Abstract
The present study evaluated behavioral and histopathological outcome after controlled cortical impact (CCI) brain injury in mice deficient in tumor necrosis factor [TNF(−/−)] and their wild-type (wt) littermates. Mice were subjected to CCI brain injury [TNF(−/−), n = 10; wt, n = 10] or served as uninjured controls [TNF(−/−), n = 10; wt, n = 10] and were evaluated for deficits in memory retention at 7 days postinjury. Although both brain-injured wt and TNF(−/−) mice exhibited significant memory dysfunction compared to uninjured controls (P < 0.02), the deficits in memory retention in injured TNF(−/−) mice were significantly less severe than in injured wt mice (P < 0.02). A second group of mice was subjected to CCI brain injury [TNF(−/−), n = 20; wt, n = 20] or served as uninjured controls [TNF(−/−), n = 15; wt, n = 15] and were evaluated over a 4-week period for neurological motor function. In the acute posttraumatic period (48 h postinjury), brain-injured TNF(−/−) mice were significantly less impaired than injured wt mice on composite neuroscore (P < 0.001), rotarod (P < 0.05), and beam balance (P < 0.02) tests. However, wt mice recovered from brain injury by 2–3 weeks postinjury, whereas TNF(−/−) mice continued to demonstrate persistent motor deficits up to 4 weeks postinjury. Histopathological analysis at 2 and 4 weeks postinjury revealed that brain-injured TNF(−/−) mice had significantly more cortical tissue loss than wt mice (P < 0.02). Our results suggest that although the presence of TNF in the acute posttraumatic period may be deleterious, this cytokine may play a role in facilitating long-term behavioral recovery and histological repair after brain injury.
Keywords: cytokines, cognition, neuromotor function, histopathology, central nervous system trauma
Tumor necrosis factor α (TNF-α), one of the central mediators of tissue injury and inflammation, has been implicated in the pathogenesis of several central nervous system disorders including cerebral ischemia, Parkinson’s disease, and brain trauma (1–4). In patients with ischemic brain damage, concentrations of TNF in peripheral blood have been correlated with brain lesion volume and associated with poor functional and neurological outcome (5). Increased levels of TNF-α have also been observed in plasma and cerebrospinal fluid levels of patients after traumatic brain injury (TBI) (6), whereas acute increases in TNF-α protein and mRNA expression have been observed in selected brain regions after experimental brain injury in the rat (7–10). In a weight-drop model of experimental TBI, acute inhibition of posttraumatic TNF-α production or blockade of TNF-α action with TNF-binding proteins has been reported to improve behavioral deficits and attenuate edema, cortical tissue loss, and hippocampal neurodegeneration in the acute posttraumatic period (9, 10).
Whereas TNF-α has been suggested to be toxic to neurons (11–13) and glia (14), it has also been reported to prevent cell death in vitro after exposure of neurons to β-amyloid peptide (15) and in vivo after administration of excitotoxins (16), peripheral nerve injury (17), and cerebral ischemia (18). TNF-α has also been associated with the regulation of tissue remodeling, gliosis, and scar formation (19–21). Because the effects of TNF-α or TNF-α inhibition in the chronic postinjury period have not been evaluated to date, the present study was designed to more fully characterize the role of TNF in both the acute posttraumatic period and in the chronic pathophysiological response to brain trauma. We investigated the neurobehavioral responses and extent of histopathological cell damage after controlled cortical impact (CCI) brain injury in mice genetically engineered to be deficient in TNF.
METHODS
Animals.
The production and specific details regarding the mice deficient in TNF used in the present study have been described (22). In brief, a genomic clone containing the complete coding sequences of lymphotoxin-α and TNF-α (GenBank accession no. Y00467, a gift of C.-V. Jongeneel, University of Lausanne) has been sequenced and each nucleotide assigned a unique numeral ranging from 1 to 7,208. A replacement-type targeting vector was constructed composed of nucleotides 1,052 (AvrII) to 6,463 (EcoRI) in which a phosphoglycerate kinase (PGK)–neomycin expression cassette replaced nucleotides 3,704–5,364 of the TNF-α gene in the plasmid pGEM-52. Transfection of W9.5 embryonic stem cells and blastocyst injection were performed as described (22). Disruption of the TNF-α allele was confirmed by using Southern blot analysis of BamHI-digested genomic DNA after hybridization with a SmaI–SmaI fragment. Southern analysis of the leukotriene a gene was accomplished by using a PCR-generated probe corresponding to the region between nucleotides 908 and 1,032. The introduced PGK–neo cassette was analyzed by using a BamHI–StuI fragment of the neomycin gene. The TNF(+/+) and TNF(−/−) mice were derived from heterozygous matings of mice backcrossed to C57BL/6 for seven successive generations. Mice were housed in a specific-pathogen-free environment and tested monthly to confirm their pathogen-free status.
Male TNF-deficient (TNF−/−) mice (n = 55), 6–9 weeks old, weighing 25–30 g, and their wild-type (wt) littermates (n = 55), weighing 23–30 g, were housed on a 12 h light/dark cycle with access to food and water ad libitum. All procedures described herein were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Surgical Procedure.
