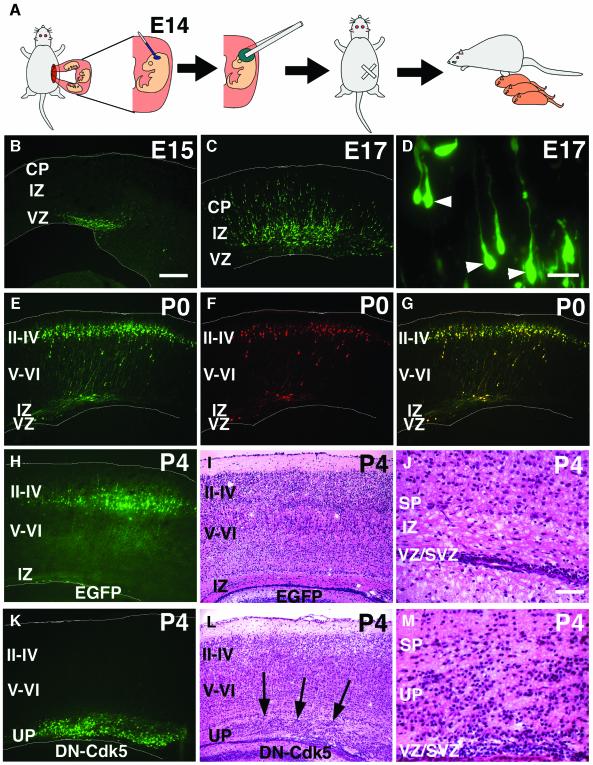
Fig. 1. In utero electroporation. (A) Schematic of in utero electroporation (see Materials and methods). (B–M) In utero electroporation of pEGFP (B–D, H–J), pEGFP plus pDsRed (E–G) or pDN-Cdk5–IRES–EGFP (K–M) into VZ cells of the cerebral cortex. Animals were killed at E15 (B), E17 (C and D), P0 (E–G) and P4 (H–M). EGFP or DsRed fluorescence was viewed in fixed coronal sections. Normal development of VZ cells and their progeny are not affected (B–J). (D) A higher magnification of (C). (E–G) Simultaneous electroporation of pEGFP [(E), green] and pDsRed [(F), red] showed high efficiency of co-expression [(G), merged]. (H–M) Introduction of DN-Cdk5 abolished normal neuronal migration (K–M), mimicking the phenotype of Cdk5-deficient mice, in contrast to controls (H–J). (I and L) HE staining of the sections in (H) and (K), respectively. (J) and (M) are higher magnifications of (I) and (L), around the IZ, respectively. Underplate-like structure (UP) (Gilmore et al., 1998) was observed in DN-Cdk5 transfected animals [arrows in (L)]. White lines in (B), (C), (E)–(H) and (K) represent pial and ventricular surfaces. CP, cortical plate; IZ, intermediate zone; VZ, ventricular zone; SVZ, subventricular zone; II–IV, layers II–IV of the CP; V–VI, layers V and VI of the CP; SP, subplate. Scale bars: 200 µm in (B), (C), (E)–(I), (K) and (L); 10 µm in (D); 50 µm in (J) and (M).
Previously, we identified a Rac1-specific guanine nucleotide exchange factor (GEF), Sif and Tiam1-like exchange factor (STEF) (Hoshino et al., 1999), as a mammalian homolog of Drosophila Still life (SIF), which is involved in synaptic growth (Sone et al., 1997; Sone et al., 2000). Tiam1 (the invasion inducing T-lymphoma and metastasis 1) is another mammalian homolog of Drosophila SIF, originally isolated as an invasion-inducing gene product (Habets et al., 1994). Transcripts of stef and Tiam1 genes are detectable in defined regions of the developing brain where active neuronal migration and neurite growth occur (Ehler et al., 1997; Yoshizawa et al., 2002). Although we and others have revealed that Rac1 and its activators, STEF/Tiam1, are required for neurite growth in N1E-115 neuroblastoma cells as well as primary hippocampal neurons through regulation of cytoskeletal reorganization (Leeuwen et al., 1997; Matsuo et al., 2002, 2003), the roles of these proteins in neuronal migration have not yet been assessed. Previous studies suggested that cortical neuronal migration requires dynamic rearrangement of the cytoskeletal network (Feng and Walsh, 2001) and that in the developing cerebral cortex, Rac1 interacts with Cdk5, an essential molecule for neuronal migration (Nikolic et al., 1998). These facts raise the possibility that the STEF/Tiam1–Rac1 pathway plays an important role in cortical neuronal migration.
In this report, by utilizing in utero electroporation, we introduced dominant-negative (DN) forms for STEF/Tiam1 and Rac1 into the developing cerebral cortex to reveal their pivotal functions in neuronal migration, in vivo. Furthermore, we investigate the role of c-Jun N-terminal kinase (JNK), one of the downstream molecules of Rac1, in the migrating neurons, highlighting the complex signaling cascade involved in neuronal migration.
Results
In utero electroporation
All electroporations in this report were performed on E14 embryos. After a pEGFP plasmid (described below) was electroporated into VZ cells of E14 mice, animals were killed at subsequent developmental stages to observe the location and morphology of transfected cells and their progeny by means of the enhanced green fluorescent protein (EGFP). This plasmid was designed to express EGFP under the control of an ubiquitous CAG promoter (Niwa et al., 1991), which was also used for all other expression plasmids in this study. At E15, 24 h after electroporation, EGFP-expressing cells were observed within the VZ (Figure 1B). They subsequently migrated out, crossing the IZ to reach the superficial part of the CP that gives rise to layers II–IV of the cerebral cortex (Figure 1C–E and H). The consistency of this migration, as well as the morphology of the transfected cells with previous reports of radial neuronal migration (Rakic, 1990; Nadarajah and Parnavelas, 2002), indicate that this gene transfer technique itself does not affect normal development of the cerebral cortex, under our experimental conditions. In addition, electroporation of an EGFP-expressing plasmid enables us not only to detect the transfected cells, but also to observe their detailed morphology (Figure 1D). Furthermore, electroporation of two plasmids encoding EGFP and DsRed revealed that the efficiency of co-transfection was extremely high: 98.3 ± 0.3% of DsRed positive cells were EGFP positive (Figure 1E–G).
We electroporated pDN-Cdk5–IRES–EGFP, a plasmid encoding a DN form of cyclin-dependent kinase 5 (Cdk5) (Nikolic et al., 1996), into E14 VZ cells to suppress Cdk5 function, since Cdk5-deficient mice have been reported to exhibit neuronal migration defects in the cerebral cortex (Gilmore et al., 1998). This plasmid was also designed to express EGFP via an internal ribosome entry site (IRES) to facilitate monitoring of the location and morphology of transfected cells. Consistent with the Cdk5-deficient cortical phenotype, DN-Cdk5-expressing cells could not migrate to the superficial layer of the CP. Hematoxylin–eosin (HE) staining showed that transfected cells were unable to migrate beyond the subplate and remained localized in the IZ, forming an underplate-like structure (Gilmore et al., 1998), even up to postnatal day 4 (P4) (Figure 1K–M), while control cells transfected only with pEGFP migrated normally to the CP (Figure 1H–J). This result strongly indicates that in utero electroporation of DN forms for certain genes may effectively suppress their functions in transfected cells, and therefore suggests that this gene transfer method provides an efficient and convenient tool to study the molecular mechanisms of neuronal migration in vivo.
