Abstract
Daily αvβ5 integrin-dependent phagocytosis of spent photoreceptor outer segment fragments by the retinal pigment epithelium (RPE) is critical for retinal function. This study identifies a key role for focal adhesion kinase (FAK) in RPE phagocytosis. Particle binding increases FAK complex formation with αvβ5 receptors at the apical, phagocytic RPE surface and activates FAK. Subsequent particle engulfment coincides with dissociation of activated FAK from αvβ5. Mutant FAK retaining focal adhesion targeting but lacking kinase activity interferes with recruitment of full-length FAK to αvβ5 and abrogates FAK activation in response to RPE phagocytic challenge. Such inhibition of FAK signaling has no effect on αvβ5-dependent binding of particles but blocks their engulfment. Conversely, FAK re-expression promotes particle engulfment by FAK null fibroblasts. Selective ligation of αvβ5 receptors at the apical RPE surface is sufficient to phosphorylate and mobilize FAK. Furthermore, FAK phagocytic signaling is independent of the internalization receptor MerTK. In contrast, inhibition of FAK signaling diminishes MerTK phosphorylation. These results demonstrate that FAK provides an essential link between binding and engulfment mechanisms of integrin-mediated phagocytosis.
Keywords: focal adhesion kinase/integrins/MerTK/phagocytosis/retinal pigment epithelium
Introduction
In the retina, the circadian renewal program of photoreceptor rod and cone outer segments (OS) involves daily shedding of distal OS tips (Young, 1967). Prompt and complete disposal of spent OS by phagocytosis is the task of the adjacent retinal pigment epithelium (RPE) (Young and Bok, 1969). Each RPE cell disposes of several thousand shed membranous disks per day, rendering RPE cells the most active phagocytes in the body. Failure of RPE cells to phagocytose OS causes retinal dystrophy in rodents and in humans (Edwards and Szamier, 1977; Gal et al., 2000).
αvβ5 is the only integrin receptor that localizes to the apical, phagocytic surface of RPE in vitro and in vivo (Finnemann et al., 1997). Antibodies blocking αvβ5 abolish OS binding by RPE but have no effect on internalization of OS prebound to the RPE surface (Finnemann et al., 1997; Miceli et al., 1997). In contrast, the scavenger receptor CD36 and the tyrosine kinase MerTK mediate internalization of OS after they are surface bound by RPE (Finnemann and Silverstein, 2001; Feng et al., 2002). OS challenge increases tyrosine phosphorylation of MerTK (Feng et al., 2002), whose absence abolishes internalization of bound OS by RPE (Chaitin and Hall, 1983). This suggests that OS binding via αvβ5 integrin by RPE initiates a signaling response that activates MerTK and other components of the internalization machinery. Such molecular links between binding and internalization functions are thus far unknown for any phagocytic mechanism.
Focal adhesion kinase (FAK) is a cytoplasmic tyrosine kinase that colocalizes with integrins at focal contacts. Activation of FAK is critical for integrin functions that involve cytoskeletal reorganization (Ilic et al., 1995; Sieg et al., 1999). While phosphorylation of any of its six tyrosine residues may regulate FAK function, phosphorylation of tyrosines at positions 576 and 577 (Tyr576 and Tyr577) is thought to directly reflect increased FAK enzymatic activity (Calalb et al., 1995; Maa and Leu, 1998). Tyrosine 397 (Tyr397) is the sole autophosphorylation site of FAK (Schlaepfer et al., 1999). Increased levels of phosphorylated Tyr397 (P-Tyr397) thus correlate well with FAK activation. Furthermore, recent studies have shown that FAK phosphorylation at Tyr861 promotes both FAK binding to αvβ5 integrin (Eliceiri et al., 2002) and Tyr397 phosphorylation (Leu and Maa, 2002).
This study identifies a novel function for FAK in an αvβ5 integrin-dependent signaling pathway that is required for particle engulfment. RPE phagocytosis is a slow process with a distinct phase of particle binding (during the first 2 h following OS challenge) preceding a phase of internalization of bound particles (between 2 and 6 h following OS challenge) (Finnemann et al., 1997; Finnemann and Rodriguez-Boulan, 1999). This study takes advantage of this unique feature of RPE phagocytosis to discriminate the activities of signaling molecules during binding and internalization. The data show that phagocytic challenge activates and mobilizes a pool of FAK complexed with αvβ5 receptors at the apical RPE surface. Furthermore, FAK signaling is essential for internalization of bound particles by RPE, and FAK expression is sufficient to promote particle engulfment by fibroblasts. Finally, analysis of RPE defective in either FAK or MerTK function shows that FAK signaling upon OS binding is independent of and required for MerTK tyrosine phosphorylation, which is important for particle engulfment. Taken together, these results provide the first evidence for a role for FAK in a mechanism used by phagocytic cells to transduce signals from integrin particle binding receptors to trigger particle engulfment.
Results
RPE phagocytosis induces αvβ5 integrin complex phosphorylation
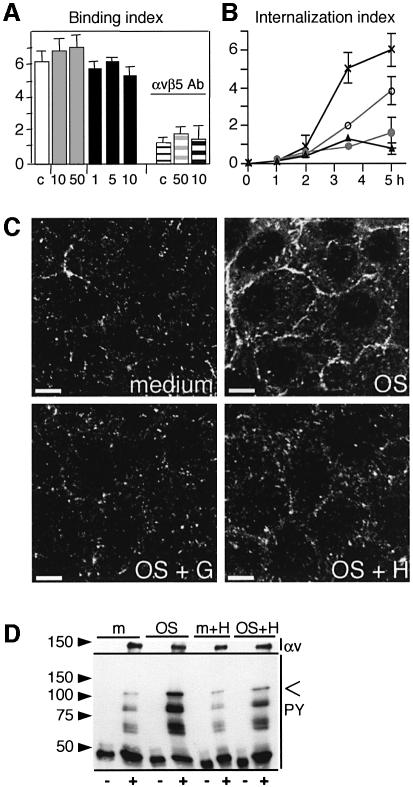
Phagocytic challenge of RPE cells with OS particles causes a strong increase in RPE protein phosphorylation (Heth and Schmidt, 1992). To test whether protein tyrosine phosphorylation was required for binding or engulfment of OS, stable RPE-J cells received a phagocytic challenge with isolated OS in the presence of the tyrosine kinase inhibitors herbimycin A or genistein. The effects of both reagents on OS uptake by RPE cells were identical. Even with increasing inhibitor concentrations, RPE cells bound OS normally within 2 h of OS challenge, via their primary binding receptor αvβ5 integrin (Figure 1A). At this time point, 85% of particles were surface bound (Finnemann and Rodriguez-Boulan, 1999). However, both genistein and herbimycin A strongly inhibited subsequent internalization of bound OS in a concentration-dependent manner (Figure 1B). Immunofluorescence labeling showed that 1 h of OS incubation increased tyrosine phosphorylation of RPE proteins (Figure 1C, compare medium with OS) and that this increase was almost completely abolished by genistein and strongly reduced by herbimycin A (Figure 1C, OS + G and OS + H). Furthermore, immunoprecipitates of αvβ5 receptors from RPE harvested after 1 h of OS challenge contained tyrosine phosphorylated proteins whose mobility in SDS–PAGE did not match that of αv and β5 proteins (Figure 1D). Thus, early after OS challenge, during the OS binding phase of RPE phagocytosis, RPE proteins residing in a complex with αvβ5 are phosphorylated, or RPE proteins that are phosphorylated upon OS challenge associate with αvβ5.