All animals were anesthetized with an i.p. injection of sodium pentobarbital (65 mg/kg) and placed in a stereotaxic head holder. Eye lubricant was applied to reduce drying of corneal membrane during surgery. After exposing the skull, a 5-mm craniotomy was performed over the left parietotemporal cortex between lambda and bregma, keeping the dura mater intact (23). CCI brain injury was produced as modified from the original model of Dixon et al. (24) in 60 mice [n = 30 wt; n = 30 TNF(−/−)] by using a 3-mm metal impounder at a velocity of 5.0 m/sec and at a depth of 1.0 mm as described (23, 25, 26). The duration was kept constant at 100 msec, and the impactor rod was angled 20° from the vertical plane so that the impactor was perpendicular to the exposed dura mater. A transducer attached to the device provided an analog signal recorded by a computer program (R.C. Electronics, Santa Barbara, CA) for analysis of displacement parameters of the impactor. Uninjured control mice [n = 25 TNF(−/−); n = 25 wt] were surgically prepared but not subjected to CCI injury. After CCI brain injury, the craniotomy was covered with a cranioplasty, and the scalp was sutured. All mice were allowed to recover on a heating pad maintained at 37°C. Two groups of mice were used: one group of TNF(−/−) (n = 10 brain injured; n = 10 uninjured) or wt mice (n = 10 brain injured; n = 10 uninjured) was tested for posttraumatic memory function and sacrificed at 1 week postinjury for histological evaluation, whereas the second group was tested for motor function over a 4-week postinjury period (see Table 1 for numbers of animals).
Table 1.
Number of animals evaluated for neuromotor function at each time point
| Time postinjury, days | Sham wt | Sham TNF (−/−) | Injured wt | Injured TNF (−/−) |
|---|---|---|---|---|
| 2 | 15 | 15 | 20 | 20 |
| 7 | 15 | 15 | 20 | 20 |
| 14 | 12 | 11 | 16 | 16 |
| 21 | 10 | 10 | 10 | 10 |
| 28 | 10 | 10 | 10 | 10 |
The number of animals in each group decreased over time because subgroups of animals were sacrificed at specific time points for histological analysis.
Evaluation of Memory Retention.
One day before injury, TNF(−/−) mice (n = 20) and their wt littermates (n = 20) were pretrained in a Morris water maze to locate a submerged platform by using external visual cues as described (25, 27). The maze was filled with water (21°C), and nontoxic white paint was used to obscure the platform from view. Each animal was placed in the maze at four sites 90° apart, along the periphery of the maze. Each animal performed a series of 10 training trials on each of 2 consecutive days. During training, animals were given 60 sec to find the hidden platform, and those who did not find the platform were then placed on it. All animals were allowed to remain on the platform for 30 sec on the first trial and 15 sec on the subsequent trials. Two hours after the final training, animals were anesthetized and subject to brain injury or sham surgery.
One week after injury, brain-injured (n = 10 TNF(−/−); n = 10 wt) and uninjured (n = 10 TNF(−/−); n = 10 wt) animals were tested in the Morris water maze. Each animal was given two 60-sec trials to swim in the maze with the platform removed, while a video camera recorded their swimming pattern and a computer calculated the swim distance. A memory score was derived as described (25, 27) by assessing the animal’s swimming time throughout different zones by a computerized tracking system (Omnitech Electronics, Columbus, OH). Each zone was ranked in a weighted fashion according to its proximity to the platform location and the total time (sec) spent in each zone was multiplied by the ranked score to derive the animal’s memory score (25, 27, 28).
Evaluation of Neurological Motor Function.
The total numbers of animals used to evaluate neurologic motor deficits at specific posttraumatic time intervals are presented in Table 1. Animals were tested for motor function at 48 h postinjury and again at weekly intervals from 1 to 4 weeks postinjury. The number of animals in each group decreased over time because subgroups of animals were sacrificed at specific timepoints for histological analysis. All tests were conducted by an independent investigator who was blinded to genotype and injury status of the mice.
Composite neuroscore.
Neurologic function was tested by using standard tasks originally characterized for the rat (29, 30) but modified successfully for mice (23, 26). The mice were scored on a 4 (normal) to 0 (severely impaired) integral scale for (i) right forelimb flexion on suspension by the tail and (ii) resistance to lateral pulsion to the right. Right hindlimb function was scored on the three aspects of limb extension (limb splay, toe spread, and toe extension) with a maximal possible score of 3 (30). Mice were also tested for their ability to stand on an inclined plane in face-down, face-up, right, and left horizontal directions with the maximal angle beginning with 50°. The maximum angle at which the animal could maintain balance on the inclined plane was recorded. Mice received a score (0–4) in each direction based on the difference between the achieved angle and baseline performance (0° difference = 4, 2.5° = 3, 5° = 2, 7.5° = 1, and 10° or more = 0). The composite neuroscore was determined by totaling scores of the forelimb flexion, hindlimb flexion, lateral pulsion, and the average of the score of the 4 directions in the angle board test to yield a maximum score of 15.
Beam balance.
The beam-balance task was used to assess the more complex components of vestibulomotor function and coordination as described for mice (31). Briefly, the mouse was placed on a narrow wooden beam (0.7 cm), and its ability to maintain equilibrium was scored as follows: 0, does not attempt to balance; 1, hangs on the beam and falls off; 2, hangs on the beam without falling; 3, hugs the beam without falling; 4, grasps the side of the beam and/or has unsteady movements; and 5, steady posture on the beam. Mice were pretrained 24 h before injury so they could balance on the beam with steady posture for 1 min.
Rotarod.
The rotarod task described by Hamm et al. (32) for rats was modified for use in mice (23, 26). The rotarod device consists of a Plexiglas frame with a 36-mm outer diameter motorized rotating rod. Two different accelerations, both with an initial velocity of 5 rpm, were used: 17.3 rpm/10 sec for the fast acceleration test, and 5 rpm/10 sec for the slow acceleration test. Performance on the task was assessed by measuring the latency until the mouse fell completely off or gripped the device and spun around without attempting to walk on the rod. All animals were acclimated to the rotarod for 2 days before injury. Initial rotarod tests were performed 24 h before brain injury to record the baseline latencies for each animal. Four trials for the fast-acceleration rotarod test were performed, and the average of the two middle latency values was taken during baseline evaluation and postinjury testing, whereas the average latency from two trials was used for the slow-acceleration rotarod test. Postinjury latencies were expressed as a percentage of their respective baseline values.