Roles of STEF/Tiam1 and Rac1 in neuronal migration
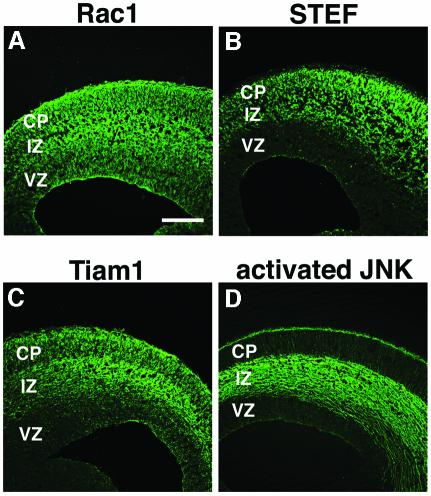
To determine the precise distribution patterns of STEF, Tiam1 and Rac1, immunohistochemistry with specific antibodies was performed on E15 cerebral cortices. Rac1 was ubiquitously detected throughout the cortex (Figure 2A), whereas STEF and Tiam1 were observed predominantly in CP and IZ, but very weakly in VZ (Figure 2B and C) consistent with their mRNA distribution (Ehler et al., 1997; Yoshizawa et al., 2002). Previous reports that Rac1 is required for migration of many types of cells in vitro (fibroblasts, neutrophils, etc.) (Ridley, 2001) support the likelihood that the STEF/Tiam1–Rac1 pathway may be involved in neuronal migration in the cerebral cortex.
Fig. 2. Distribution patterns of Rac1 (A), STEF (B), Tiam1 (C) and activated JNK (D) in the cerebral cortex of E15 embryos visualized by specific antibodies. Scale bar, 200 µm.
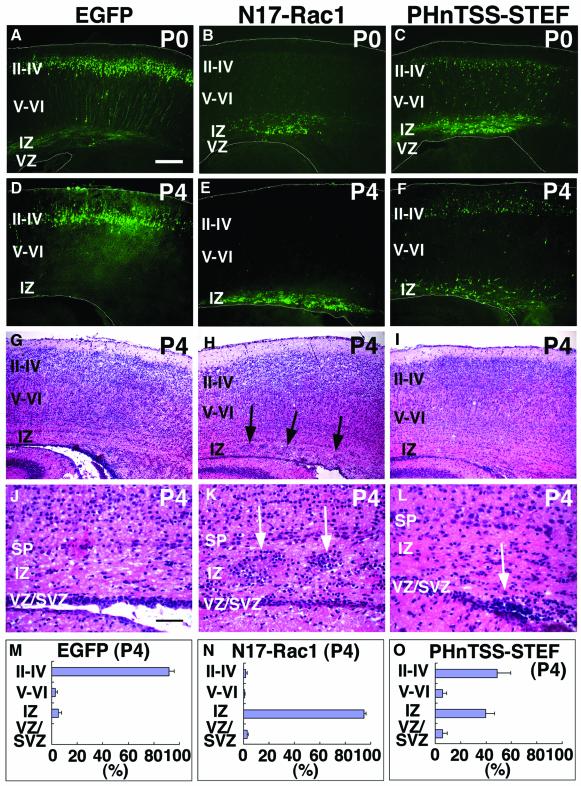
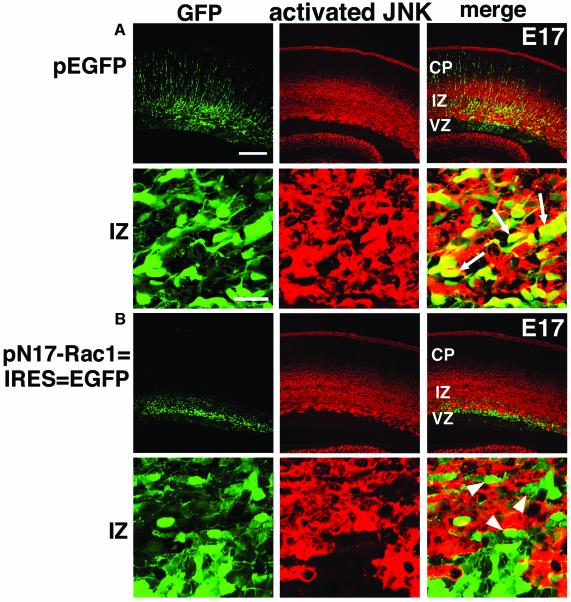
To test this, we tried to introduce DN forms for Rac1 (N17-Rac1) (Ridley et al., 1992) and STEF/Tiam1 into the cerebral cortex during development. A fragment encompassing the PHnTSS domain of STEF (PHnTSS-STEF) has been shown to work as a specific DN form for both STEF and Tiam1 (Matsuo et al., 2002). pN17-Rac1–IRES–EGFP and pPHnTSS-STEF–IRES–EGFP plasmids were electroporated into E14 VZ cells, and embryos were killed at various developmental stages. These plasmids were designed to express N17-Rac1 and PHnTSS-STEF together with EGFP via the IRES. At P0, 5 days after electroporation, most of the N17-Rac1 transfected cells were stalled in the IZ of the cortex, while control (pEGFP) transfected cells migrated normally to the superficial layers of CP (Figure 3A and B). Similarly, many PHnTSS-STEF-transfected cells were also observed in the IZ of P0 mice, although the extent was less than that of N17-Rac1 cells (Figure 3C). At P4, 9 days after electroporation, most N17-Rac1 transfected cells and many PHnTSS-STEF transfected cells remained in the IZ (Figure 3E and F). After EGFP signals were recorded, sections of P4 samples were counterstained by HE staining and revealed abnormally located cells within the IZ of N17-Rac1- and PHnTSS-STEF-transfected mice (Figure 3G–L).
Fig. 3. DN effects of Rac1 and STEF/Tiam1 on the developing cerebral cortex. pEGFP (A, D, G, J and M), pN17-Rac1–IRES–EGFP (B, E, H, K and N) or pPHnTSS-STEF–IRES–EGFP (C, F, I, L and O) was introduced into E14 VZ cells. At P0 (A–C) or P4 (D–O), frozen brain sections were examined for EGFP fluorescence (A–F). White lines in (A)–(F) represent pial and ventricular surfaces. After EGFP signals of P4 samples (D–F) were recorded, sections were subjected to HE staining (G–L). (J–L) Higher magnifications of (G)–(I), around the IZ, respectively. Arrows indicate abnormal accumulation of cells in the IZ of N17-Rac1 or PHnTSS-STEF electroporated brains, respectively. Most of these cells were found to be EGFP positive. Each DN form used in this experiment (N17-Rac1, PHnTSS STEF) contains an epitope tag. By immunostaining, expression of each DN form was confirmed in EGFP-positive cells (data not shown). (M–O) Mice subjected to electroporation were killed at P4 to estimate the extent of migration by recording fluorescence intensities of EGFP in distinct parts of the cerebral cortex; layers II–IV, layers V–VI, IZ and VZ/SVZ. Each score represents mean percentage of relative intensity ± SE. (M) n = 5; (N) n = 7; (O) n = 8. Scale bars: 200 µm in (A)–(I); 50 µm in (J)–(L).
The extent of cell migration was statistically estimated by the fluorescence intensities in distinct layers of the cerebral cortex. In brains transfected with control plasmid (pEGFP), 92.1 ± 3.9% of EGFP fluorescence was detected in layers II–IV of the CP (Figure 3M). In contrast, 94.9 ± 1.2% of the fluorescence was detected in the IZ of N17-Rac1-transfected brains, and 39.7 ± 6.9% in PHnTSS-STEF-expressing animals (Figure 3N and O). These findings indicate that suppression of Rac1 or STEF/Tiam1 function leads to inhibition of normal cortical migration, resulting in an increase of ectopic cells in IZ. Two possible explanations present themselves: one is that the STEF/Tiam1–Rac1 pathway is involved in cortical neuronal migration and the other is that functional suppression of Rac1 or STEF/Tiam1 affects the lineage of cells produced in VZ, consequently resulting in ectopic localization of transfected cells. To discriminate between the two possibilities, we performed the following experiments.