Fig. 1. OS internalization requires tyrosine kinase activity. OS binding induces tyrosine phosphorylation of αvβ5 complexes. (A) OS binding by RPE-J cells during 2 h of OS incubation was determined in the presence of solvent (white bar), 10 and 50 µM genistein (10, 50, gray bars) or 1–10 µg/ml herbimycin A (1, 5, 10, black bars). αvβ5 antibody P1F6 (50 µg/ml) inhibited OS binding similarly in the presence of solvent (white striped bar, c), 50 µM genistein (gray striped bar, 50) or 10 µg/ml herbimycin (black striped bar, 10). (B) OS internalization by RPE was determined after 1–5 h of OS challenge in the presence of solvent as control (×), genistein [10 µM (open circle), 50 µM (closed circle)], or 5 µg/ml herbimycin A (closed triangle). (A and B) show average OS indices ± SD (n = 3). (C) Tyrosine-phosphorylated proteins in RPE were detected by immunofluorescence staining after 1 h of challenge with medium (medium), OS (OS), OS in the presence of 50 µM genistein (OS + G), or OS in the presence of 5 µg/ml herbimycin A (OS + H). Three-dimensional (3D) projections are shown. Scale bars: 10 µm. (D) Immunoblotting was used to detect tyrosine-phosphorylated proteins in immunoprecipitates with non-immune IgG (–) and αvβ5 (+) IgG (panel PY). Reprobing of blots with αv antibody showed similar amounts of precipitated αvβ5 (panels αv). RPE cells were lysed after 1 h of challenge with OS (OS) or medium (m) in the absence or presence of 5 µg/ml herbimycin A as indicated (m + H, OS + H). The open arrowhead indicates the molecular size of FAK.
FAK localizes to the apical, phagocytic surface of RPE
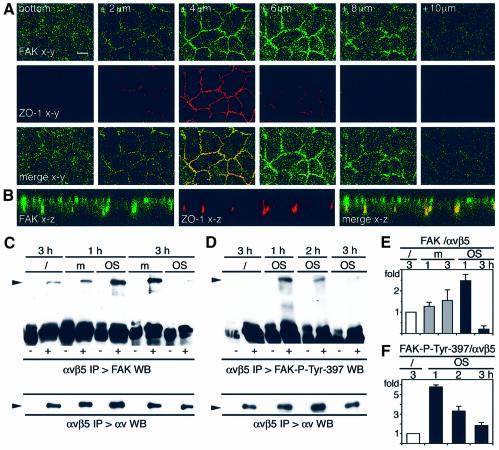
Interestingly, one of the tyrosine phosphorylated proteins associated with αvβ5 following OS challenge shared the electrophoretic mobility of FAK, with a molecular size of ∼120 kDa (Figure 1D, open arrowhead). Therefore, I used confocal microscopy to establish the subcellular distribution of FAK in polarized RPE monolayers. As expected, FAK was present at the basal RPE surface where RPE cells, like other epithelial cells, adhere to extracellular matrix substrates via basal integrin receptors (Finnemann et al., 1997) (Figure 2A, bottom). Serial x–y sections at 2 µm intervals showed additional FAK at higher focal planes (Figure 2A). Comparison of the distribution of FAK and the tight junction protein ZO-1 revealed that FAK was most prominent at and above the focal plane of the tight junction of RPE cells. Since RPE-J cells localize their tight junction at the center rather than the apical end of the lateral membrane (Bonilha et al., 1999), apical plasma membrane labeling appears apical and lateral in confocal x–y scans. z-axis confocal scans confirmed that in polarized RPE, FAK localized to the entire apical plasma membrane domain, the site of OS phagocytosis (Figure 2B).
Fig. 2. FAK localizes to the apical, phagocytic RPE surface. OS uptake controls FAK complex formation with αvβ5 and its phosphorylation at Tyr397 in the complex. (A) Polarized RPE-J cells were labeled with FAK and ZO-1 tight-junction antibodies. Confocal x–y scans were acquired at 2 µm intervals starting at the basal surface (bottom) as indicated. Scale bar: 10 µm. (B) z-axis confocal scan of the same samples further reveals apical localization of FAK. (A and B) show one representative field each. (C) RPE lysates were harvested after 1 and 3 h of incubation with OS (OS) or with medium (m). Immunoblotting served to detect FAK in immunoprecipitations with non-immune (–) and αvβ5 (+) IgG. (D) The same (–) and (+) immuno precipitations were performed after 1 and 3 h of OS challenge (OS) before immunoblotting to detect complexed FAK-P-Tyr397. Lower panels in (C) and (D) show blot reprobed with αv antibody. (E) FAK and αv band intensities were compared in immunoprecipitates to quantify FAK–αvβ5 complexes. (F) FAK-P-Tyr397 and αv band intensities were compared in immunoprecipitates to quantify FAK-P-Tyr397–αvβ5 complexes. Bars in (E and F) represent averages ± SD, n = 4.
FAK dissociates from αvβ5 upon OS binding
To determine whether FAK associated with αvβ5 in RPE, immunoblotting was used to detect FAK in immunoprecipitates of αvβ5 from RPE cells harvested before and after OS challenge. FAK coprecipitated with αvβ5 (but not with non-immune IgG) in resting RPE even before they received OS (Figure 2C, /). FAK remained in the αvβ5 complex 1 h after challenge with OS (Figure 2C, 1 h), during the OS binding phase when αvβ5 complexes were increasingly phosphorylated (Figure 1D). However, FAK no longer resided in the αvβ5 complex 3 h after OS challenge of RPE, during the OS internalization phase (Figure 2C, 3 h). This dissociation of FAK from αvβ5 was specific to OS contact since cells receiving assay medium alone retained FAK–αvβ5 complexes at all times (Figure 2C, m). Quantification of FAK and αv integrin in immunoprecipitates revealed that the relative amount of FAK in the αvβ5 complex as compared with untreated cells increased 2.5-fold after 1 h, before strongly declining to 0.2-fold after 3 h of OS challenge (Figure 2E). Differences at both time points were significant with P < 0.001. In contrast, assay medium alone weakly increased the relative amount of complexed FAK to 1.3-fold after 1 h and to 1.6-fold at 3 h. These medium effects were not significant at 1 h (P = 0.1) and significant with P < 0.05 at 3 h. While their cause remains unknown, only OS challenge promoted prompt additional recruitment of FAK to αvβ5 during OS binding and subsequent dissociation of FAK from αvβ5 during OS internalization. To determine whether the OS-dependent increase in FAK–αvβ5 integrin complexes, shown in Figure 2C, was responsible for the increase in the 120 kDa phosphoprotein coprecipitate with αvβ5, shown in Figure 1D, αvβ5 immune complexes were next tested for the presence of autophosphorylated FAK by immunoblotting with antibodies specific for FAK phosphorylated at Tyr397. Interestingly, only very low levels of FAK-P-Tyr397 resided in αvβ5 complexes in resting RPE (Figure 2D, /), but FAK-P-Tyr397 levels in αvβ5 complexes increased 5.8-fold after 1 h of OS challenge before declining to background levels by 3 h (Figure 2D and F, OS). Medium incubation did not alter FAK-P-Tyr397 levels in αvβ5 complexes (data not shown). The more pronounced change in FAK-P-Tyr397 levels compared with FAK protein levels in the complexes suggested that FAK recruitment was not the sole effect of OS challenge but that phosphorylation of FAK-Tyr397 residues occurred in αvβ5 complexes.