Histological Evaluation of Cortical Tissue Loss.
At 1, 2, and 4 weeks after brain injury, TNF(−/−) (n = 6 per time point) and wt (n = 6 per time point) mice were anesthetized with sodium pentobarbital (200 mg/kg), perfused with 4% paraformaldehyde, and decapitated. Brains were rapidly removed, processed, and embedded in paraffin. Brains were cut in consecutive 6-μm sections in the coronal plane and mounted on poly-l-lysine-coated slides. For analysis of cortical cavity volume, tissue sections were taken every 0.5 mm between −0.15 mm and 4.35 mm posterior to bregma (33) and stained with hematoxylin and eosin. Each section was digitalized by using a charge-coupled device camera (XC-711, Sony), and the area of the cavity in each section was measured directly by using an image analysis system (MCID/M4 image software, Imaging Research, St. Catherine’s, ON). The total volume of the cortical cavity was calculated by integrating the area obtained from each section with the distance between each level.
Data Analysis.
Memory scores from the Morris water maze were expressed as means (±SD) and subjected to a two-way ANOVA followed by a post-hoc Bonferroni t test. Composite neuroscore, rotarod scores, and beam-balance scores were expressed as medians and analyzed nonparametrically by using the Kruskal–Wallis test followed by post-hoc Mann–Whitney U tests for comparisons between each group. Differences in the cavity volume were assessed by using an unpaired Student’s t test. Significance levels were set at a P < 0.05.
RESULTS
Memory Retention.
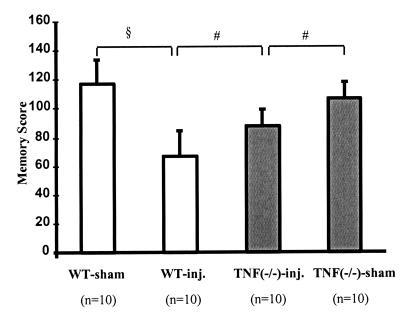
There was no difference in the learning latencies between wt mice and TNF(−/−) mice before injury or in the swim distances between uninjured and injured wt and TNF(−/−) animals at 1 week postinjury (data not shown). CCI brain injury resulted in significant memory deficits at 1 week postinjury in both wt mice (P < 0.001) and TNF(−/−) mice (P < 0.02) when compared with their uninjured controls (Fig. 1). However, injury-induced deficits in memory retention in brain-injured TNF(−/−) mice were significantly less severe than in brain-injured wt mice (P < 0.02).
Figure 1.
Evaluation of memory function in the Morris water maze at 1 week postinjury. Bars depict mean scores, and error bars represent SD. Open bars are wt animals; filled bars are TNF(−/−) animals. Two-way ANOVA was followed by Bonferroni-corrected t tests between sham and injured (inj.) mice of similar genotype and between injured wt and TNF(−/−) mice. §, P < 0.001; and #, P < 0.02
Neurological Motor Function.
Composite neuroscore. Before injury, no differences were observed between TNF(−/−) and wt mice with respect to their forelimb flexion, lateral pulsion, hindlimb function, and baseline angles in the angle-board test. At 48 h postinjury, both brain-injured wt and TNF(−/−) mice exhibited significant neurological deficits when compared with their uninjured controls (P < 0.001; Fig. 2A). However, motor deficits in the injured TNF(−/−) mice were significantly less severe than in injured wt mice (P < 0.001). By 1 week postinjury, neurological motor function of brain-injured wt mice had begun to recover, and no differences were observed between composite neuroscores of injured TNF(−/−) mice and injured wt mice, although both groups remained significantly impaired with respect to uninjured animals (P < 0.05; Fig. 2A). Brain-injured wt mice continued to recover motor function over time, and by 3 weeks, no appreciable deficit in neurologic motor function was observed compared with uninjured controls. In contrast, no improvement in neurological motor score was observed in brain-injured TNF(−/−) mice during the 4-week postinjury observation period [P < 0.05 compared with sham TNF(−/−) mice] at each observation time point. The composite neuroscore of brain-injured TNF(−/−) mice also remained significantly lower than that of brain-injured wt mice at 2 (P < 0.001), 3 (P < 0.05) and 4 (P < 0.05) weeks postinjury (Fig. 2A). The results of the composite neuroscore analysis were consistently replicated when the individual neuroscore tests were evaluated separately (data not shown).
Figure 2.
Evaluation of motor function over a 4-week postinjury time period. Median scores are shown for sham (open symbols) and brain-injured (inj., filled symbols) wt (squares) and TNF(−/−) (triangles) mice. (A) Composite neuromotor function was assessed on a 15-point scale as described in Methods. Statistical comparisons between sham and injured groups of similar genotype are given by ∗ (P < 0.05) and ∗∗ (P < 0.001), whereas comparisons between injured groups are indicated by # (P < 0.05) and ## (P < 0.001). (B) Ability to balance on a beam was assessed on a 5-point scale. Statistical comparisons between sham and injured groups of similar genotype are given by ∗∗ (P < 0.005) and ∗ (P < 0.05), whereas comparisons between injured groups are indicated by # (P < 0.02).
Beam balance.
All naive wt and TNF(−/−) mice learned to balance on the narrow beam. Whereas both injured wt and TNF(−/−) mice were significantly impaired in their performance on the beam balance at 48 h postinjury compared with their respective uninjured controls, brain-injured TNF(−/−) mice performed significantly better at this task than brain-injured wt mice (P < 0.02, Fig. 2B). Beam-balance scores of injured wt mice improved over time to reach uninjured control levels by 3 weeks postinjury. However, no recovery of brain-injured TNF(−/−) mice was evident, leading to persistent beam-balance deficits up to 4 weeks postinjury (Fig. 2B).