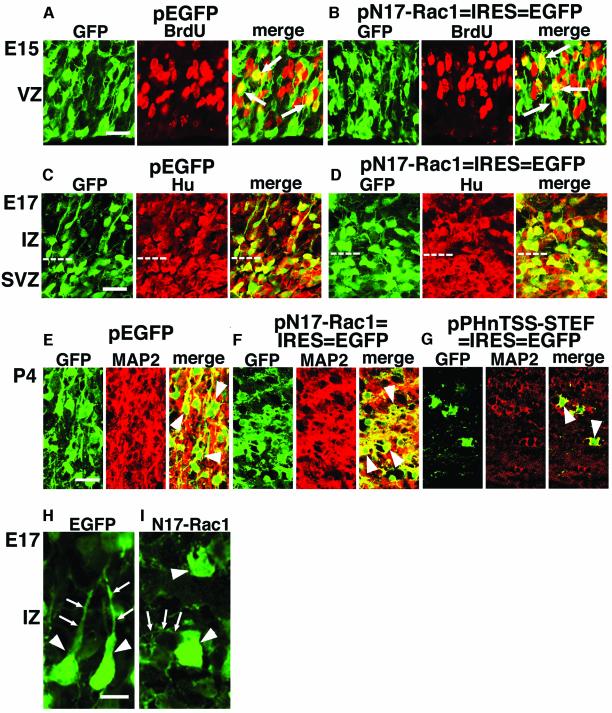
First, we examined whether N17-Rac1-transfected cells could normally proliferate in VZ. Embryos were electroporated with pN17-Rac1–IRES–EGFP or pEGFP at E14, subjected to BrdU labeling for 1 h at E15, and killed to observe incorporation. This experiment revealed that N17-Rac1-expressing cells were able to enter S phase (Figure 4B) to an extent similar to control pEGFP-transfected cells (Figure 4A), implying that expression of N17-Rac1 did not affect VZ cell division. Similar results were obtained from animals electroporated with pPHnTSS-STEF–IRES–EGFP (data not shown).
Fig. 4. N17-Rac1 did not affect cell division in VZ cells nor differentiation of their progenies. (A and B) BrdU incorporation in VZ cells in animals electroporated with pEGFP (A) or pN17-Rac1–IRES–EGFP (B). Twenty-four hours after electroporation to E14 embryos, BrdU was intraperitoneally administered to the animals for 1 h and animals were killed immediately afterwards. Frozen sections of brains were immunostained with anti-GFP (green) and anti-BrdU (red) antibodies. Arrows indicate cells co-stained with both antibodies. BrdU incorporation rates (BrdU+ cells/EGFP+ cells) in VZ were 27.0 ± 2.2% in (A) and 25.8 ± 1.6% in (B). Scale bar, 20 µm. (C and D) Expression of an early neuronal marker, Hu. E14 embryos were electroporated with the indicated plasmids and killed at E17. Frozen sections were immunostained with anti-GFP (green) and anti-Hu (red) antibodies. White dotted lines represent the boundary of IZ and SVZ. Scale bar, 20 µm. (E–G) Expression of MAP2. E14 embryos were electroporated and killed at P4. Frozen sections were immunostained with anti-GFP (green) and anti-MAP2 (red) antibodies. (E) shows the region around the CP, and (F) and (G) show the region around the IZ. Arrowheads indicate cells co-expressing the transgene and MAP2. Ectopically located cells differentiated to MAP2-positive neurons, although it could not be determined whether these neurons maintained their original layer specificity. Scale bar, 20 µm. (H and I) Morphology of EGFP-positive cells in IZ of E17 brains electroporated with pEGFP or pN17-Rac1–IRES–EGFP at E14. Cells were stained with anti-EGFP antibody to observe detailed morphology. While control cells [arrowheads in (H)] exhibited a spindle-like morphology with a leading process [arrows in (H)] toward the pial surface, N17-Rac1-expressing cells [arrowheads in (I)] showed a round morphology with minor randomly directed processes [arrows in (I)]. Scale bar, 10 µm.
Next, an early stage neuronal marker, Hu, was examined in embryos electroporated with pN17-Rac1–IRES–EGFP or pEGFP at E14. Brains were sectioned at E17, and subjected to immunostaining with an anti-Hu antibody. It has been shown that postmitotic neurons start to express Hu just after they arise in the developing cortex (Okano and Darnell, 1997). In control embryos, most of the EGFP expressing cells in IZ and subventricular zone (SVZ) expressed Hu (Figure 4C). Similarly, N17-Rac1-transfected cells were Hu-positive within IZ and SVZ (Figure 4D), suggesting that normal differentiation into neurons occurred. Similar results were obtained from animals electroporated with pPHnTSS-STEF–IRES–EGFP (data not shown).
We also investigated a marker for mature neurons, MAP2, in animals electroporated at E14 and killed 9 days later at P4. In control animals, EGFP-positive cells in the CP highly expressed MAP2 (Figure 4E). In animals electroporated with pN17-Rac1–IRES–EGFP, transfected cells that were stalled in IZ significantly expressed MAP2 (Figure 4F). Similar results were seen in animals electroporated with pPHnTSS-STEF–IRES–EGFP (Figure 4G). These findings suggest that cells transfected with N17-Rac1 or PHnTSS-STEF were able to differentiate into mature neurons, albeit the loss of normal migration. These data indicate that the STEF/Tiam1–Rac1 pathway is involved in neuronal migration, rather than in proliferation or differentiation of VZ cells.
We further investigated the morphology of cells that express N17-Rac1. Animals were electroporated at E14 and killed at E17. While control cells (pEGFP) exhibited proper polarization, extending a leading process to the pial surface in the IZ (Figure 4H), N17-Rac1-expressing cells in IZ were round, with short and irregular processes (Figure 4I). This result suggests that Rac1 activity is required for acquisition of the spindle-like morphology and the leading process of migrating neurons.
Involvement of JNK in neuronal migration
Next, we focused on one of the downstream molecules of Rac1, JNK. Immunostaining for activated JNK revealed a strong signal in the IZ of E15 cerebral cortex (Figure 2D), suggesting a role for JNK in cortical neuronal migration. We investigated the effect of N17-Rac1 on JNK activity in vivo. Embryos were electroporated at E14 with pN17-Rac1–IRES–EGFP or control pEGFP, killed at E17 and subjected to immunostaining. Activated JNK was detected in control IZ cells (Figure 5A) but rare in IZ cells expressing N17-Rac1 (Figure 5B). These findings suggest that suppression of Rac1 activity not only perturbed the cell morphology but also dramatically decreased JNK activity in migrating neurons in the IZ.
Fig. 5. JNK is activated in migrating neurons in the IZ in a Rac1-dependent manner. (A and B) N17-Rac1 suppresses the activation of JNK in the developing cerebral cortex. E14 brains were electroporated with pEGFP (A) or pN17-Rac1–IRES–EGFP (B) and analyzed at E17. Frozen sections were immunostained with anti-EGFP (green) and anti-activated JNK (red) antibodies. Lower panels are higher magnifications of upper panels. Activated JNK was observed in many control pEGFP-transfected cells [arrows in (A)], but rarely in N17-Rac1 expressing cells in IZ [arrowheads in (B)]. To explain this phenomenon, there exists the possibility that the N17-Rac1-expressing cells are undergoing normal differentiation in an ectopic site (the IZ), rather than N17-Rac1 preventing JNK activation. However, this seemed unlikely because these N17-Rac1-expressing cells were not MAP2-positive at this stage (data not shown). Scale bars: 200 µm in upper panels; 20 µm in lower panels.