OS phagocytic challenge activates FAK
To investigate in more detail the effect of OS challenge on FAK phosphorylation, phosphotyrosine immunoblotting was performed on FAK immunoprecipitated from RPE. As expected, FAK phosphorylation was significantly increased by 1 h after OS addition (Figure 3A, arrowhead indicates size of FAK). Additional phosphotyrosine bands may represent FAK complexed proteins. The attempt to identify β5 integrin (molecular size ∼90 kDa) in FAK precipitates has thus far been unsuccessful, but may require more sensitive antibodies for immunodetection than currently available. Assay medium alone did not cause significant FAK phosphorylation at any time point (data not shown). Total tyrosine phosphorylation of FAK remained at a maximum of 3.5-fold over background levels in RPE up to 4.5 h following OS addition. Interestingly, FAK tyrosine phosphorylation decreased to background levels only ∼6 h after OS challenge, 3 h after FAK dissociated from the αvβ5 complex (compare Figure 3A with 2C–F).
Fig. 3. Phagocytic challenge phosphorylates FAK at tyrosines 397, 576 and 861. RPE-J cells received OS for up to 7.5 h as indicated before lysis. (A) FAK immunoprecipitates were first probed with phosphotyrosine antibody and subsequently with total FAK (C-terminus) antibody. Phosphotyrosine bands colocalizing with FAK (arrowhead) and total FAK bands were compared with quantify FAK phosphorylation at each time point. (B) Whole-cell lysate was immunoblotted for FAK-P-Tyr397, FAK-P-Tyr576, FAK-P-Tyr861 and total FAK protein (C-terminus). Bars show relative amounts of FAK phosphoresidues compared with total FAK at each time point. For (A and B), data points in graphs represent averages ± SD, n = 3.
Comparative immunoblotting of whole-cell lysates using antibodies specific for FAK’s phosphorylated residues Tyr397, Tyr576 and Tyr861 was used to further characterize the phosphorylation profile of FAK (Figure 3B, panels as indicated, with corresponding quantification below). By 2 h after OS challenge of RPE, FAK-Tyr397 and FAK-Tyr576 phosphorylation increased 3.2- and 2.0-fold over control cells, respectively, indicating activation of FAK. Like the total phosphotyrosine content of FAK, levels of Tyr397 and Tyr576 phosphorylation were still maximal after 4.5 h but declined by 6 h after OS challenge. In contrast, phosphorylation of FAK at Tyr861 was maximally increased 2.2-fold by 1 h after OS challenge but declined to 0.6-fold as compared with untreated controls by 3 h. Immunoblotting of the same lysates using antibodies specific to the FAK C-terminus showed that the amount of FAK protein in the cells remained constant during the experiment (Figure 3B, total FAK). These results indicate that FAK in RPE is activated during OS binding and remains active during internalization of bound OS.
OS challenge phosphorylates and relocalizes apical FAK
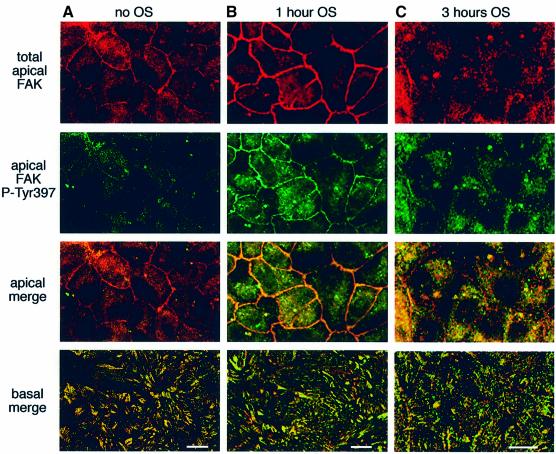
To distinguish the fraction of FAK functionally associated with OS phagocytosis at the RPE apical surface from FAK involved in basal substrate adhesion, RPE cells were next immunostained with FAK kinase domain and FAK-P-Tyr397 antibodies. In resting confluent RPE monolayers, FAK residing at the apical plasma membrane was not phosphorylated at Tyr397 (Figure 4A). One hour of challenge with unlabeled OS induced Tyr397 phosphorylation of apical FAK, as shown by the overlap of FAK protein and FAK-P-Tyr397 fluorescence (Figure 4B). Prominent apical plasma membrane labeling of total FAK after 1 h of phagocytic challenge further confirmed that OS incubation recuited additional FAK to the phagocytic surface of RPE (compare Figure 4A with B, total FAK). In contrast, 3 h after OS addition, FAK association with the apical plasma membrane appeared greatly reduced. Apical FAK remained phosphorylated but redistributed from the plasma membrane to the RPE cytoplasm (Figure 4C). As expected, basal FAK was phosphorylated at Tyr397 and showed no change with OS challenge, confirming its activity in focal contacts of RPE (Figure 4A–C, basal merge). These results demonstrate that phagocytic challenge specifically activates and mobilizes FAK that localizes to the apical surface in resting RPE.
Fig. 4. Phagocytic challenge phosphorylates and relocalizes apical FAK. RPE-J cells received medium (A) or OS (B) for 1 h or OS for 3 h (C) before fixation. Each column shows one representative field. The three upper rows show 3D projections of three planes, each acquired at 1 µm intervals from the apical surface. Double labeling of FAK (first row) and of FAK-P-Tyr397 (second row) was detected in confocal x–y scans. The third row shows merged first and second rows (apical merge). The fourth row shows a single x–y scan of merged FAK and FAK-P-Tyr397 fluorescence signals at the basal surface of the same fields (basal merge). Scale bars: 10 µm.
β5 but not FAK colocalizes with internalized OS
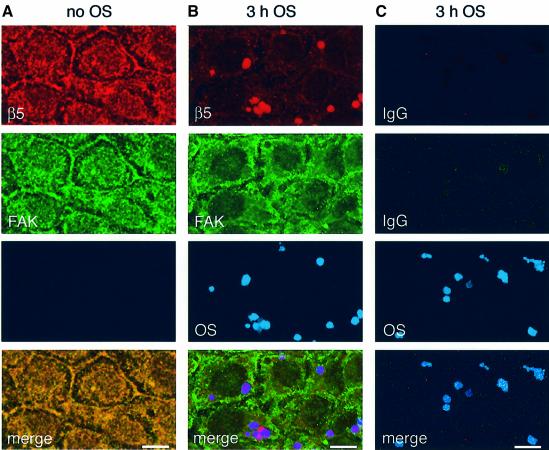
To further investigate the subcellular localization of FAK and αvβ5 integrin during OS uptake, RPE cells received fluorescent OS or assay medium for 3 h before double immunolabeling with FAK and β5 antibodies (labeling αvβ5 receptors and individual β5 subunits). β5 and FAK colocalized at the apical surface of medium-treated control cells, confirming their presence in a protein complex (Figure 5A). In contrast, following 3 h of OS challenge, β5 was partially recruited to sites of internalized OS while FAK did not colocalize with either β5 or OS (Figure 5B). Control labeling confirmed that secondary antibodies did not stain OS non-specifically (Figure 5C). Thus, when αvβ5–FAK complexes dissociate during OS uptake, β5 redistributes to OS phagosomes and FAK redistributes to distinct, currently uncharacterized cytoplasmic sites.
Fig. 5. β5 but not FAK colocalizes with internalized OS. RPE-J cells received medium (A) or OS (B and C) for 3 h before fixation. Each column shows one representative field. (A and B) Signals of β5 (first row) and FAK (second row) immunolabeling and of Cy5-OS (third row) were detected in confocal x–y scans. (A) 3D projection of the apical cell area acquired as in Figure 4 shows colocalization of FAK and β5 in RPE that did not receive OS. (B) 3D projection of three x–y scans acquired at focal planes 2–4 µm below the apical surface shows overlap of β5 but not of FAK labeling with signals of internalized OS. (C) Negative control shows immunolabeling, substituting non-immune antibodies for β5 and FAK antibodies. Phagocytosis and image acquisition were the same as in (B). Merge shows overlap of all three signals. Scale bars: 10 µm.