Rotarod.
Before injury, no significant differences were observed in the latencies in the fast-acceleration rotarod test between TNF(−/−) mice (15.7 ± 3.5 sec; mean ± SD) and wt mice (15.1 ± 3.1 sec) or in the slow-acceleration test [TNF(−/−) mice = 83.5 ± 8.2 sec; wt mice = 79.8 ± 9.2 sec]. At 48 h postinjury, brain-injured wt and TNF(−/−) mice showed significantly decreased latencies on the rotarod compared to their respective uninjured controls (Fig. 3 A and B). As observed for the composite neuroscore and beam-balance task, injured TNF(−/−) mice were not as severely impaired in the ability to perform on the rotarod as their wt counterparts during the acute postinjury period (i.e., at 48 h postinjury, P < 0.05). By 1 week postinjury, rotarod latencies of brain-injured wt mice had recovered to baseline values. In contrast, brain-injured TNF(−/−) mice continued to exhibit significantly impaired rotarod latencies on both fast acceleration (P < 0.05) and slow acceleration (P < 0.001). This rotarod performance deficit persisted in the injured TNF(−/−) mice for both the fast and slow accelerations through the 4-week observation period.
Figure 3.
Rotarod performance over a 4-week postinjury time period after brain injury in mice. Rotarod scores, calculated as a percent of preinjury latencies, are expressed as median scores for fast-acceleration (A) and slow-acceleration (B) paradigms for sham (open symbols) and brain-injured (inj., filled symbols) wt (squares) and TNF(−/−) (triangles) mice. Statistical comparisons between sham and injured groups of similar genotype are given by ∗∗ (P < 0.001) and ∗ (P < 0.05), whereas comparisons between injured groups are indicated by ## (P < 0.001) and # (P < 0.05).
Posttraumatic Cell Loss in the Cortex.
By 1 week postinjury, all brain-injured mice had a well developed cavity within the ipsilateral cortex. At 4 weeks postinjury, the trauma-induced cavity in the brain-injured wt mice never extended beyond the cortex (Fig. 4A), whereas in the brain-injured TNF(−/−) mice, it often reached the white matter or even became confluent with the ventricle (Fig. 4B). Furthermore, the areas of the cavities between 0.85 mm and 1.85 mm posterior to bregma were significantly larger in brain-injured, TNF (−/−) mice (Fig. 5A). Although no significant differences were observed in the volume of the injury cavity between brain-injured wt mice (4.9 ± 0.3 mm3, mean ± SD) and TNF(−/−) mice (5.5 ± 0.7 mm3) at 1 week postinjury (Fig. 5B), by 2 weeks postinjury, the TNF(−/−) mice exhibited a greater cortical injury cavity volume (6.1 ± 0.4 mm3) than their wt counterparts (5.1 ± 0.5 mm3, P < 0.05). The difference in cavity volume was more prominent by 4 weeks postinjury with 6.4 ± 0.4 mm3 for the TNF(−/−) mice and 5.3 ± 0.6 mm3 for the wt mice (P < 0.005).
Figure 4.
Photomicrographs of representative coronal brain slices obtained at 4 weeks postinjury were selected from a wt mouse (A) and a TNF-deficient mouse (B) at approximately −1.85 mm bregma.
Figure 5.
Quantitation of injury cavity in mice after controlled cortical impact brain injury. (A) Cavity area was calculated from hematoxylin and eosin-stained coronal sections from −1.5 to 4.35 mm posterior to bregma for wt (squares) and TNF (−/−) (triangles)at 4 weeks postinjury. Symbols represent mean areas; error bars denote SD. ∗, P < 0.05 (B) Lesion volume was calculated at 1,2, and 4 weeks postinjury. Mean volumes are presented for wt mice (open bars) and TNF(−/−) mice (filled bars). Error bars depict SD. ∗∗, P < 0.005; ∗, P < 0.05 when compared with wild-type mice.
DISCUSSION
In the acute posttraumatic period, brain-injured TNF(−/−) mice exhibited significantly reduced deficits in both memory function (1 week) and neuromotor function (48 h) compared to brain-injured wt mice. Whereas injured wt mice recovered motor function within 2–3 weeks, we were surprised to find that brain-injured TNF(−/−) mice showed no recovery of motor function over the 4 week postinjury study period. These behavioral observations were supported by the demonstration of significantly greater cortical tissue loss in the chronic postinjury period (2–4 weeks) in injured TNF(−/−) mice when compared with injured wt mice. These data suggest that whereas TNF may play a deleterious role in the acute response of the traumatically injured brain, this cytokine may also have important beneficial effects in the delayed, chronic response of the injured brain.
TNF has been suggested to be one of the central mediators of tissue injury and inflammation (1–3). A profound inflammatory response has been documented, initiated immediately after experimental TBI (34, 35), which is characterized by the release of several cytokines (4). It has been suggested that TNF plays a detrimental role in the acute pathophysiology of TBI and ischemia (2, 8, 16, 36, 37). TNF promotes inflammation by stimulation of capillary endothelial cell proinflammatory responses, thereby promoting leukocyte adhesion and infiltration into the ischemic brain (1). Up-regulation of TNF-α protein and mRNA has been reported to occur in models of cerebral ischemia (38) and in the injured cortex and hippocampus between 1 and 6 h after experimental brain injury (7, 8, 10). Posttraumatic expression is typically observed before the accumulation of infiltrating immune cells, suggesting that TNF-α is synthesized directly by neurons (39–41). Increased levels of TNF-α have also been observed in plasma and cerebrospinal fluid of human head-injured patients (6).