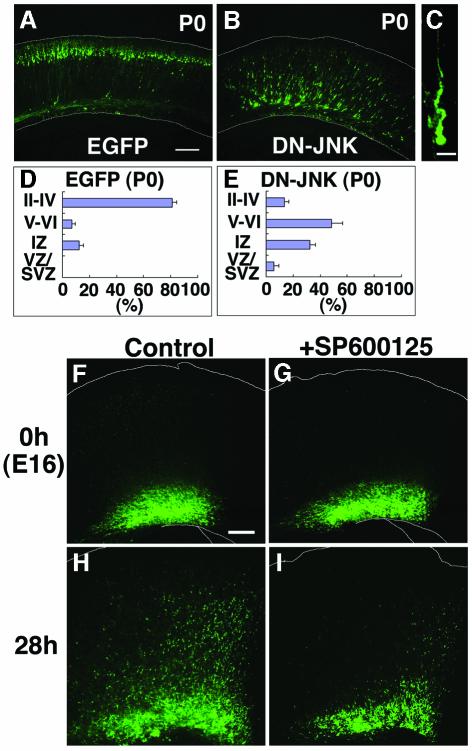
To elucidate the role of JNK in neuronal migration, we introduced a DN form for JNK (DN-JNK) (Derijard et al., 1994) into the developing cerebral cortex. Embryos were co-electroporated with pDN-JNK and pEGFP plasmids at E14. Five days after electroporation (P0), control EGFP-expressing cells had migrated to the superficial layer of CP (Figure 6A), but most of the DN-JNK expressing cells remained trapped in the lower part of the cerebral cortex (Figure 6B). These cells were observed in IZ and the lower CP, in contrast to most of the N17-Rac1 expressing cells, which could not migrate beyond the subplate (Figure 3B). The extent of cell migration was statistically estimated by the fluorescence intensities in distinct layers of the cortex. In brains expressing DN-JNK, EGFP fluorescence was mainly detected in the IZ and layers V–VI of the CP (32.3 ± 4.2 and 48.4 ± 7.9%, respectively) (Figure 6E), whereas fluorescence was largely observed in layers II–IV (81.0 ± 3.4%) in control animals (Figure 6D). Furthermore, in animals that were electroporated at E14 and killed at P4, more DN-JNK expressing cells in layers V–VI of CP (68.3 ± 2.4%) and fewer DN-JNK expressing cells in IZ (7.9 ± 3.2%) were observed than in animals killed at P0 (data not shown). These findings indicate that suppression of JNK function retarded rather than inhibited migration. DN-JNK transfected cells still exhibited a spindle-like morphology, although the leading process appeared twisted and irregular (Figure 6C).
Fig. 6. Involvement of JNK in migrating neurons. (A–E) Effects of DN-JNK on cortical neuronal migration. E14 brains were electroporated with pEGFP (A and D) or pDN-JNK plus pEGFP (B, C and E) and analyzed at P0 by fluorescence microscopy. (C) A high magnification of (B), an IZ cell. DN-JNK expression was confirmed in EGFP-positive cells by anti-epitope immunostaining (data not shown). Scale bars: 200 µm in (A) and (B); 10 µm in (C). (D and E) Estimation of neuronal migration in cerebral cortices transfected with indicated plasmids as described in Figure 3. Each score represents the mean percentage of relative intensity ± SE. (D) n = 4; (E) n = 5. Similar results were obtained by introduction of pDN-JNK–IRES–EGFP, which was designed to elicit simultaneous expression of DN-JNK and EGFP (data not shown). (F–I) E14 brains were electroporated with pEGFP and sectioned into coronal slices at E16. The slices were cultured on insert membrane for 3 h (F and G) and subjected to an additional 28 h incubation ±SP600125 in the culture media [(H) or (I), respectively]. Addition of SP600125 resulted in suppression of neuronal migration in vitro. Similar results were obtained from four independent experiments.
To further confirm the involvement of JNK in neuronal migration, we performed slice cultures on electroporated brains. Embryos were electroporated with pEGFP at E14 and coronal slices (300 µM) were taken at E16 and cultured for 28 h to observe the migration of EGFP-positive cells. While EGFP-positive cells migrated toward the pial surface in the control slices, migration was suppressed when an inhibitor of JNK (SP600125) (Bennett et al., 2001) was added to the culture medium (Figure 6F–I), supporting the involvement of JNK in neuronal migration.
Possible downstream events of JNK in migrating neurons
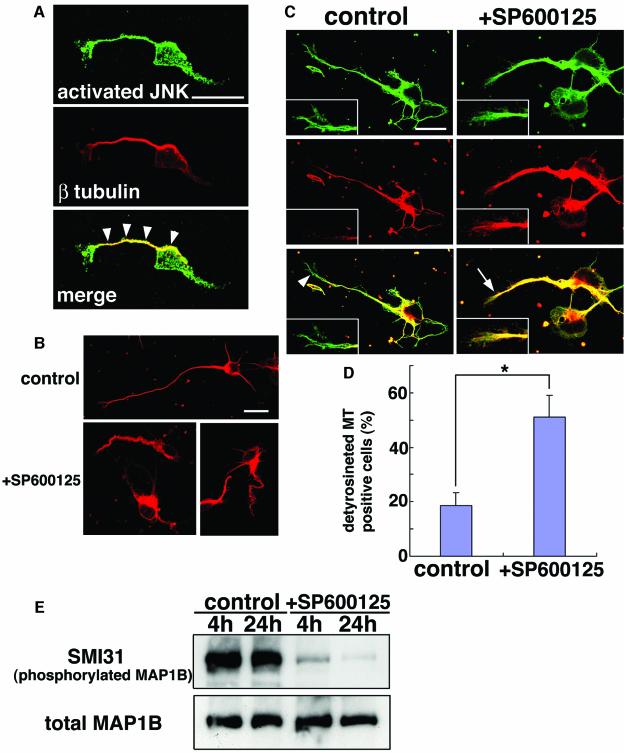
In Figure 5A, activated JNK does not appear to be confined to nuclei, but distributed in many subcellular regions of migrating cells. To further investigate the subcellular localization of activated JNK, dissociated cultures were performed. E15 brains were cut into thick coronal slices and the IZ was removed and dissociated for culture on poly-d-lysine-coated plastic dishes for 24 h. Immunocytochemistry revealed that activated JNK was strongly detected in the cytoplasmic region of the cultured cells, particularly in processes (Figure 7A, upper panel). Co-staining with an anti-β-tubulin antibody showed co-localization of activated JNK with microtubules (Figure 7A). A JNK inhibitor, SP600125, was added to the culture and resulting phenotypes were observed by immunostaining with anti-β-tubulin antibody (Figure 7B). Most β-tubulin-positive cells were also stained with the neuron-specific anti-β-III-tubulin antibody (data not shown), indicating their neuronal identity. Compared with control cells, SP600125-treated cells exhibited odd morphology, resembling that of DN-JNK-expressing cells in IZ (Figure 6C). Microtubules in processes seemed more twisted, irregular and thick in SP600125-treated cells than in control cells. Moreover, their processes were significantly shorter than control; the mean length of the longest neurites was 84.4 ± 14.6 µm in control cells but 45.4 ± 5.1 µm in neurons treated with SP600125 for 24 h. These results suggest that JNK is required for proper microtubule formation in cultured cortical cells.