Engulfment of bound OS requires FAK activity
To test whether inhibition of FAK signaling during phagocytosis affected OS binding and/or OS internalization, RPE cells were infected with a recombinant adenovirus encoding the C-terminal portion of FAK, termed FRNK (‘FAK-related non-kinase’). Since FRNK lacks kinase activity but retains the focal contact targeting and binding site of FAK, it acts as a specific dominant-negative inhibitor of signaling by full-length FAK (Schaller et al., 1993). Control cells were infected with adenovirus encoding GFP. Surface labeling of αvβ5 receptors confirmed that levels of αvβ5 receptors were similar at the apical surface of cells infected with either virus and of uninfected cells (see Supplementary figure available at The EMBO Journal Online). Strikingly, FRNK adenovirus-infected RPE bound OS normally but did not internalize bound OS (Figure 6A). Immunoblotting of RPE lysates showed that adenoviral FRNK expression at similar levels as endogenous FAK in control cells did not affect full-length FAK protein levels (Figure 6B). However, in cells expressing FRNK, but not in control cells, full-length FAK was largely extracted by non-ionic detergent (Figure 6B and C). In contrast, FRNK was resistant to non-ionic detergent extraction, suggesting that exogenous FRNK disrupted the cytoskeletal anchorage of endogenous FAK by competing for its focal adhesion targeting (Figure 6B and C). FRNK also reduced the levels of full-length FAK that formed a complex with αvβ5 in RPE (Figure 6D and E). Moreover, FRNK suppressed the induction of Tyr397 phosphorylation in response to OS binding (Figure 6F and G). FAK phosphorylation of its substrate p130CAS promotes p130CAS complex formation with the adaptor protein CrkII (Vuori et al., 1996). Here, FRNK prevented the association of p130CAS with CrkII that was specifically induced by OS challenge in control cells (Figure 6H and I). Thus, FRNK not only interfered with the activity of FAK itself, but also effectively inhibited FAK signaling to downstream targets. Taken together, these results demonstrate that FRNK abrogated the signaling response of FAK to OS phagocytic challenge that is essential for OS internalization by RPE.
Fig. 6. Inhibition of FAK signaling blocks internalization but not binding of OS. RPE-J cells were infected with FRNK (F) or GFP (c) adenovirus. (A) Fluorescence scanning was used to establish OS binding indices after 2 h of OS challenge (gray bars) and OS internalization indices after 5 h of OS challenge (black bars). Bars show averages ± SD, n = 4. (B) Immunoblotting with FAK C-terminus antibody detected full-length endogenous FAK (FAK) and exogenous FRNK (FRNK) in whole-cell lysates (total), and in soluble (s) and insoluble (i) fractions prepared by differential detergent extraction. (C) Bars show the relative amount of insoluble FAK. Bars represent averages ± SD, n = 3. (D) Full-length FAK was detected in αvβ5 integrin immunoprecipitations. (E) Bars show average amounts of αvβ5-complexed FAK ± SD, n = 3. (F) Whole-cell lysates of untreated cells (/) or cells receiving medium (m) or OS (OS) for 3 h were immunoblotted for FAK-P-Tyr397 and for total FAK as indicated. (G) Bars show quantification of FAK-P-Tyr397 compared with total FAK within each sample. Bars represent averages ± SD, n = 3. (H) Immunoprecipitates with non-immune IgG (–) or Crk antibody (+) were isolated from lysates of untreated cells (/) or cells receiving medium (m) or OS (OS) for 3 h. Immunoblotting with p130CAS antibody detected co-precipitated p130CAS (1st WB:p130CAS). Blots were stripped and re-probed with CrkII antibodies (2nd WB:CrkII). (I) Bars show relative amounts of p130CAS complexed with Crk. Bars represent averages ± SD, n = 3.
De novo FAK expression is sufficient to promote OS engulfment
The experiments showed that specific inhibition of FAK by FRNK inhibited OS uptake. Next, I set out to determine whether, conversely, expression of full-length FAK would promote OS uptake. To this end, FAK null fibroblasts were transiently transfected with plasmids encoding either GFP or full-length FAK. Interestingly, FAK-expressing fibroblasts responded to OS incubation with an increase in FAK autophosphorylation, as determined by FAK-P-Tyr397 immunoblotting (Figure 7A and B, OS). This effect was specific for OS since it did not occur in response to medium alone (Figure 7A and B, medium). Quantification of OS binding showed that both GFP- and FAK-expressing fibroblasts bound similar numbers of OS (Figure 7C, RADS, black bars). OS binding by both types of cells was significantly reduced in the presence of peptides containing the integrin recognition motif RGD, suggesting that these cells used an integrin-mediated mechanism to bind OS (Figure 7C, RGDS, black bars). Strikingly, OS internalization was increased 6-fold in cells expressing FAK as compared with cells expressing GFP (Figure 7C, RADS, gray bars). Figure 7D shows representative high-magnification images of individual fibroblasts that were transfected with GFP or with FAK. Following uptake of Cy5-labeled OS (shown in blue) and fixation without permeabilization, surface-bound OS alone were immunostained with OS antibodies (shown in red). This allowed surface-bound (blue and red) OS to be distinguished from internal (only red) OS. GFP fluorescence and HA-FAK immunostaining (both shown in green) confirmed expression of transfected cDNAs. Taken together, these data show that expression of FAK is sufficient to promote engulfment of bound OS.
Fig. 7. FAK expression in FAK null fibroblasts increases internalization of bound OS. GFP- or FAK-transfected fibroblasts received medium (m) or OS (OS) for 3 h. (A) Comparative immunoblotting of lysates as indicated for each panel shows levels of FAK-P-Tyr397, of transfected GFP and FAK, and of actin, which was used as a loading control. Quantification in (B) shows an average 2.5-fold increase in FAK-P-Tyr397 in response to OS ± SD, n = 3. (C) OS bound (black bars) and internalized (gray bars) by GFP- and FAK-transfected cells were quantified by OS counting. OS binding was significantly reduced (P < 0.05) by 1 mM RGDS peptides compared with inactive RADS peptides. Bars show averages ± SD, n = 3. (D) Micrographs show fluorescence signals from surface-bound OS, total (bound plus internal) OS, GFP or HA tag, marking FAK (HA-FAK) in representative GFP- (upper row) or FAK- (lower row) transfected cells as indicated. Merge shows overlap of all three signals. Scale bars: 10 µm.