Among the acute consequences of TBI are the breakdown of the blood–brain barrier and glutamate-mediated toxicity. TNF-α has been suggested to participate in blood–brain barrier breakdown after TBI (4, 10, 42). TNF-α modulates capillary permeability by inducing the transcription of proteolytic enzymes, which may result in increased blood–brain barrier permeability and increased vasogenic brain edema (43). Moreover, TNF-α has been shown to potentiate glutamate neurotoxicity via glutamatergic receptor mechanisms (44). TNF-α has been reported to inhibit glutamate uptake and has been shown to modulate the accumulation of extracellular glutamate by a pathway that involves the liberation of nitric oxide (45). In vitro, TNF-α has been observed to be cytotoxic to oligodendrocytes (14) and human or murine neurons (13, 46) and may also participate in acute posttraumatic apoptotic and necrotic cell death (47), both of which have been associated with progressive regional cell death after brain trauma (48–52). In vivo, exogenous TNF-α has been shown to exacerbate focal ischemic injury in a dose-dependent manner if given shortly after the event (37). In the present study, improved memory retention and motor function observed in the TNF(−/−) mice may reflect the pathogenic and neurotoxic effects of the cytokine in the acute posttraumatic period.
Acute inhibition of TNF-α activity with the naturally occurring TNF receptor antagonist, TNF-binding protein, after experimental allergic encephalomyelitis in rats has been shown to result in attenuation of inflammatory lesions and behavioral dysfunction (53). Administration of TNF-binding protein after focal cerebral ischemia reduced the volume of ischemic infarct in rats by attenuating impairment of microvessel perfusion, particularly in perifocal/penumbral regions of the cortex (54). Furthermore, circulating antibody against TNF-α protected rat brain from reperfusion injury (55). After weight-drop brain trauma in rats, attenuation of TNF-α gene transcription by pentoxyfilline or inhibition of TNF activity with TNF-binding protein (both administered 5 min after injury) reduced posttraumatic disruption of the blood–brain barrier, edema formation, and hippocampal neurodegeneration, and improved neurological function (9, 10). The synthetic cannabinoid and N-methyl-d-aspartate receptor antagonist, dexabinol (HU-211), was also reported to inhibit brain TNF-α production and improve neuropathological and behavioral outcome after weight-drop brain injury in the rat (10). Taken together, the above data suggest that TNF may be an ideal target for therapeutic intervention in the acute posttraumatic period to reduce secondary brain injury. Our data, illustrating that brain-injured TNF(−/−) mice exhibit attenuated deficits in motor function at 2 days and memory retention at 1 week postinjury, are consistent with these observations that TNF may mediate, in part, the pathophysiological response to TBI in the acute posttraumatic period.
In contrast to its pathologic role in the acute posttraumatic period, our data suggest that TNF may play a beneficial role in the chronic period after TBI. Whereas motor function in brain-injured wt mice recovered to preinjury control levels by 2–3 weeks postinjury, significant motor deficits in brain-injured TNF(−/−) mice persisted for up to 4 weeks, and trauma-induced cortical cell loss was markedly exacerbated at both 2 and 4 weeks in the TNF(−/−) mice. In vitro and in vivo evidence suggest that, in contrast to the proinflammatory and cytotoxic effects attributed to excessive production of TNF-α, low concentrations of these cytokines may be neuroprotective, suggestive of a potential dual role with respect to neuronal survival (56). Alternatively, the timing of TNF production (e.g., delayed production of modest or even significant levels) may be an important factor related to the neuroprotective effects of TNF. Whereas up-regulation of TNF mRNA and increased synthesis of TNF protein have been demonstrated in the acute (hours) posttraumatic period (7–10), alterations in TNF in the chronic period have not yet been evaluated. Other studies have suggested a protective role for TNF. Pretreatment of mice with TNF-α has been reported to reduce infarct volume after focal ischemia (18). Indirect evidence for a neuroprotective role for TNF-α has been provided in a recent report by Bruce et al. (16), who observed that mice lacking functional TNF receptors were more susceptible to excitotoxic or ischemic damage. In contrast to its proinflammatory effects, a recent study showed that TNF-α might also possess some antiinflammatory effects in autoimmune-mediated demyelination (57).
The mechanism whereby endogenous TNF-α protects neurons against ischemic and excitotoxic insults may involve the induction of antioxidant pathways. Levels of lipid peroxidation induced by kainic acid have been reported to be increased in neurons of TNF-receptor-deficient mice relative to controls (11). Moreover, in vitro, TNF-α has been observed to up-regulate the antioxidant enzyme, superoxide dismutase (58), and the calcium-binding protein, calbindin-D28k (59), suggesting that the neuroprotective action of TNF may, in part, result from attenuation of posttraumatic oxidative damage (36) and/or stabilization of intracellular calcium flux known to be associated with TBI (60–62).
TNF-α may also mediate repair processes and neuronal plasticity in the chronic posttraumatic period. Recent reports have indicated that TNF-α may participate in the repair of peripheral nerves in rats (63), blood vessels in humans (21), and wound healing in mice (64). TNF-α has been shown to increase the spontaneous synaptic currents in hippocampal neurons in vitro, suggestive of a role for TNF-α in mediating synaptic plasticity in vivo (65). TNF-α may also enhance the recovery process by increasing the expression of antiinflammatory cytokines, such as IL-10 and TGF-α (66), and this cytokine network may potentially play a role in chronic central nervous system repair mechanisms by triggering proliferative responses of astrocytes after injury (67–69).