Fig. 7. JNK regulates microtubule dynamics and MAP1B phosphorylation status in cultured neurons. (A) Cells in E15 IZ were dissociated and cultured for 24 h and stained with anti-activated JNK (green) and anti-β-tubulin (red) antibodies. Activated JNK was observed in nuclei, cytoplasm and processes, particularly along microtubules (arrowheads). Scale bars, 16 µm. (B) Influence of the JNK inhibitor on primary cultured cells. E15 cerebral cortices were dissociated and cultured for 24 h and then subjected to additional 24 h incubation ±SP600125 (upper panel or lower panels, respectively). Cells were stained with an anti-β-tubulin antibody (red). Scale bars, 20 µm. (C and D) E15 cerebral cortices were dissociated and cultured for 20 h and then subjected to additional 8 h incubation ±SP600125. Cells were stained with anti-α-tubulin (green) and anti-detyrosinated tubulin (red) antibodies. Insets represent higher magnifications of tips of processes. Detyrosinated microtubules were barely seen at the process tips (arrowhead) in the control cell, but were significantly detected at the distal ends of the microtubules (arrow) in SP600125-treated cells. Scale bars, 16 µm. (D) Ratio of cells whose longest neurites had detyrosinated microtubules at their tips. When the distance between the distal ends of detyrosinated microtubules [red in (C)] and entire microtubules [green in (C)] at the tip of the longest neurite was <5 µm, that cell was counted as a ‘detyrosinated MT-positive cell’. Scores represent mean percentage ± SE; n = 4 brains; *P = 0.012. (E) Influence of SP600125 on the phosphorylation status of MAP1B. Primary culture of E15 cerebral cortex (2DIV) was treated ±SP600125 for the indicated periods and subjected to immunoblot analyses with the indicated antibodies.
Previous reports suggested that there exist two types of microtubules, the stable type with a longer half-life and the dynamic type with a shorter half-life (Bulinski and Gundersen, 1991). Dynamic microtubules are predominantly localized to tips of neuronal processes where active tubulin dynamics are thought to occur (Goold et al., 1999). Cultured cells were stained with anti-detyrosinated α-tubulin antibody, a marker for stable microtubules (Bulinski and Gundersen, 1991), as well as with anti-α-tubulin antibody, which labels both stable and dynamic microtubules. Although detyrosinated α-tubulin signal was observed along processes in all cells, it was detected only in a small portion of cells (18.5 ± 4.8%) at the distal ends of microtubules within process tips in controls. In contrast, detyrosinated α-tubulin was significantly detected up to the distal ends of microtubules in many SP600125-treated cells (51.2 ± 7.8%), indicating reduced dynamic microtubules at process tips (Figure 7C and D). These results suggest that JNK is required for regulation of microtubule dynamics in processes of cultured embryonic neurons.
Microtubule-associated proteins such as MAP2, MAP1B and tau are known to play important roles in regulating the stability of microtubules (Takemura et al., 1992). Because MAP1B is expressed in radially migrating IZ cells (Cheng et al., 1999) and because knockout mice exhibited abnormal cortical neuronal migration (Gonzalez-Billault et al., 2000), we suspected that MAP1B might regulate tubulin dynamics under the control of the JNK in radially migrating IZ cells. It was demonstrated that GSK3β can phosphorylate MAP1B, the phosphorylated isoform of which can be specifically recognized by the SMI31 antibody, resulting in reduction of the microtubule-stabilizing activity of MAP1B (Lucas et al., 1998; Goold et al., 1999). To investigate the influence of JNK on MAP1B in the developing cortical neurons, we monitored the phosphorylation status of MAP1B in cultured neurons when JNK activity was suppressed. Dissociated cells from E15 cerebral cortex were cultured for 2 days with or without SP600125 for the last 4 or 24 h, and subjected to immunoblot analysis with the SMI31 antibody. As shown in Figure 7E, treatment with SP600125 resulted in significant reduction of phosphorylated MAP1B recognized by SMI31. These results suggest an involvement of JNK in phosphorylation of MAP1B, reducing microtubule stabilization, and, therefore, in regulation of tubulin dynamics in processes of migrating neurons, potentially contributing to proper neuronal migration.
Discussion
In this study, we showed essential roles for STEF/Tiam1, Rac1 and JNK in neuronal migration in vivo by utilizing an in utero electroporation technique. This technique enabled us to introduce genes of interest into VZ cells of mouse embryos in utero, allowing us to observe resulting phenotypes at later stages (Inoue and Krumlauf, 2001; Saito and Nakatsuji, 2001; Tabata and Nakajima, 2001). An advantage of this method is that the technique itself does not affect normal cerebral cortical development under our experimental conditions. Although several in vitro culture systems have been developed to monitor neuronal migration, they cannot precisely mimic normal development. For instance, no difference was observed in in vitro migration assays between neurons in explants from Cdk5-deficient and wild-type mice, whereas considerable abnormal neuronal migration was found in many regions of the nervous system in the Cdk5 mutant animals, in vivo (Ohshima et al., 1996; Gilmore et al., 1998; Gilmore and Herrup, 2001). Our experiment showed that electroporation of DN-Cdk5 into VZ cells in the cerebral cortex could mimic the phenotype of the Cdk5-deficient mice; cells transfected at E14 could not migrate beyond the subplate. This result suggests that DN-Cdk5 could appropriately suppress the function of endogeneous Cdk5 and further suggests that Cdk5 kinase activity is essential for neuronal migration because the DN-Cdk5 used in this study (Cdk5 N144) was kinase defective (Nikolic et al., 1996). Therefore, in utero electroporation of DN forms of certain genes can be a strong tool to investigate the molecular mechanisms of neuronal migration in vivo. Furthermore, this method is certainly applicable for analysis of genes such as Rac1 and JNK, whose mutants exhibit lethality before cerebral cortex formation (Sugihara et al., 1998; Kuan et al., 1999).
However, expression of DN forms of certain molecules sometimes causes interference with the function of other endogenous proteins, leading to phenotypes different from loss-of-function ones, although still providing clues to understanding the molecular function. For example, the phenotype of Drosophila expressing the DN form of Rac is similar, but not identical, to that of Rac-deficient fruitflies in the nervous system (Luo et al., 1994; Hakeda-Suzuki et al., 2002; Ng et al., 2002). In this study, we used four DN constructs; DN-Cdk5, -STEF/Tiam1, -Rac1 and -JNK. These DN forms seemed to work as expected in vivo and the phenotypes likely reflected loss-of-function. Introduction of the DN form of Cdk5 in the developing cerebral cortex caused inhibition of radial neuronal migration resembling that of Cdk5-deficient mice. Use of DN-JNK, like application of a JNK inhibitor to cultured slices, resulted in suppression of neuronal migration, suggesting that JNK is required for cortical neuronal migration. Some phenomena observed in DN animals were consistent with results from previous in vitro studies. For example, N17-Rac1-expressing cells in the developing cortex were round, lacked processes and had reduced JNK activity, all of which are known to be under the control of Rac1 in some cultured cell lines. DN forms for both Rac1 and STEF/Tiam1 suppressed radial neuronal migration. Our previous in vitro study showed that STEF/Tiam1 work as strong activators for Rac1 and, therefore, it is reasonable that similar phenotypes are observed with the two DN-forms. The circumstantial evidence strongly suggests that the DN forms used in this study appropriately suppressed the functions of the relevant molecules in vivo, although subtle side effects cannot be excluded.
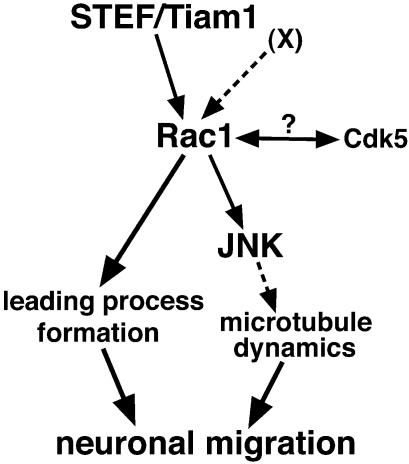
Our experiment showed that functional suppression of Rac1 and its GEFs, STEF/Tiam1, caused inhibition of neuronal migration in the cerebral cortex without affecting proper differentiation. This suggests that the STEF/Tiam1–Rac1 pathway is involved in neuronal migration in the cerebral cortex (Figure 8). Interestingly, a milder phenotype was observed in PHnTSS-STEF transfected cortices. This implies that another Rac1 GEF(s) may also be involved in this process. Another possible candidate is βPIX, which is also expressed in the IZ and the CP during development (Yoshizawa et al., 2003). However, other possibilities may explain the different phenotypes. For example, the PHnTSS-STEF construct might not completely inhibit STEF/Tiam1, although our previous study showed that this construct was very efficient, at least in vitro (Matsuo et al., 2002).
