Abstract
Maintaining glutamate at low extracellular concentrations in the central nervous system is necessary to protect neurons from excitotoxic injury and to ensure a high signal-to-noise ratio for glutamatergic synaptic transmission. We have used dl-threo-β-benzyloxyaspartate (TBOA), an inhibitor of glutamate uptake, to determine the role of glutamate transporters in the regulation of extracellular glutamate concentration. By using the N-methyl-d-aspartate receptors of patched CA3 hippocampal neurons as “glutamate sensors,” we observed that application of TBOA onto organotypic hippocampal slices led to a rapid increase in extracellular glutamate concentration. This increase was Ca2+-independent and was observed in the presence of tetrodotoxin. Moreover, prevention of vesicular glutamate release with clostridial toxins did not affect the accumulation of glutamate when uptake was inhibited. Inhibition of glutamine synthase, however, increased the rate of accumulation of extracellular glutamate, indicating that glial glutamate stores can serve as a source in this process. TBOA blocked synaptically evoked transporter currents in astrocytes without inducing a current mediated by the glutamate transporter. This indicates that this inhibitor is not transportable and does not release glutamate by heteroexchange. These results show that under basal conditions, the activity of glutamate transporters compensates for the continuous, nonvesicular release of glutamate from the intracellular compartment. As a consequence, acute disruption of transporter activity immediately results in significant accumulation of extracellular glutamate.
Present knowledge of Na+-dependent glutamate transporter function derives from experiments involving two types of approaches: genetic manipulations, in which the gene for a selected transporter subtype is disrupted, and pharmacological blockade, in which transport function is inhibited with appropriate drugs. In mice in which the GLT-1 or the GLAST gene was knocked out, the extracellular glutamate concentration ([glu]o) was increased and animals were susceptible to seizures and excitotoxic cell death (1). Although such studies, by using genetic approaches, underline the important role of the glutamate transporters in regulating glutamate concentrations in the central nervous system (2), they do not provide information on the dynamics of glutamate homeostasis in response to acute disruption of uptake systems.
Through pharmacological intervention, the short-term consequences of inhibiting glutamate transporter function can be analyzed. Until recently, however, such strategies have been limited by the lack of adequate uptake inhibitors. Indeed, several glutamate uptake inhibitors, such as l-trans-pyrrolidine-2,4-dicarboxylic acid (tPDC), or threo-β-hydroxy-aspartic acid (THA), act as competitive antagonists that are transported in place of glutamate (3, 4). This results in the release of glutamate through heteroexchange (5). Thus, although these inhibitors have been widely used in studies of glutamatergic synaptic transmission, they are not suitable for examining the regulation of [glu]o, which they artifactually increase. Nontransportable inhibitors such as dihydrokainate (DHK) (6) circumvent the problem of heteroexchange, but the latter blocks mainly GLT-1, leaving the other subtypes of glutamate transporters relatively unaffected. An additional concern with some of these inhibitors (e.g., THA or DHK) is the potential interaction with glutamatergic receptors, because these substances are usually glutamate analogs.
In this study, we used a newly synthesized, nontransportable inhibitor of glutamate uptake, dl-threo-β-benzyloxyaspartate (TBOA) to investigate the role of glutamate transporters in regulating [glu]o on a time scale of seconds to minutes. This compound is a potent inhibitor of glutamate uptake by EAAT1 (IC50, 70 μM), EAAT2 (IC50, 6 μM), and EAAT3 (IC50, 6 μM), the three main subtypes of glutamate transporters present in the human hippocampus (ref. 7 and K.S., unpublished data).
By applying TBOA to organotypic hippocampal slice cultures we observed that acute pharmacological disruption of glutamate uptake rapidly leads to an increase in [glu]o that is sufficient to activate N-methyl-d-aspartate receptors (NMDARs). We have examined the mechanisms and the implications of this accumulation of glutamate.
MATERIALS AND METHODS
Preparation.
Experiments were performed on rat organotypic hippocampal slice cultures. Tissue slices of 400-μm thickness were prepared and cultured by means of the roller-tube technique as described previously (8).
Electrophysiological Recordings.