We have shown that mice deficient in TNF exhibited milder behavioral dysfunction than wt littermates in the acute posttraumatic period. In contrast, Bruce et al. (16) have reported that mice deficient in both p55 and p75 TNF receptors were more susceptible in the acute period to ischemic and excitotoxic injury. In their study, Bruce et al. (16) observed similar injury-induced increases in TNF protein in both wt and TNF receptor-deficient mice. The possibility exists that the prolonged presence of unbound TNF may induce pathologic cellular changes in a receptor-independent fashion (71, 72). Alternatively, the differences between our observation and those of Bruce et al. may be due, in part, to differences in the injury models, the time course of the pathological changes, and/or the strain of mice used. Moreover, broader alterations, secondary to the defined mutations in TNF receptor or TNF ligand, might explain these differences.
In conclusion, the mechanisms underlying the acute pathology and behavioral deficits after TBI have yet to be fully elucidated. The attenuation of posttraumatic behavioral deficits during the first postinjury week in TNF(−/−) mice support the hypothesis that the inflammatory cascade, including the production of proinflammatory cytokines, may be deleterious in the acute posttraumatic period. Additionally, this report suggests that TNF may be beneficial in the chronic period after TBI and that long-term disruption of TNF may be deleterious to functional recovery in the context of central nervous system injury. These data suggest a critical therapeutic time window for treatments related to TNF-α blockade intended to reduce traumatic brain damage and behavioral dysfunction.
Acknowledgments
We thank Jeanne Marks and Laura Meehan for their assistance with manuscript preparation and Desirée Davis for housekeeping. This study is supported, in part, by National Institutes of Health Grants (National Institute of Neurological Disorders and Stroke) P01-NS08803, R01-NS26818, (National Institute of General Medical Sciences) R01-GM34690 (T.K.M.), (National Institute of Aging) AG-09215, AG-10124 (J.Q.T.), and a Veterans Administration Merit Review grant (T.K.M.).
ABBREVIATIONS
- CCI
controlled cortical impact
- TNF-α
tumor necrosis factor α
- wt
wild type
- TBI
traumatic brain injury
References
- 1.Feuerstein G Z, Liu T, Barone F C. Cerebrovasc Brain Metab Rev. 1994;6:341–360. [PubMed] [Google Scholar]
- 2.Rothwell N J, Hopkins S J. Trends Neurosci. 1995;18:130–136. doi: 10.1016/0166-2236(95)93890-a. [DOI] [PubMed] [Google Scholar]
- 3.Arvin B, Neville L F, Barone F C, Feuerstein G Z. Neurosci Biobehav Rev. 1996;20:445–452. doi: 10.1016/0149-7634(95)00026-7. [DOI] [PubMed] [Google Scholar]
- 4.Morganti-Kossmann M C, Lenzlinger P M, Stahel P, Csuka E, Ammann E, Stocker R, Trentz O, Kossmann T. Mol Psychiatry. 1997;2:133–136. doi: 10.1038/sj.mp.4000227. [DOI] [PubMed] [Google Scholar]
- 5.Fassbender K, Rossol S, Kammer T, Daffertshofer M, Wirth S, Dollman M, Hennerici M. J Neurol Sci. 1994;122:135–139. doi: 10.1016/0022-510x(94)90289-5. [DOI] [PubMed] [Google Scholar]
- 6.Ross S A, Halliday M I, Campbell G C, Byrnes D P, Rowlands B J. Br J Neurosurg. 1994;8:419–425. doi: 10.3109/02688699408995109. [DOI] [PubMed] [Google Scholar]
- 7.Taupin V, Toulmond S, Serrano A, Benavides J, Zavala F. J Neuroimmunol. 1993;42:177–186. doi: 10.1016/0165-5728(93)90008-m. [DOI] [PubMed] [Google Scholar]
- 8.Fan L, Young P R, Barone F C, Feuerstein G Z, Smith D H, McIntosh T K. Mol Brain Res. 1996;36:287–291. doi: 10.1016/0169-328x(95)00274-v. [DOI] [PubMed] [Google Scholar]
- 9.Shohami E, Bass R, Wallach D, Yamin A, Gallily R. J Cerebr Blood Flow Metab. 1996;16:378–384. doi: 10.1097/00004647-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Shohami E, Gallily R, Mechoulam R, Bass R, Ben-Hur T. J Neuroimmunol. 1997;72:169–177. doi: 10.1016/s0165-5728(96)00181-6. [DOI] [PubMed] [Google Scholar]
- 11.Gelbhard H A, Dzenko K A, Epstein L G. Dev Neurosci. 1993;15:417–422. doi: 10.1159/000111367. [DOI] [PubMed] [Google Scholar]
- 12.Yeung M C, Pulliam L, Lau A S. AIDS. 1995;9:137–143. [PubMed] [Google Scholar]
- 13.Westmoreland S V, Kolson D, Gonzalez-Scarano F. J Neurovirol. 1996;2:118–226. doi: 10.3109/13550289609146545. [DOI] [PubMed] [Google Scholar]
- 14.Robbins D S, Shirazi Y, Drysdale B-E. J Immunol. 1987;139:2593–2597. [PubMed] [Google Scholar]