αvβ5 ligation is sufficient to mobilize and activate FAK
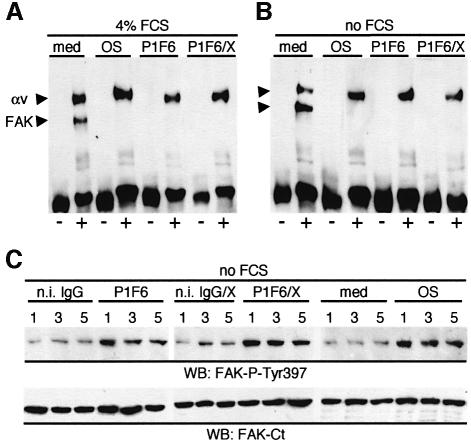
RPE cells in culture bind OS normally, via αvβ5 integrin, in serum-free medium (Finnemann and Silverstein, 2001). In contrast, they fail to efficiently internalize bound OS unless heat-inactivated serum is present (Finnemann and Silverstein, 2001; Hall et al., 2001). This is thought to be a consequence of the absence of ligands for internalization receptors such as MerTK. Such ligands remain incompletely characterized but include the serum protein Gas6, which is a ligand for MerTK (Hall et al., 2001). To assess whether serum factors played a role in FAK activation during OS phagocytosis, RPE cells were challenged with OS in the absence of serum. Moreover, OS particles may directly interact with RPE surface receptors other than αvβ5 to activate FAK. To determine the signaling response by RPE to specific and exclusive ligation of αvβ5 receptors, αvβ5 antibody P1F6 was therefore substituted for OS to selectively ligate αvβ5 at the apical RPE surface. Figure 8 shows that FAK dissociated from αvβ5 in response to treatment with αvβ5 antibody alone (Figure 8A, P1F6), as well as in response to αvβ5 antibody in the presence of crosslinker (Figure 8A, P1F6/X). These effects were independent of the presence of serum (Figure 8B). Ligation of αvβ5 receptors alone in the absence of serum also increased FAK-P-Tyr397 levels in RPE over those in cells receiving appropriate control treatment (Figure 8C). These data imply that ligation of αvβ5 receptors at the apical surface of RPE is sufficient to activate FAK signaling.
Fig. 8. αvβ5 ligation is sufficient to phosphorylate and mobilize FAK. RPE-J cells received assay medium (med), OS (OS), αvβ5 receptor mouse IgG P1F6 (P1F6), non-immune mouse IgG (n.i. IgG), P1F6 plus crosslinking anti-mouse IgG antibodies (P1F6/X) or non-immune IgG plus crosslinking antibodies (n.i. IgG/X) for 3 h (A and B) or 1–5 h as indicated (C) before lysis. Experiments were performed in the presence or absence of 4% FCS as indicated. (A) Immunoprecipitations with non-immune IgG (–) or αvβ5 IgG (+) were probed with FAK-antibody detecting FAK at 120 kDa (FAK) and with αv-antibody (αv) detecting αv at 140 kDa. (B) The same experiment as in (A) was performed in the absence of serum. (C) Identical whole-cell lysate immunoblots were probed with FAK-P-Tyr397 and FAK C-terminus antibodies to determine FAK autophosphorylation.
FAK signaling is upstream of and mediates MerTK phosphorylation
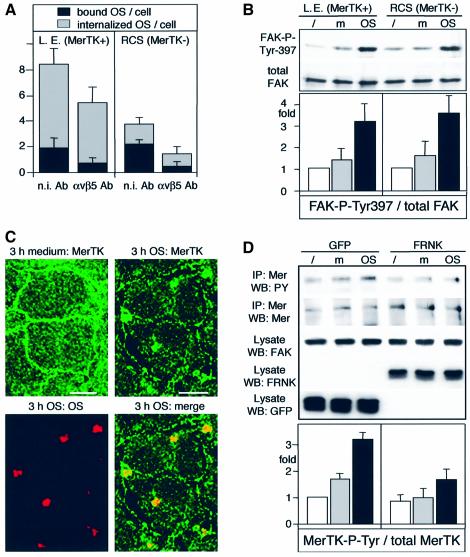
To determine specifically whether MerTK played a role in FAK activation downstream of αvβ5, FAK and αvβ5 activities were explored in RPE derived from the Royal College of Surgeons (RCS) rat strain. RCS rats are a well-studied animal model of retinal degeneration. A loss-of-function mutation in MerTK (D’Cruz et al., 2000; Nandrot et al., 2000) abrogates the OS internalization function of RPE (Chaitin and Hall, 1983). Figure 9A shows that RCS rat RPE cells in culture bound similar numbers of OS as wild-type Long Evans strain-derived rat RPE cells that have functional MerTK. Moreover, both Long Evans and RCS rat RPE used αvβ5 integrin to bind OS, as αvβ5 blocking antibodies reduced the numbers of OS bound per cell by 63 and 82%, respectively, on average (Figure 9A, black bars). As expected, RCS RPE cells were impaired in OS internalization, confirming their mutant phenotype (Figure 9A, gray bars). However, FAK activation, as determined by increased FAK-P-Tyr397 levels, occurred as efficiently in RCS RPE as in Long Evans RPE (Figure 9B). This demonstrates that OS binding via αvβ5 integrin activates FAK independently of MerTK.
Fig. 9. FAK signaling is upstream of and required for MerTK phosphorylation. (A) Long Evans or RCS RPE received OS for 2 h in the presence of 50 µg/ml non-immune (n.i. Ab) or αvβ5 antibody (αvβ5 Ab) before labeling of surface-bound OS and counting of bound (black bars) and internal (gray bars) OS. Bars show averages ± SD, n = 4. (B) Comparative immunoblotting of lysates as indicated for each panel showed increased FAK phosphorylation at Tyr397 in response to OS (OS) but not to medium (m) over untreated controls (/) in Long Evans as well as in RCS RPE. Bars show average FAK-P-Tyr397 levels relative to untreated RPE ± SD, n = 3. (C) RPE-J cells received medium (3 h medium: MerTK) or Cy5-labeled OS (3 h OS panels) for 3 h before fixation and labeling of MerTK. 3D projections representing apical cell domains acquired as described in Figure 4 are shown. Scale bars: 10 µm. (D) RPE-J cells expressing FRNK, as characterized in Figure 6, received medium (m) or (OS) before lysis and immuno precipitation of MerTK. Immunoprecipitates were blotted and probed first with phosphotyrosine antibody (panel IP:MerTK WB:PY) and reprobed with MerTK antibody (panel IP:MerTK WB:MerTK). Expression of transfected GFP and FRNK as well as full-length FAK and actin as controls in cell lysates are also shown, as indicated in the panels. Bars show average MerTK tyrosine phosphorylation ± SD, n = 3. FRNK significantly inhibited OS-induced MerTK tyrosine phosphorylation with P < 0.01.
Unfortunately, incompatible fixation conditions for FAK and available MerTK antibodies prevented double immunofluorescence labeling of both proteins in the same samples. However, confocal microscopy revealed that MerTK localized to the apical surface of resting or medium-fed confluent RPE cells (Figure 9C). Thus, MerTK, like αvβ5 and FAK, resides at the apical, phagocytic surface of resting RPE. Confirming earlier observations by Feng et al. (2002), MerTK partially colocalized with phagocytosed OS after 3 h of OS challenge, similar to β5 integrin but unlike FAK (compare Figure 9C with 5).
Finally, MerTK activation was tested in RPE in which FAK signaling in response to OS was abolished by FRNK expression. MerTK activation is only poorly understood at the time, but its tyrosine phosphorylation correlates with and has been suggested to indicate its activity during RPE phagocytosis (Feng et al., 2002). Since MerTK activation-specific reagents do not exist, I immunoprecipitated MerTK from RPE and determined MerTK phosphorylation by immunoblotting with phosphotyrosine antibodies. Strikingly, expression of FRNK, which abolishes the signaling response by full-length FAK in response to OS (Figure 6), diminished the robust increase in MerTK phosphorylation shown by control cells expressing GFP (Figure 9D). This exciting finding suggests that the phagocytosis receptor MerTK is a direct or indirect target of FAK signaling during OS phagocytosis.
Discussion
This study identifies a critical function for FAK in RPE phagocytosis: FAK signaling in response to αvβ5 integrin-dependent OS binding by RPE is essential for the subsequent activation of the OS engulfment machinery, including the tyrosine phosphorylation of the internalization receptor MerTK.