After 12–25 days in vitro, the cultures were transferred to a recording chamber mounted onto the stage of an upright microscope (Axioskop 2 FS; Zeiss) and superfused with an external solution (31°C, pH 7.4) containing 137 mM Na+, 2.7 mM K+, 146.2 mM Cl−, 2.8 mM Ca2+, 0.5 mM Mg2+, 11.6 mM HCO3, 0.4 mM H2PO4, and 5.6 mM d-glucose. Patch-clamp recordings were obtained from CA3 pyramidal cells and CA3 stratum radiatum astrocytes (Axopatch 200B amplifier; Axon Instruments, Foster City, CA) with patch pipettes (2–5 MΩ) filled with 122.5 mM Cs gluconate/10 mM Hepes/8 mM NaCl/10 mM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate (BAPTA). Cells were held at +40 mV and were considered acceptable if the holding current was stable and less than 600 pA, with access resistances from 8 to 16 MΩ. During the experiment, input resistance was assessed by applying 0.4-s voltage commands of +10 or –10 mV. l-Glutamate was applied locally by pressure-ejection as indicated (NeuroPhore; Medical Systems, Greenvale, NY). Cultures treated with clostridial toxins [botulinum A (BoNT A) and tetanus (TeNT) toxins; 100 ng/ml], as well as their respective controls, were incubated during 3 days in serum-free medium.
Drugs and Chemicals.
1,2,3,4-Tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide (NBQX, 25 μM) and picrotoxin (300 μM) were always present in the bathing fluid, except for experiments described in Fig. 4B2, where NBQX was omitted. Tetrodotoxin (TTX, 0.5 μM), (+)-S-α-methyl-4-carboxyphenylglycine (MCPG), d-2-amino-5-phosphonovaleric acid (DAPV), 4-acetamido-4′-isothiocyanatostilbene-2,2′-disulfonic acid sodium salt (SITS) (Sigma), 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB), and l-methionine sulfoximine (MSO) were applied as indicated. TBOA was prepared by K.S. as described previously (7). NBQX, MCPG, and DAPV were purchased from Tocris (Bristol, U.K.); BAPTA, picrotoxin, glutamate, SITS, and MSO were from Sigma; TTX was obtained from Latoxan (Rossans, France); and NPPB from Biomol (Plymouth Meeting, PA).
Figure 4.
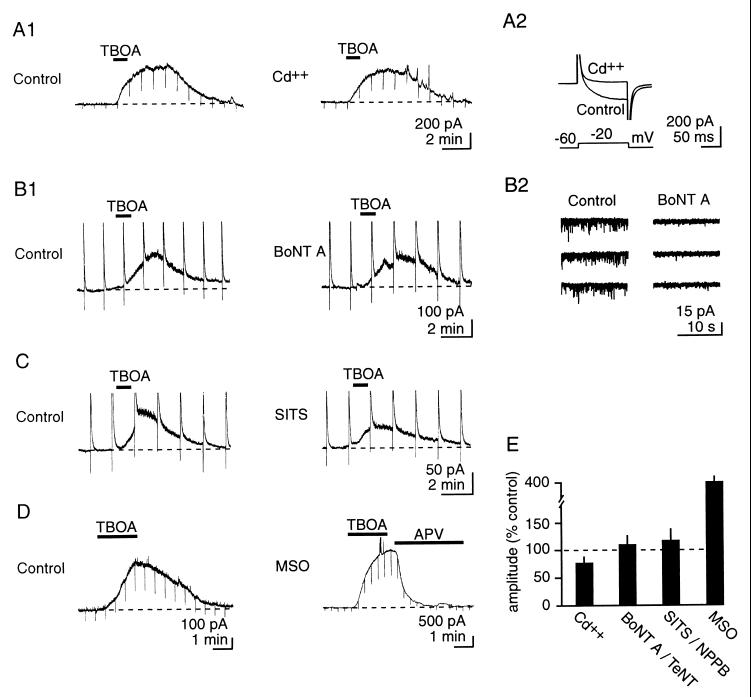
The source of glutamate that accumulates during transporter blockade is nonvesicular. (A1) Left and Right traces: same cell. Cd2+ (200 μM) does not significantly modify extracellular glutamate accumulation during inhibition of uptake with TBOA (200 μM). (A1, B1, C, and D) Representative current traces. (A2) Cd2+ abolishes voltage-gated Ca2+ currents. (B) BoNT A (100 ng/ml), which blocks vesicular release of glutamate (B2, AMPA receptor-mediated miniature excitatory postsynaptic currents at −60 mV, same two cells as B1), does not alter responses to TBOA (C) Left and Right traces: same cell. The anion channel inhibitor SITS (2 mM) does not prevent extracellular glutamate accumulation after transporter blockade. In B1 and C, upward deflections represent truncated responses to brief focal application of NMDA (500 μM in puffer pipette, 40 ms). (D) Inhibition of glutamine synthase with MSO increases the extent of extracellular glutamate accumulation on inhibition of uptake. Note the scale for the MSO recording. (E) Pooled results for the responses to Cd2+, BoNT A or TeNT, SITS or NPPB, and MSO.