- 15.Barger S W, Horster D, Mattson M P. Proc Natl Acad Sci USA. 1995;92:9328–9332. doi: 10.1073/pnas.92.20.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruce A J, Boling W, Kindy M S, Peschon J, Kraemer P J, Carpenter M K, Holtsberg F W, Mattson M P. Nat Med. 1996;2:788–794. doi: 10.1038/nm0796-788. [DOI] [PubMed] [Google Scholar]
- 17.Chen L E, Seaber A V, Wong G H W, Urbaniak J R. Neurochem Int. 1996;29:197–203. doi: 10.1016/0197-0186(95)00121-2. [DOI] [PubMed] [Google Scholar]
- 18.Nawashiro H, Tasaki K, Ruetzler C A, Hallenbeck J M. J Cerebr Blood Flow Metab. 1997;17:483–490. doi: 10.1097/00004647-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Gordon H M, Kucera G, Salvo R, Boss J. J Immunol. 1992;148:4021–4027. [PubMed] [Google Scholar]
- 20.La Fleur M, Underwood J L, Rappolee D A, Werb Z. J Exp Med. 1996;184:2311–2326. doi: 10.1084/jem.184.6.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindner H, Holler E, Ertl B, Multhoff G, Schreglmann M, Klauke I, Schultz-Hector S, Eissner G. Blood. 1997;89:1931–1938. [PubMed] [Google Scholar]
- 22.Marino M, Dunn A, Grail D, Inglese M, Noguchi Y, Richards E, Jungbluth A, Moore M, Williamson B, Basu S, Old L. Proc Natl Acad Sci USA. 1997;94:8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murai H, Pierce J E S, Raghupathi R, Smith D H, Saatman K E, Trojanowski J Q, Lee V M Y, Loring J F, Eckman C, Younkin S, McIntosh T K. J Comp Neurol. 1998;392:428–438. doi: 10.1002/(sici)1096-9861(19980323)392:4<428::aid-cne2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 24.Dixon C E, Clifton G L, Lighthall J W, Yaghmai A A, Hayes R L. J Neurosci Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- 25.Smith D H, Soares H D, Pierce J E S, Perlman K G, Saatman K E, Meaney D F, Dixon C E, McIntosh T K. J Neurotrauma. 1995;12:169–178. doi: 10.1089/neu.1995.12.169. [DOI] [PubMed] [Google Scholar]
- 26.Raghupathi R, Fernandez S C, Murai H, Trusko S P, Scott R W, Nishioka W K, McIntosh T K. J Cereb Blood Flow Metab. 1998;18:1259–1269. doi: 10.1097/00004647-199811000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Smith D H, Okiyama K, Thomas M J, Claussen B, McIntosh T K. J Neurotrauma. 1991;8:259–269. doi: 10.1089/neu.1991.8.259. [DOI] [PubMed] [Google Scholar]
- 28.Smith D H, Lowenstein D H, Gennarelli T A, McIntosh T K. Neurosci Lett. 1994;168:151–154. doi: 10.1016/0304-3940(94)90438-3. [DOI] [PubMed] [Google Scholar]
- 29.McIntosh T K, Vink R, Noble L, Yamakami I, Fernyak S, Faden A I. Neuroscience. 1989;28:233–244. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- 30.Gruner J A, Yee A K, Blight A R. Brain Res. 1996;729:90–101. [PubMed] [Google Scholar]
- 31.Mikawa S, Kinouchi H, Kamii H, Gobbel G T, Chen S, Carlson E, Epstein C J, Chan P H. J Neurosurg. 1996;85:885–891. doi: 10.3171/jns.1996.85.5.0885. [DOI] [PubMed] [Google Scholar]
- 32.Hamm R J, Pike B R, O’Dell D M, Lyeth B G, Jenkins L W. J Neurotrauma. 1994;11:187–196. doi: 10.1089/neu.1994.11.187. [DOI] [PubMed] [Google Scholar]
- 33.Franklin K B J, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic; 1997. [Google Scholar]
- 34.Clark R S, Schiding J K, Kaczorowski S L, Marion D W, Kochanek P M. J Neurotrauma. 1994;11:499–506. doi: 10.1089/neu.1994.11.499. [DOI] [PubMed] [Google Scholar]
- 35.Soares H D, Hicks R R, Smith D H, McIntosh T K. J Neurosci. 1995;15:8223–8233. doi: 10.1523/JNEUROSCI.15-12-08223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shohami E, Beit-Yannai E, Horowitz M, Kohen R. J Cereb Blood Flow Metab. 1997;17:1007–1019. doi: 10.1097/00004647-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Barone F C, Arvin B, White R F, Miller A, Webb C L, Willete R N, Lysko P G, Feuerstein G Z. Stroke. 1998;28:1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- 38.Liu T, Clark R K, McDonnell P C, Young P R, White R F, Barone F C, Feuerstein G Z. Stroke. 1994;25:1481–1488. doi: 10.1161/01.str.25.7.1481. [DOI] [PubMed] [Google Scholar]
- 39.Tchelingerian J L, Vignais L, Jacque C. NeuroReport. 1994;5:585–588. doi: 10.1097/00001756-199401000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Villarroya H, Marie Y, Ouallet J C, Le Saux F, Tchelingerian J L, Bauman N. J Neurosci Res. 1997;49:975–982. doi: 10.1002/(SICI)1097-4547(19970901)49:5<592::AID-JNR9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 41.Tchelingerian J L, Quinonero J, Booss J, Jacque C. Neuron. 1993;10:213–224. doi: 10.1016/0896-6273(93)90312-f. [DOI] [PubMed] [Google Scholar]
- 42.Probert L, Selmaj K. J Neuroimmunol. 1997;72:117. doi: 10.1016/s0165-5728(96)00176-2. [DOI] [PubMed] [Google Scholar]