Phosphorylation of FAK Tyr861 promotes direct interaction of FAK with the β5 integrin cytoplasmic tail in vitro (Eliceiri et al., 2002). The experiments presented here did not discern whether FAK interacts with αvβ5 in RPE directly or indirectly. However, FAK–αvβ5 complex levels during RPE phagocytosis correlated well with fluctuations of Tyr861 phosphorylation. It is thus likely that Tyr861 phosphorylation, presumably via Src family kinases (Eliceiri et al., 2002), plays a role in the regulation of αvβ5–FAK association in RPE.
Normal OS binding in the presence of FRNK, which interfered with FAK–αvβ5 association, argues that the OS binding activity of αvβ5 in RPE is independent of FAK. In contrast, both pharmacological inhibition of tyrosine kinase activity and specific inhibition of FAK by FRNK abolished engulfment of surface-bound OS that occurs between 2 and 6 h following OS challenge. This suggests that OS binding via αvβ5 integrin initiates a signaling response by FAK in the αvβ5 complex that is required for subsequent OS engulfment.
The simultaneous formation of additional FAK–αvβ5 complexes and increase of FAK activity (as measured by increased phosphorylation of Tyr397 and Tyr576) during OS binding suggests that FAK activation in RPE phagocytosis may require its association with αvβ5. In contrast, FAK activity remained elevated during OS internalization even after FAK dissociated from the αvβ5 integrin complex. The long lifespan of increased levels of phosphorylated Tyr397 and Tyr576 suggests that FAK signaling activity during RPE phagocytosis may extend beyond the time and the site of OS binding to αvβ5 at the apical surface of RPE. Alternatively, while FAK may remain phosphorylated during the later times of OS phagocytosis, it may fulfill its signal transduction function during the period of time it resides in the complex with αvβ5. The experiments here have begun to unravel the downstream elements of FAK signaling in RPE phagocytosis, providing evidence for a role of FAK in regulation of p130CAS/Crk and of MerTK. MerTK and αvβ5–FAK complexes are present jointly at the apical surface of RPE before OS challenge. In contrast, during OS internalization, FAK distributes to—currently uncharacterized—cytoplasmic sites. These are distinct from phagocytosed OS, to which both MerTK and αvβ5 are recruited. This suggests that FAK may functionally interact with MerTK during the OS binding phase while both proteins still reside at the apical plasma membrane of RPE. Such activation of MerTK during the OS binding phase would be in agreement with its role as a master regulator whose activation is essential to initiate OS engulfment. Despite its importance, it is thus far not understood how MerTK function promotes particle internalization during phagocytosis. This study provides the first evidence for a functional interaction of αvβ5 integrin and MerTK phagocytosis receptors via FAK. This intriguing finding opens numerous questions regarding precisely how αvβ5-induced FAK signaling regulates the OS internalization machinery. Does FAK phosphorylate MerTK? Does MerTK associate with either αvβ5 or FAK directly? Is MerTK phosphorylation a prerequisite for its localization to phagocytosed OS? Future studies will be needed to explore these important issues.
The RPE phagocytic pathway belongs to a group of conserved phagocytosis mechanisms that have evolved to provide efficient, non-inflammatory clearance of spent cells (undergoing apoptosis) or spent photoreceptor OS fragments (shed daily in the retina). Macrophages and dendritic cells can utilize αvβ3 or αvβ5 integrin receptors to clear apoptotic cells (Savill et al., 1990; Albert et al., 1998; Finnemann and Rodriguez-Boulan, 1999). Phagocytosis of apoptotic cells by dendritic cells and fibroblasts also involves αvβ5-dependent phosphorylation of p130CAS and CrkII and requires activity of MerTK (Albert et al., 2000; Scott et al., 2001; Tosello-Trampont et al., 2001). Moreover, the nematode homolog of CrkII, Ced2, is a necessary element of a phagocytosis pathway in Caenorhabditis elegans (Reddien and Horvitz, 2000). The results shown in this study demonstrate that formation of p130CAS–CrkII complexes and MerTK phosphorylation that occur during OS phagocytosis by RPE cells require FAK signaling. Signaling proteins that associate with a particle binding receptor and signal upstream of p130CAS–CrkII or of MerTK to promote particle engulfment are yet unknown in any other related phagocytic pathway. FAK provides a critical molecular link between particle binding and particle engulfment via MerTK in phagocytosis by RPE cells. The results also show that expression of FAK can induce internalization of surface-bound OS by fibroblasts, which are not professional phagocytes. This strongly suggests that FAK or an equivalent signaling molecule, such as PYK2, may fulfill a similar function in integrin-dependent phagocytosis by other phagocytic cell types.
Materials and methods
Cell culture and transfection
Rat RPE-J cells (ATCC, Manassas, VA) were maintained in DMEM with 4% FCS (ICN, Costa Mesa, CA) (Nabi et al., 1993). For experiments, cells were seeded at 50% confluence and grown for 7–8 days before use. A murine FAK null fibroblast cell line (Ilic et al., 1995) was obtained from ATCC and grown in DMEM with 10% FCS. To express FAK or GFP, 106 fibroblasts were resuspended in nucleofection reagent V (Amaxa, Cologne, Germany) and transfected with 3 µg of pEGFP-N1 (BD Clontech, Palo Alto, CA) or pKH3-HA-FAK (a gift from J.-L.Guan, Cornell University, Ithaca, NY; Chen et al., 1996) plasmid DNA using an Amaxa nucleofector™ at program T27 according to the manufacturer’s instructions (Amaxa). Transfected cells were seeded on glass coverslips and used for experiments after 24 h.
Mutant (MerTK–) RCS-rdy/rdy-p rats were obtained from the National Center for Research Resources (NIH, Bethesda, MD) and bred. Wild-type (MerTK+) Long Evans rat mothers with litters were obtained from Charles River (Wilmington, MA). RPE was isolated from 12-day-old rat pups according to established procedures (Bonilha et al., 1999). Briefly, cornea, lens, iris and vitreous body were removed from eyes enucleated from CO2 asphyxiated rats. Eyecups were incubated in 1 mg/ml hyaluronidase (Sigma, St Louis, MO) in Hank’s balanced saline solution (HBSS; Sigma) for 1 h at 37°C. The neural retina was removed and eyecups were incubated in 2 mg/ml trypsin (Sigma) in HBSS for 1 h at 37°C. RPE sheets were collected from the underlying choroid and plated in 96-well plates with or without coverslips at a density of ∼10 000 cells/well. Cells were cultured in DMEM with 10% FCS for 4–7 days before use.
OS binding and phagocytosis assays
OS were isolated according to established protocols from bovine eyes obtained fresh from the slaughterhouse (Molday et al., 1987). For detection by fluorescence-based assays, OS were covalently prelabeled with FITC (Molecular Probes, Eugene, OR) or Cy5 (Amersham, Piscataway, NJ) as described previously (Finnemann et al., 1997). Polarized RPE cells or transfected fibroblasts were challenged with 10 OS per cell in DMEM with 4% FCS for the duration of the experiment, chilled, washed three times with PBS containing 1 mM MgCl2 and 0.2 mM CaCl2 (PBS-CM) to remove excess OS, and lysed or fixed.