Data Acquisition and Analysis.
Signals were filtered at 2 kHz, digitally recorded on a computer by using clampex 7 (Axon Instruments) and stored on tape for later analysis. Numerical data in the text are expressed as means ± SEM. Student’s t test was used to compare values when appropriate. P < 0.05 was considered significant.
RESULTS
[glu]o in the slice cultures was monitored by using the NMDARs of the patched neuron as “glutamate sensors.” NMDAR responses were isolated pharmacologically by using NBQX (25 μM) and picrotoxin (300 μM) and recorded in CA3 pyramidal cells held at +40 mV. Under these conditions, application of l-glutamate or NMDA induced an outward current blocked by DAPV (9).
Inhibition of Uptake Rapidly Increases [glu]o.
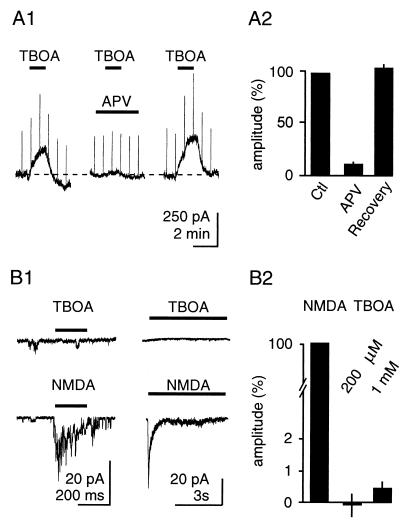
TBOA (200 μM, 2 min) led to an activation of NMDARs within seconds of its application, apparent as an outward current associated with an increase in membrane conductance. NMDAR currents increased progressively in the presence of TBOA, reaching 331 ± 60 pA 2 min after application, and did not occur in the presence of the NMDAR antagonist DAPV (70 μM) (Fig. 1A; n = 6). Larger currents were observed when TBOA application was prolonged (data not shown). This effect was not due to direct stimulation of NMDARs because TBOA at concentrations of up to 1 mM did not induce currents in outside-out patches from neurons containing NMDARs (Fig. 1B, n = 9). These findings are consistent with TBOA leading to a rapid rise in glutamate concentration that is sufficient to activate NMDARs.
Figure 1.
TBOA increases [glu]o. (A) Application of TBOA (200 μM) leads to a rapid activation of NMDARs in CA3 pyramidal neurons held at +40 mV. The response is blocked by DAPV. (A1) Representative traces. (A2) Pooled results. (B) TBOA is not an agonist at NMDARs, because it does not activate NMDARs in outside-out patches. (B1 Left) Single traces; brief application (NMDA: 100 μM). (Right) Averaged traces from the same cell; longer application. (B2) Pooled results.
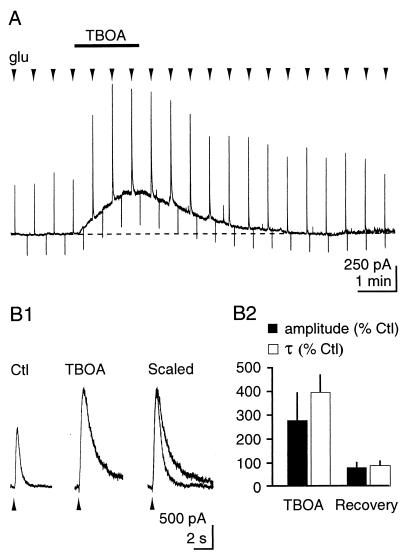
To assess whether the increase in glutamate concentration was a result of the block of transporter function, we next examined the effect of TBOA on the kinetics of NMDAR responses to brief (50- to 200-ms), repetitive pressure application of glutamate close to the patched neuron (Fig. 2, n = 6). TBOA (200 μM) increased the amplitude [Control (Ctl), 289 ± 61 pA; TBOA, 609 ± 136 pA; P = 0.03] and the decay time constant (τ) (Ctl, 254 ± 76 ms; TBOA, 662 ± 100 ms; P = 0.004) of NMDAR responses, indicating that the clearance of puffed extracellular glutamate was delayed significantly. TBOA had no effect on the time course of responses to pressure-applied NMDA, which is not a substrate of the glutamate transporters (data not shown).