- 43.Rosenberg G A, Estrada E Y, Dencoff J E, Stetler-Stevenson W G. Brain Res. 1995;703:151–155. doi: 10.1016/0006-8993(95)01089-0. [DOI] [PubMed] [Google Scholar]
- 44.Chao C C, Hu S. Dev Neurosci. 1994;16:172–179. doi: 10.1159/000112104. [DOI] [PubMed] [Google Scholar]
- 45.Ye Z C, Sontheimer H. NeuroReport. 1996;7:2181–2185. doi: 10.1097/00001756-199609020-00025. [DOI] [PubMed] [Google Scholar]
- 46.Sipe K J, Srisawasdi D, Dantzer R, Kelley K W, Weyhenmeyer J A. Brain Res. 1996;38:222–232. doi: 10.1016/0169-328x(95)00310-o. [DOI] [PubMed] [Google Scholar]
- 47.Laster S M, Wood J G, Gooding C R. J Immunol. 1988;141:2629–2635. [PubMed] [Google Scholar]
- 48.Dietrich W D, Alonso O, Halley M. J Neurotrauma. 1994;11:289–301. doi: 10.1089/neu.1994.11.289. [DOI] [PubMed] [Google Scholar]
- 49.Rink A D, Fung K-M, Trojanowski J Q, Lee V, Neugebauer E, McIntosh T K. Am J Pathol. 1995;147:1575–1583. [PMC free article] [PubMed] [Google Scholar]
- 50.Hicks R R, Soares H D, Smith D H, McIntosh T K. Acta Neuropathol. 1996;91:236–246. doi: 10.1007/s004010050421. [DOI] [PubMed] [Google Scholar]
- 51.Colicos M A, Dash P K. Brain Res. 1996;739:120–131. doi: 10.1016/s0006-8993(96)00824-4. [DOI] [PubMed] [Google Scholar]
- 52.Conti A C, Raghupathi R, Trojanowski J Q, McIntosh T K. J Neurosci. 1998;18:5663–5672. doi: 10.1523/JNEUROSCI.18-15-05663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin D, Near S L, Bendele A, Russell D A. Exp Neurol. 1995;131:221–228. doi: 10.1016/0014-4886(95)90044-6. [DOI] [PubMed] [Google Scholar]
- 54.Dawson D A, Martin D, Hallenbeck J M. Neurosci Lett. 1996;218:41–44. doi: 10.1016/0304-3940(96)13116-5. [DOI] [PubMed] [Google Scholar]
- 55.Lavine S D, Hoffman F M, Zlokovic B V. J Cerebr Blood Flow Metab. 1998;18:52–58. doi: 10.1097/00004647-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 56.Rothwell N J, Strijbos P J. Int J Dev Neurosci. 1995;13:179–185. doi: 10.1016/0736-5748(95)00018-c. [DOI] [PubMed] [Google Scholar]
- 57.Liu J, Marino M W, Wong G, Grail D, Dunn A, Bettadapura J, Slavin A J, Old L, Bernard C C. Nat Med. 1998;4:78–83. doi: 10.1038/nm0198-078. [DOI] [PubMed] [Google Scholar]
- 58.Wong G H. Biochem Biophys Acta. 1995;1271:205–209. doi: 10.1016/0925-4439(95)00029-4. [DOI] [PubMed] [Google Scholar]
- 59.Mattson M P, Cheng B, Baldwin S A, Smith-Swintosky V L, Keller J, Geddes J W, Scheff S W, Christakos S. J Neurosci Res. 1995;42:357–370. doi: 10.1002/jnr.490420310. [DOI] [PubMed] [Google Scholar]
- 60.Fineman I, Hovda D A, Smith M, Yoshino A, Becker D P. Brain Res. 1993;624:94–102. doi: 10.1016/0006-8993(93)90064-t. [DOI] [PubMed] [Google Scholar]
- 61.Nilsson P, Laursen H, Hillered L, Hansen A J. J Cerebr Blood Flow Metab. 1996;16:262–270. doi: 10.1097/00004647-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 62.Shapira Y, Yadid G, Cotev S, Shohami E. Neurol Res. 1989;11:169–171. doi: 10.1080/01616412.1989.11739885. [DOI] [PubMed] [Google Scholar]
- 63.Bizette C, Chan-Chi-Song P, Fontaine M, Tadie M. Chirurgie. 1996;121:474–481. [PubMed] [Google Scholar]
- 64.Hubner G, Brauchle M, Smola H, Madlener M, Fassler R, Werner S. Cytokine. 1996;8:548–556. doi: 10.1006/cyto.1996.0074. [DOI] [PubMed] [Google Scholar]
- 65.Grassi F, Mileo A M, Punturieri A, Eusebi F. Brain Res. 1994;659:226–230. doi: 10.1016/0006-8993(94)90883-4. [DOI] [PubMed] [Google Scholar]
- 66.McCarthy P L. Baillieres Clin Haematol. 1994;7:153–177. doi: 10.1016/s0950-3536(05)80011-3. [DOI] [PubMed] [Google Scholar]
- 67.Appel E, Kolman O, Kazimirsky G, Blumberg P M, Brodie C. NeuroReport. 1997;8:3309–3312. doi: 10.1097/00001756-199710200-00023. [DOI] [PubMed] [Google Scholar]
- 68.Aloisi F, Borsellino G, Care A, Testa U, Gallo P, Russo G, Peschle C, Levi G. Int J Dev Neurosci. 1995;13:265–274. doi: 10.1016/0736-5748(94)00071-a. [DOI] [PubMed] [Google Scholar]
- 69.Hattori A, Tanaka E, Murase K, Ishida N, Chatani Y, Tsujimoto M, Hayashi K. J Biol Chem. 1993;268:2577–2582. [PubMed] [Google Scholar]
- 70.Neumann B, Luz A, Pfeffer K, Holzmann B. J Exp Med. 1996;184:259–264. doi: 10.1084/jem.184.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kagan B, Baldwin R, Munoz D, Wisnieski B. Science. 1992;255:1427–1430. doi: 10.1126/science.1371890. [DOI] [PubMed] [Google Scholar]
- 72.Baldwin R, Stolowitz M, Hood L, Wisnieski B. Proc Natl Acad Sci USA. 1996;93:1021–1026. doi: 10.1073/pnas.93.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]