To quantify OS binding or internalization by fluorescence scanning, cells on glass coverslips received FITC-labeled OS. In some cases, fluorescence derived from externally bound particles was quenched using trypan blue. Trypan blue enters surface-bound particles and quenches their FITC signals (Hed, 1986; Finnemann et al., 1997). Selective immunofluorescence labeling of surface-bound OS, which can also distinguish bound and internalized OS (see below), was used to confirm that trypan blue extinguished emission by FITC–OS bound by RPE but did not affect signals by FITC–OS internalized by RPE. Cells incubated with OS on ice served as background controls since RPE cells do not bind or internalize OS at 4°C (Finnemann and Rodriguez-Boulan, 1999). All samples were fixed with ice-cold methanol and rehydrated with PBS-CM. Nuclei were counterstained with propidium iodide or DAPI at 1 µg/ml, respectively. Fluorescence signals were recorded by fluorescence scanning of coverslips mounted on glass slides, using a STORM 860 scanner and quantified using ImageQuant 1.2 (both Molecular Dynamics, Sunnyvale, CA) (Finnemann et al., 1997). The uptake index (representing bound plus internalized particles) or the internalization index was calculated by dividing particle counts by nuclei counts of each area, thereby normalizing for cell numbers. The correlation of phagocytic indices with phagosome numbers has been discussed in detail previously (Finnemann and Rodriguez-Boulan, 1999).
To distinguish bound and internalized OS by immunofluorescence microscopy, cells were fed with Cy5-labeled OS before fixation in 4% paraformaldehyde in PBS-CM, which fixes but does not permeabilize cells. Surface-bound OS alone were labeled using OS-specific antiserum (a gift from E.Rakoczy, University of Western Australia, Perth) and AlexaFluor405-conjugated secondary antibody (Molecular Probes). Nuclei were labeled with propidium iodide. Fluorescence images of Cy5 (representing bound plus internalized OS) and Alexa405 (representing only bound OS), and of propidium iodide were taken of random areas showing ∼100 cells each. Bound OS, internalized OS and nuclei were counted in each area. Five areas of each sample were counted per experiment and each experiment was performed in triplicate.
Adenoviral infection
Recombinant adenovirus encoding the C-terminal FAK fragment FRNK was a gift from D.Schlaepfer (The Scripps Research Institute, La Jolla, CA). Control adenovirus encoding GFP was provided by E.Rodriguez-Boulan (Weill Medical College, New York, NY). Five- to six-day-old RPE-J monolayers received purified adenovirus at 5–10 infectious particles/cell for 2 h in serum-free medium. Following infection, cells were further incubated for ∼40 h in growth medium before phagocytic challenge. At this time, monolayers of infected cells carried apical αvβ5 receptors and were morphologically normal (see Supplementary figure).
Cell lysis, immunoprecipitation and western blotting
Cells were solubilized by agitation for 30 min at 4°C in 50 mM Tris–HCl pH 7.8, 150 mM NaCl, 2 mM CaCl2, 1 mM MgCl2, 1% NP-40, 1% Triton X-100, freshly supplemented with 1 mM PMSF and 1% phosphatase inhibitor cocktail II (Sigma). For each immunoprecipitation, cleared lysate from 500 000 RPE cells was used. Immunoprecipitation of αvβ5 integrin with 2.5 µg of P1F6 αvβ5 heterodimer-specific antibody (Covance, Richmond, CA) was performed exactly as described previously (Finnemann and Rodriguez-Boulan, 1999). MerTK, Crk and FAK immunoprecipitations used 5 µg of MerTK (a gift from D.Vollrath, Stanford University, Palo Alto, CA), Crk (BD Transduction, Lexington, KY) or FAK 2A7 (Upstate, Lake Placid, NY) antibody, respectively, and were collected with protein A/G plus agarose (Santa Cruz, Santa Cruz, CA) and washed three times with 50 mM HEPES pH 7.4, 150 mM NaCl, 10% glycerol, 1.5 mM MgCl2, 1% Triton X-100 before elution in SDS sample buffer. Control immunoprecipitations were performed with 2.5–5 µg of non-immune mouse or rabbit IgG (Rockland, Gilbertsville, PA). Whole-cell lysates (representing 20 000 RPE cells) or immunoprecipitates were separated by 7.5% SDS–PAGE under reducing conditions and transferred to nitrocellulose. Blots were incubated as indicated in the figures with primary antibodies to p130CAS, αv integrin, FAK kinase domain (all BD Transduction), CrkII (Santa Cruz), FAK C-terminus (C20; Santa Cruz), MerTK, phosphotyrosine (PY100; Cell Signaling, Beverly, MA), FAK-P-Tyr397, FAK-P-Tyr576 or FAK-P-Tyr861 (all Biosource, Camarillo, CA) and appropriate peroxidase-conjugated secondary antibodies followed by ECL detection (NEN, Boston, MA). X-ray films were scanned and signals quantified using NIH Image 1.61.
Cell fractionation
Resistance of protein to extraction with 50 mM MES pH 6.4, 5 mM MgCl2, 3 mM EGTA and 0.5% Triton X-100 indicates cytoskeletal association of proteins according to established protocols (Fey et al., 1984; Finnemann and Rodriguez-Boulan, 1999). Here, polarized RPE-J cells in 24-well plates (600 000 cells/well) were first incubated for 40 s at room temperature with 160 µl of the above extraction buffer supplemented with 1 mM PMSF and 1% phosphatase inhibitor cocktail II (soluble fraction, s). Subsequently, remaining proteins were solubilized exactly as described above for whole-cell lysates (insoluble fraction, i). Extracts s or i containing protein of 40 000 cells per lane were analyzed by immunoblotting.
Immunofluorescence microscopy
Samples were fixed in ice-cold 5% acetic acid, 95% ethanol (MerTK) or 100% methanol (all others) and processed as described previously (Bonilha et al., 1999). Antibodies used were monoclonal antibodies to phosphotyrosine (PY100) and FAK (BD Transduction), and polyclonal antibodies to β5 integrin (a gift from L.F.Reichardt, University of California, San Francisco, CA), FAK-P-Tyr397, MerTK and ZO-1 (Zymed, South San Francisco, CA). Secondary antibodies were from Molecular Probes. Images were acquired on a Zeiss LSM510 confocal microscope and recompiled in Adobe Photoshop 6.0.
Following surface OS labeling of transfected FAK fibroblasts (see OS binding and phagocytosis), cells were permeabilized using 0.5% Triton X-100 in PBS-CM before labeling with HA antibody 16B12 (Covance) and anti-mouse AlexaFluor594 antibody (Molecular Probes) to detect HA-FAK. Images of bound OS-, GFP-, HA- and total OS-derived fluorescence signals were acquired using sequential scanning on a Leica TCS SP2 spectral confocal system.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
I thank David D.Schlaepfer for generously providing reagents and helpful discussion. I further thank Jun-Lin Guan, Elizabeth P.Rakoczy, Louis F.Reichardt, Enrique Rodriguez-Boulan, and Douglas Vollrath for providing reagents. I also thank Maria Febbraio and Geri Kreitzer for valuable comments on this manuscript. Yoonhee Kim provided excellent technical assistance. This work was supported by a Career Development Award by Research to Prevent Blindness and by grants from the NEI/NIH.