Fig. 8. The possible Rac1 pathway involved in neuronal migration in vivo (see Discussion). X represents a putative Rac1 GEF(s) other than STEF/Tiam1 involved in neuronal migration.
Studies have reported that in vitro Rac1 induces rearrangement of the actin cytoskeleton and activation of JNK in many cell types (Bishop and Hall, 2000). We showed here that suppression of Rac1 activity in electroporated cells caused down-regulation of JNK activity, revealing that the JNK activity in migrating neurons is controlled by Rac1. Although previous reports suggested that Rac1 and its influence on the actin cytoskeletal dynamics are important in regulating cell migration, the significance of JNK activity in neuronal migration is still unknown. We propose a crucial role for JNK in neuronal migration and show that its functional suppression causes abnormal neuronal migration in the cerebral cortex. Interestingly, the morphology of the DN-JNK transfected cells is different from that of N17-Rac1-transfected cells. While N17-Rac1-expressing cells exhibited a round shape lacking a leading process, DN-JNK-expressing cells displayed a spindle-like morphology with a distinct leading process. This implies that acquisition of the leading process requires Rac1 but not JNK, and further suggests that additional downstream pathways of Rac1, other than JNK, are involved in this process (Figure 8). Fukata et al. (2002) showed that Rac1 is involved in initiation of protruding process formation in restricted parts of cells via the IQGAP–Clip170 pathway. This process does not seem to require JNK activity.
However, the leading processes in DN-JNK expressing cells appeared twisted and irregular, suggesting that JNK is required for acquiring proper leading process morphology. The major cytoskeletal component of the leading process has been shown to be the microtubule (Hatten, 2002). Previous reports have shown that microtubule dynamics are very important for neuronal migration, since genetic mutations of Lis1 and doublecortin, both thought to regulate microtubule dynamics, cause abnormal cortical neuronal migration in mammals (Gupta et al., 2002; Olson and Walsh, 2002). In cultured IZ neurons, we showed that activated JNK was localized along microtubules, especially in processes. Furthermore, we showed that addition of a JNK inhibitor to the culture media resulted in abnormal morphology and reduced dynamics of microtubules in processes of cultured cells from developing cerebral cortex. These facts suggest that JNK is involved in proper microtubule formation through regulating tubulin dynamics.
We showed that addition of a JNK inhibitor to the cultured neurons resulted in suppression of MAP1B phosphorylation. Although this indicates the involvement of JNK, whether direct phosphorylation occurs is still not clear. Because GSK3β can directly phosphorylate MAP1B (Lucas et al., 1998) and because both GSK3β and JNK are members of proline-directed kinases, we suspect that JNK may directly phosphorylate MAP1B. Since the phosphorylation of MAP1B, recognized by SMI-31, causes reduced microtubule-stabilizing activity of MAP1B (Goold et al., 1999), JNK might be able to control dynamics of microtubules through regulating the phosphorylation status of MAP1B. Together with the fact that MAP1B-deficient mice exhibited abnormal cortical migration (Gonzalez-Billault et al., 2000), these facts may indicate that JNK is involved in proper neuronal migration through phosphorylation of MAP1B, contributing to proper microtubule dynamics in the processes of migrating neurons (Figure 8).
Previous genetic studies suggested the involvement of several molecules in neuronal migration (Cdk5, Lis1, Reelin, ApoER2/VLDLR, mDab1, etc.; Gupta et al., 2002; Olson and Walsh, 2002). In particular, Cdk5 is thought to play a central role, because it has been shown to regulate a microtubule-associated protein, Lis1, and to phosphorylate mDab1. In addition, regulation of cell adhesion is also known to be important in neuronal migration. Cdk5 is reported to regulate N-cadherin-mediated adhesion (Kwon et al., 2000) and Rac1 has been also shown to be involved in cell adhesion in vitro (Fukata and Kaibuchi, 2001). As Rac1 has been reported to interact with Cdk5 in the developing cerebral cortex (Nikolic et al., 1998) and because N17-Rac1- and DN-Cdk5-expressing cells were detained in the same region in the cerebral cortex, as shown in this report, interaction between these two proteins may account for cortical neuronal migration (Figure 8).
Materials and methods
Plasmids
Plasmid DNA were prepared using Endo Free plasmid purification kit (Qiagen). All plasmids contained the CAG promoter (Niwa et al., 1991) that drives efficient expression in the in utero electroporation system (Saito and Nakatsuji, 2001). The CMV promoter region of pcDNA3 (Clontech) was replaced with the promoter/enhancer region of pCAGGS (provided by Dr J.Miyazaki) to generate pcCAG. The IRES2–EGFP sequence from pIRES2–EGFP (Clontech) was inserted just after the cloning site of pcCAG, producing pCAG–IRES–EGFP. N17-Rac1, PHnTSS-STEF or DN-Cdk5 (Nikolic et al., 1996) cDNA sequence was inserted into the cloning site of pCAG–IRES–EGFP to generate pN17-Rac1–IRES–EGFP, pPHnTSS-STEF–IRES–EGFP or pDN-Cdk5–IRES–EGFP, respectively. pEGFP was constructed by inserting EGFP cDNA sequence (Clontech) into the cloning site of pcCAG. pDN-JNK was a generous gift from Dr S.Tamura [originally named pCX-Flag-JNK(APF)] (Wang et al., 2001).
In utero electroporation
Pregnant ICR mice were purchased from Charles River Japan. Animals were handled in accordance with guidelines established by Kyoto University. Pregnant mice carrying E14 embryos were anesthetized and a right dorsal incision was made to access the uterus. One microliter of plasmid DNA (2 µg/µl) in TE (pH 7.5) containing Fast Green was injected into the lateral ventricles of embryonic brains from outside the uteri with a glass micropipette (G-1.0; Narishige). Holding the embryo in utero with forceps-type electrodes (NEPA GENE), 50 ms of 40 V electronic pulses were delivered five times at intervals of 450 ms with a square electroporator (NEPA GENE). After electroporation, uteri were placed back in the abdominal cavity, allowing embryos to continue developing. At desired stages, electroporated brains were sectioned coronally by cryostat, microtome or vibratome at the level of the rostral half of the hippocampus to obtain the dorso-lateral region of the cortices.
All electroporated vectors were expression plasmids generally used for transfection into cultured cells. Little integration or replication was seen in electroporated cells. Vectors were introduced into VZ cells because the DNA solution was injected into the ventricles. In VZ cells, the DNA copy number (as visualized by EGFP signal) is reduced dramatically with each cell cycle. However, EGFP signal in post-mitotic cells in the cerebral cortex is maintained for a long period. This is unlikely to be attributed to the presence of stable EGFP in post-mitotic cells but to the presence of the expression vector, because EGFP is not very stable when expressed in the developing cerebral cortex (Kawaguchi et al., 2001). In addition, not only the EGFP signals but also epitope-tagged molecules (N17-Rac1, PHnTSS-STEF, DN-JNK) could be detected in the cerebral cortices at P4 (data not shown).
Immunohistochemistry
Frozen sections of fixed embryonic brains were treated with 10% goat serum in phosphate-buffered saline (GS-PBS) containing 0.05% Triton X-100 for 1 h at room temperature and subsequently incubated with diluted primary antibodies in GS-PBT (GS-PBS containing 0.1% Tween 20) at 4°C overnight. After several washes in PBS, sections were treated with Alexa488- or Alexa594-conjugated secondary antibodies (Molecular Probes) diluted in GS-PBT for 45 min at room temperature, followed by washes in PBS. Fluorescent images were obtained by TCS SL laser scanning confocal microscopy (Leica).
Primary antibodies
Primary antibodies used in this study were anti-Rac1 (1/50; Upstate Biotechnology), anti-STEF (1/100; Matsuo et al., 2002), anti-Tiam1 (1/100; SantaCruz C-16), anti-active JNK (1/200; Promega), anti-Hu (1/250; Molecular Probes), anti-MAP2 (1/100; Chemicon), anti-GFP (1/1500, polyclonal; Molecular Probes), anti-GFP (1/10, monoclonal; provided by Dr A.Imura), SMI31 (1/1000; Sternberger Monoclonals, Inc.), anti-MAP1B (1/50; SantaCruz), anti-β-tubulin (1/400; Sigma), anti-α-tubulin (1/100; Sigma) and anti-detyrosinated α-tubulin (1/100; Chemicon) antibodies.
BrdU incorporation experiment
Twenty-four hours after electroporation in utero, pregnant mice (E15) were given two intraperitoneal injections of BrdU at 40 mg/kg with a 30 min interval. One hour after the first BrdU injection, mice were killed and embryonic brains were fixed. Frozen sections were sequentially treated with anti-GFP polyclonal antibody and a biotinylated secondary antibody (1/500; Vector Laboratory), re-fixed with 4% PFA briefly, incubated with 2 N HCl for 1 h at 37°C and incubated with anti-BrdU antibody (1/75; Becton Dickinson) at 4°C overnight, followed by treatment with Alexa594-conjugated secondary antibody and streptavidin-FITC (1/100; Vector Laboratory).
Quantification of fluorescence intensities
Fluorescent images of frozen sections of EGFP-expressing mouse brains were captured by TCS SL laser scanning confocal microscopy (Leica). Fluorescence intensities inside similar width rectangles in various regions of the cerebral cortex (layers II–IV, V–VI, and IZ and VZ/SVZ) were measured by the TCS SL software. Relative intensities to the total fluorescence were calculated and plotted in the graphs with standard errors.
Slice culture
Embryos were electroporated with pEGFP in utero at E14 and killed at E17. Electroporated brains were cut into 300 µm coronal slices with a vibratome in DMEM/F-12 1:1 media (Invitrogen). Cortical slices were incubated on the insert membrane (Millipore) in 2 ml of enriched culture media (Miyata et al., 2002) in a CO2 incubator (37°C, 5% CO2). After 3 h incubation, 4 µl of DMSO with or without SP600125 (40 µM, final concentration; BIOMOL Research Laboratories) was added to the media for an additional 24 h incubation.
Primary culture, western blotting and immunocytochemistry
Primary culture, Western blotting and immunocytochemistry were performed as described previously (Matsuo et al., 2002, 2003).
Acknowledgments
Acknowledgements
We thank T.Fujimori and A.Takakura for technical advice, and S.Yoshida, M.Sone, N.Matsuo, M.Terao, T.Terashima, C.Hama, M.Hoshi, R.Yu, Y.Nishimura, N.Tamamaki and T.Obata for helpful discussions and technical help. We also thank J.Miyazaki, L.H.Tsai, K.Kaibuchi, S.Tamura, R.J.Davis, K.Yoshioka, A.Imura and O.Tohyama for providing plasmids and antibodies. This work was supported in part by Grants-in-Aid for Scientific Research on Priority Areas (C) Advanced Brain Science Project, (C) Genome Science, and (A) Research for Comprehensive Promotion of Study of Brain (to M.H.) from the Ministry of Education, Culture, Sports, and Science and Technology, Japan.
References
- Bennett B.L. et al. (2001) SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl Acad. Sci. USA, 98, 13681–13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop A.L. and Hall,A. (2000) Rho GTPases and their effector proteins. Biochem. J., 348, 241–255. [PMC free article] [PubMed] [Google Scholar]
- Bulinski J.C. and Gundersen,G.G. (1991) Stabilization of post-translational modification of microtubules during cellular morphogenesis. BioEssays, 13, 285–293. [DOI] [PubMed] [Google Scholar]
- Cheng A., Krueger,B.K. and Bambrick,L.L. (1999) MAP5 expression in proliferating neuroblasts. Brain Res. Dev. Brain Res., 113, 107–113. [DOI] [PubMed] [Google Scholar]
- Derijard B., Hibi,M., Wu,I.H., Barrett,T., Su,B., Deng,T., Karin,M. and Davis,R.J. (1994) JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell, 76, 1025–1037. [DOI] [PubMed] [Google Scholar]
- Ehler E., van Leeuwen,F., Collard,J.G. and Salinas,P.C. (1997) Expression of Tiam-1 in the developing brain suggests a role for the Tiam-1-Rac signaling pathway in cell migration and neurite outgrowth. Mol. Cell. Neurosci., 9, 1–12. [DOI] [PubMed] [Google Scholar]
- Feng Y. and Walsh,C.A. (2001) Protein–protein interactions, cytoskeletal regulation and neuronal migration. Nat. Rev. Neurosci., 2, 408–416. [DOI] [PubMed] [Google Scholar]
- Fukata M. and Kaibuchi,K. (2001) Rho-family GTPases in cadherin-mediated cell–cell adhesion. Nat. Rev. Mol. Cell. Biol., 2, 887–897. [DOI] [PubMed] [Google Scholar]
- Fukata M. et al. (2002) Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell, 109, 873–885. [DOI] [PubMed] [Google Scholar]
- Gilmore E.C. and Herrup,K. (2001) Neocortical cell migration: GABAergic neurons and cells in layers I and VI move in a cyclin-dependent kinase 5-independent manner. J. Neurosci., 21, 9690–9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore E.C., Ohshima,T., Goffinet,A.M., Kulkarni,A.B. and Herrup,K. (1998) Cyclin-dependent kinase 5-deficient mice demonstrate novel developmental arrest in cerebral cortex. J. Neurosci., 18, 6370–6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson J.G. (2001) Neuronal migration disorders. Ment. Retard. Dev. Disabil. Res. Rev., 7, 167–171. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Billault C. et al. (2000) Perinatal lethality of microtubule-associated protein 1B-deficient mice expressing alternative isoforms of the protein at low levels. Mol. Cell. Neurosci., 16, 408–421. [DOI] [PubMed] [Google Scholar]
- Goold R.G., Owen,R. and Gordon-Weeks,P.R. (1999) Glycogen synthase kinase 3beta phosphorylation of microtubule-associated protein 1B regulates the stability of microtubules in growth cones. J. Cell Sci., 112, 3373–3384. [DOI] [PubMed] [Google Scholar]
- Gupta A., Tsai,L.H. and Wynshaw-Boris,A. (2002) Life is a journey: a genetic look at neocortical development. Nat. Rev. Genet., 3, 342–355. [DOI] [PubMed] [Google Scholar]
- Habets G.G., Scholtes,E.H., Zuydgeest,D., van der Kammen,R.A., Stam,J.C., Berns,A. and Collard,J.G. (1994) Identification of an invasion-inducing gene, Tiam-1, that encodes a protein with homology to GDP–GTP exchangers for Rho-like proteins. Cell, 77, 537–549. [DOI] [PubMed] [Google Scholar]
- Hakeda-Suzuki S., Ng,J., Tzu,J., Dietzl,G., Sun,Y., Harms,M., Nardine,T., Luo,L. and Dickson,B.J. (2002) Rac function and regulation during Drosophila development. Nature, 416, 438–442. [DOI] [PubMed] [Google Scholar]