References
- Albert M.L., Pearce,S.F.A., Francisco,L.M., Sauter,B., Roy,P., Silverstein,R.L. and Bhardwaj,N. (1998) Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med., 188, 1359–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M.L., Kim,J.I. and Birge,R.B. (2000) αvβ5 integrin recruits the CrkII–Dock180–rac1 complex for phagocytosis of apoptotic cells. Nat. Cell Biol., 2, 899–905. [DOI] [PubMed] [Google Scholar]
- Bonilha V.L., Finnemann,S.C. and Rodriguez-Boulan,E. (1999) Ezrin promotes morphogenesis of apical microvilli and basal infoldings in retinal pigment epithelium. J. Cell Biol., 147, 1533–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calalb M.B., Polte,T.R. and Hanks,S.K. (1995) Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol. Cell. Biol., 15, 954–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaitin M.H. and Hall,M.O. (1983) Defective ingestion of rod outer segments by cultured dystrophic rat pigment epithelial cells. Invest. Ophthalmol. Vis. Sci., 24, 812–820. [PubMed] [Google Scholar]
- Chen H.C., Appeddu,P.A., Isoda,H. and Guan,J.L. (1996) Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J. Biol. Chem., 271, 26329–26334. [DOI] [PubMed] [Google Scholar]
- D’Cruz P.M., Yasumura,D., Weir,J., Matthes,M.T., Abderrahim,H., LaVail,M.M. and Vollrath,D. (2000) Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum. Mol. Genet., 9, 645–651. [DOI] [PubMed] [Google Scholar]
- Edwards R.B. and Szamier,R.B. (1977) Defective phagocytosis of isolated rod outer segments by RCS rat retinal pigment epithelium in culture. Science, 197, 1001–1003. [DOI] [PubMed] [Google Scholar]
- Eliceiri B.P., Puente,X.S., Hood,J.D., Stupack,D.G., Schlaepfer,D.D., Huang,X.Z., Sheppard,D. and Cheresh,D.A. (2002) Src-mediated coupling of focal adhesion kinase to integrin αvβ5 in vascular endothelial growth factor signaling. J. Cell Biol., 157, 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W., Yasumura,D., Matthes,M.T., LaVail,M.M. and Vollrath,D. (2002) Mertk triggers uptake of photoreceptor outer segments during phagocytosis by cultured retinal pigment epithelial cells. J. Biol. Chem., 277, 17016–17022. [DOI] [PubMed] [Google Scholar]
- Fey E.G., Wan,K.M. and Penman,S. (1984) Epithelial cytoskeletal framework and nuclear matrix-intermediate filament scaffold: three-dimensional organization and protein composition. J. Cell Biol., 98, 1973–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann S.C. and Rodriguez-Boulan,E. (1999) Macrophage and retinal pigment epithelium phagocytosis: apoptotic cells and photoreceptors compete for αvβ3 and αvβ5 integrins, and protein kinase C regulates αvβ5 binding and cytoskeletal linkage. J. Exp. Med., 190, 861–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann S.C. and Silverstein,R.L. (2001) Differential roles of CD36 and αvβ5 integrin in photoreceptor phagocytosis by the retinal pigment epithelium. J. Exp. Med., 194, 1289–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann S.C., Bonilha,V.L., Marmorstein,A.D. and Rodriguez-Boulan,E. (1997) Phagocytosis of rod outer segments by retinal pigment epithelial cells requires αvβ5 integrin for binding but not for internalization. Proc. Natl Acad. Sci. USA, 94, 12932–12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal A., Li,Y., Thompson,D.A., Weir,J., Orth,U., Jacobson,S.G., Apfelstedt-Sylla,E. and Vollrath,D.(2000) Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat. Genet., 26, 270–271. [DOI] [PubMed] [Google Scholar]
- Hall M.O., Prieto,A.L., Obin,M.S., Abrams,T.A., Burgess,B.L., Heeb,M.J. and Agnew,B.J. (2001) Outer segment phagocytosis by cultured retinal pigment epithelial cells requires Gas6. Exp. Eye Res., 73, 509–520. [DOI] [PubMed] [Google Scholar]
- Hed J. (1986) Methods for distinguishing ingested from adhering particles. Methods Enzymol., 132, 198–204. [DOI] [PubMed] [Google Scholar]
- Heth C.A. and Schmidt,S.Y. (1992) Protein phosphorylation in retinal pigment epithelium of Long-Evans and RCS rats. Invest. Ophthalmol. Vis. Sci., 33, 2839–2847. [PubMed] [Google Scholar]
- Ilic D. et al. (1995) Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature, 377, 539–544. [DOI] [PubMed] [Google Scholar]
- Leu T.H. and Maa,M.C. (2002) Tyr-863 phosphorylation enhances focal adhesion kinase autophosphorylation at Tyr-397. Oncogene, 21, 6992–7000. [DOI] [PubMed] [Google Scholar]
- Maa M.C. and Leu,T.H. (1998) Vanadate-dependent FAK activation is accomplished by the sustained FAK Tyr-576/577 phosphorylation. Biochem. Biophys. Res. Commun., 251, 344–349. [DOI] [PubMed] [Google Scholar]
- Miceli M.V., Newsome,D.A. and Tate,D.J. (1997) Vitronectin is responsible for serum-stimulated uptake of rod outer segments by cultured retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci., 38, 1588–1597. [PubMed] [Google Scholar]
- Molday R.S., Hicks,D. and Molday,L. (1987) Peripherin. A rim-specific membrane protein of rod outer segment discs. Invest. Ophthalmol. Vis. Sci., 28, 50–61. [PubMed] [Google Scholar]
- Nabi I.R., Mathews,A.P., Cohen-Gould,L., Gundersen,D. and Rodriguez-Boulan,E. (1993) Immortalization of polarized rat retinal pigment epithelium. J. Cell Sci., 104, 37–49. [DOI] [PubMed] [Google Scholar]
- Nandrot E. et al. (2000) Homozygous deletion in the coding sequence of the c-mer gene in RCS rats unravels general mechanisms of physiological cell adhesion and apoptosis. Neurobiol. Dis., 7, 586–599. [DOI] [PubMed] [Google Scholar]
- Reddien P.W. and Horvitz,H.R. (2000) CED-2/CrkII and CED-10/Rac control phagocytosis and cell migration in Caenorhabditis elegans. Nat. Cell Biol., 2, 131–136. [DOI] [PubMed] [Google Scholar]
- Savill J., Dransfield,I., Hogg,N. and Haslett,C. (1990) Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature, 343, 170–173. [DOI] [PubMed] [Google Scholar]
- Schaller M.D., Borgman,C.A. and Parsons,J.T. (1993) Autonomous expression of a noncatalytic domain of the focal adhesion-associated protein tyrosine kinase pp125FAK. Mol. Cell. Biol., 13, 785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer D.D., Hauck,C.R. and Sieg,D.J. (1999) Signaling through focal adhesion kinase. Prog. Biophys. Mol. Biol., 71, 435–478. [DOI] [PubMed] [Google Scholar]
- Scott R.S., McMahon,E.J., Pop,S.M., Reap,E.A., Caricchio,R., Cohen,P.L., Earp,H.S. and Matsushima,G.K. (2001) Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature, 411, 207–211. [DOI] [PubMed] [Google Scholar]
- Sieg D.J., Hauck,C.R. and Schlaepfer,D.D. (1999) Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J. Cell Sci., 112, 2677–2691. [DOI] [PubMed] [Google Scholar]
- Tosello-Trampont A.C., Brugnera,E. and Ravichandran,K.S. (2001) Evidence for a conserved role for CRKII and Rac in engulfment of apoptotic cells. J. Biol. Chem., 276, 13797–13802. [DOI] [PubMed] [Google Scholar]
- Vuori K., Hirai,H., Aizawa,S. and Ruoslahti,E. (1996) Introduction of p130cas signaling complex formation upon integrin-mediated cell adhesion: a role for Src family kinases. Mol. Cell. Biol., 16, 2606–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R.W. (1967) The renewal of photoreceptor cell outer segments. J. Cell Biol., 33, 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R.W. and Bok,D. (1969) Participation of the retinal pigment epithelium in the rod outer segment renewal process. J. Cell Biol., 42, 392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]