- Hatten M.E. (2002) New directions in neuronal migration. Science, 297, 1660–1663. [DOI] [PubMed] [Google Scholar]
- Hoshino M., Sone,M., Fukata,M., Kuroda,S., Kaibuchi,K., Nabeshima,Y. and Hama,C. (1999) Identification of the stef gene that encodes a novel guanine nucleotide exchange factor specific for Rac1. J. Biol. Chem., 274, 17837–17844. [DOI] [PubMed] [Google Scholar]
- Inoue T. and Krumlauf,R. (2001) An impulse to the brain—using in vivo electroporation. Nat. Neurosci., 4, 1156–1158. [DOI] [PubMed] [Google Scholar]
- Kawaguchi A. et al. (2001) Nestin–EGFP transgenic mice: visualization of the self-renewal and multipotency of CNS stem cells. Mol. Cell. Neurosci., 17, 259–273. [DOI] [PubMed] [Google Scholar]
- Kuan C.Y., Yang,D.D., Samanta Roy,D.R., Davis,R.J., Rakic,P. and Flavell,R.A. (1999) The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron, 22, 667–676. [DOI] [PubMed] [Google Scholar]
- Kwon Y.T., Gupta,A., Zhou,Y., Nikolic,M. and Tsai,L.H. (2000) Regulation of N-cadherin-mediated adhesion by the p35-Cdk5 kinase. Curr. Biol., 10, 363–372. [DOI] [PubMed] [Google Scholar]
- Leeuwen F.N., Kain,H.E., Kammen,R.A., Michiels,F., Kranenburg,O.W. and Collard,J.G. (1997) The guanine nucleotide exchange factor Tiam1 affects neuronal morphology; opposing roles for the small GTPases Rac and Rho. J. Cell Biol., 139, 797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas F.R., Goold,R.G., Gordon-Weeks,P.R. and Salinas,P.C. (1998) Inhibition of GSK-3beta leading to the loss of phosphorylated MAP-1B is an early event in axonal remodelling induced by WNT-7a or lithium. J. Cell Sci., 111, 1351–1361. [DOI] [PubMed] [Google Scholar]
- Luo L., Liao,Y.J., Jan,L.Y. and Jan,Y.N. (1994) Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev., 8, 1787–1802. [DOI] [PubMed] [Google Scholar]
- Matsuo N., Hoshino,M., Yoshizawa,M. and Nabeshima,Y. (2002) Characterization of STEF, a guanine nucleotide exchange factor for Rac1, required for neurite growth. J. Biol. Chem., 277, 2860–2868. [DOI] [PubMed] [Google Scholar]
- Matsuo N., Terao,M., Nabeshima,Y. and Hoshino,M. (2003) Roles of STEF/Tiam1, guanine nucleotide exchange factors for Rac1, in regulation of growth cone morphology. Mol. Cell. Neurosci., in press. [DOI] [PubMed] [Google Scholar]
- Miyata T., Kawaguchi,A., Saito,K., Kuramochi,H. and Ogawa,M. (2002) Visualization of cell cycling by an improvement in slice culture methods. J. Neurosci. Res., 69, 861–868. [DOI] [PubMed] [Google Scholar]
- Nadarajah B. and Parnavelas,J.G. (2002) Modes of neuronal migration in the developing cerebral cortex. Nat. Rev. Neurosci., 3, 423–432. [DOI] [PubMed] [Google Scholar]
- Ng J., Nardine,T., Harms,M., Tzu,J., Goldstein,A., Sun,Y., Dietzl,G., Dickson,B.J. and Luo,L. (2002) Rac GTPases control axon growth, guidance and branching. Nature, 416, 442–447. [DOI] [PubMed] [Google Scholar]
- Nikolic M., Dudek,H., Kwon,Y.T., Ramos,Y.F. and Tsai,L.H. (1996) The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev., 10, 816–825. [DOI] [PubMed] [Google Scholar]
- Nikolic M., Chou,M.M., Lu,W., Mayer,B.J. and Tsai,L.H. (1998) The p35/Cdk5 kinase is a neuron-specific Rac effector that inhibits Pak1 activity. Nature, 395, 194–198. [DOI] [PubMed] [Google Scholar]
- Niwa H., Yamamura,K. and Miyazaki,J. (1991) Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene, 108, 193–199. [DOI] [PubMed] [Google Scholar]
- Ohshima T., Ward,J.M., Huh,C.G., Longenecker,G., Veeranna, Pant,H.C., Brady,R.O., Martin,L.J. and Kulkarni,A.B. (1996) Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc. Natl Acad. Sci. USA, 93, 11173–11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano H.J. and Darnell,R.B. (1997) A hierarchy of Hu RNA binding proteins in developing and adult neurons. J. Neurosci., 17, 3024–3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E.C. and Walsh,C.A. (2002) Smooth, rough and upside-down neocortical development. Curr. Opin. Genet. Dev., 12, 320–327. [DOI] [PubMed] [Google Scholar]
- Rakic P. (1990) Principles of neural cell migration. Experientia, 46, 882–891. [DOI] [PubMed] [Google Scholar]
- Ridley A.J. (2001) Rho GTPases and cell migration. J. Cell Sci., 114, 2713–2722. [DOI] [PubMed] [Google Scholar]
- Ridley A.J., Paterson,H.F., Johnston,C.L., Diekmann,D. and Hall,A. (1992) The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell, 70, 401–410. [DOI] [PubMed] [Google Scholar]
- Saito T. and Nakatsuji,N. (2001) Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Dev. Biol., 240, 237–246. [DOI] [PubMed] [Google Scholar]
- Sone M., Hoshino,M., Suzuki,E., Kuroda,S., Kaibuchi,K., Nakagoshi,H., Saigo,K., Nabeshima,Y. and Hama,C. (1997) Still life, a protein in synaptic terminals of Drosophila homologous to GDP–GTP exchangers. Science, 275, 543–547. [DOI] [PubMed] [Google Scholar]
- Sone M. et al. (2000) Synaptic development is controlled in the periactive zones of Drosophila synapses. Development, 127, 4157–4168. [DOI] [PubMed] [Google Scholar]
- Sugihara K. et al. (1998) Rac1 is required for the formation of three germ layers during gastrulation. Oncogene, 17, 3427–3433. [DOI] [PubMed] [Google Scholar]
- Tabata H. and Nakajima,K. (2001) Efficient in utero gene transfer system to the developing mouse brain using electroporation: visualization of neuronal migration in the developing cortex. Neuroscience, 103, 865–872. [DOI] [PubMed] [Google Scholar]
- Takemura R., Okabe,S., Umeyama,T., Kanai,Y., Cowan,N.J. and Hirokawa,N. (1992) Increased microtubule stability and alpha tubulin acetylation in cells transfected with microtubule-associated proteins MAP1B, MAP2 or tau. J. Cell Sci., 103, 953–964. [DOI] [PubMed] [Google Scholar]
- Wang H., Ikeda,S., Kanno,S., Guang,L.M., Ohnishi,M., Sasaki,M., Kobayashi,T. and Tamura,S. (2001) Activation of c-Jun amino-terminal kinase is required for retinoic acid-induced neural differentiation of P19 embryonal carcinoma cells. FEBS Lett., 503, 91–96. [DOI] [PubMed] [Google Scholar]
- Yoshizawa M., Hoshino,M., Sone,M. and Nabeshima,Y. (2002) Expression of stef, an activator of Rac1, correlates with the stages of neuronal morphological development in the mouse brain. Mech. Dev., 113, 65–68. [DOI] [PubMed] [Google Scholar]
- Yoshizawa M., Sone,M., Matsuo,N., Nagase,T., Ohara,O., Nabeshima,Y. and Hoshino,M. (2003) Dynamic and coordinated expression profile of Dbl-family guanine nucleotide exchange factors in the developing mouse brain. Gene Expr. Patterns, 3, 375–381. [DOI] [PubMed] [Google Scholar]