Figure 2.
TBOA delays the clearance of extracellular glutamate. TBOA increases the amplitude and the τ of the NMDAR response to brief, local application of l-glutamate (500 μM, 50–200 ms). (A) Continuous recording. (B1) Expanded traces from another cell. (B2) Pooled results.
The Increase in [glu]o Is Not Due to Heteroexchange.
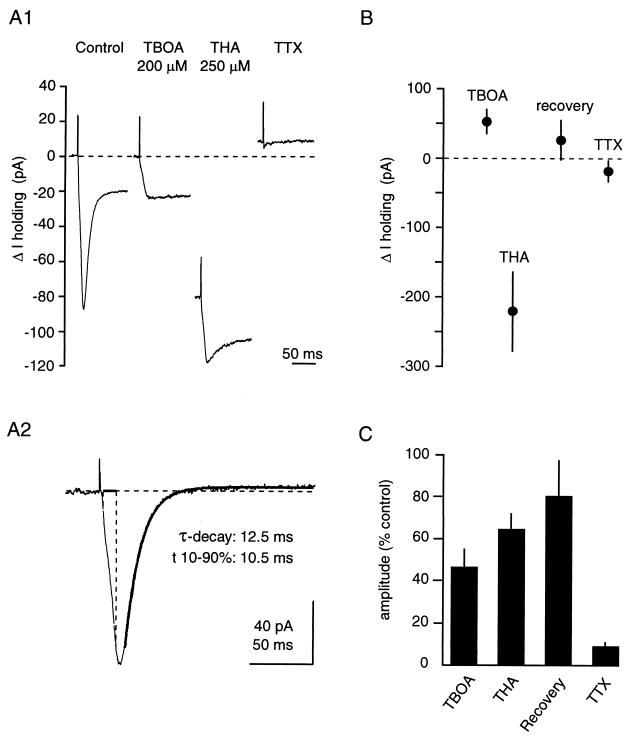
TBOA is not transported in Xenopus oocytes expressing the human glutamate transporters EAAT1, EAAT2, or EAAT3 and, consequently, does not release glutamate by heteroexchange (ref. 7 and K.S., unpublished data). The properties of this drug with regard to rat glutamate transporters in the central nervous system, however, have not been characterized. Therefore, we recorded synaptically evoked transporter currents in CA1 stratum radiatum astrocytes held at –80 mV in the presence of antagonists of ionotropic and metabotropic glutamate receptors (25 μM NBQX, 25 μM DAPV, and 1 mM MCPG). Astrocytes were identified on the basis of morphology of the soma, low resting membrane potential (<−70 mV), low input resistance (<10 MΩ), and the absence of action-potential discharge when depolarized. Under these conditions, monopolar extracellular synaptic stimulation (20–100 μA, 100 μs) elicited transient inward currents characteristic of glutamate transporters (10) (Fig. 3A1). The transportable inhibitor THA (250 μM) reduced the transporter-mediated current, but also generated a large (−222 ± 58 pA, n = 10), inward shift in the holding current, reflecting its transport by astrocytic glutamate transporters (10). In contrast, TBOA blocked transporter currents (Fig. 3 A1 and B) without inducing an inward shift in the holding current. Rather, it generated a small, outward current shift (51 ± 18 pA, n = 7). The current remaining in the presence of both uptake inhibitors relaxed with a time course of seconds, and probably reflects activity-dependent changes in extracellular potassium concentration (10).
Figure 3.
TBOA inhibits rat glutamate transporters without being transported. (A) Synaptic currents in astrocytes in the presence of glutamate receptor antagonists. Extracellular stimulation (20–100 μA, 100 μs) generates a current mediated by glutamate transporters, which is reduced by TBOA (200 μM) and THA (250 μM). In addition, THA generates an inward current reflecting its transport through the transporter, whereas TBOA does not. Responses are blocked by TTX, confirming their synaptic nature. (A1) Sample traces from one astrocyte. (A2) Subtraction of the TBOA trace from the Ctl trace reveals the net transporter current. Single-exponential fit is superimposed. (B) Pooled results for the change in holding current. Note that TBOA induces a small, outward current. (C) Pooled results for the inhibition of the transporter current.
The kinetic properties of the transporter currents were determined by subtracting the current in the presence of TBOA from the control current (Fig. 3A2). Transporter currents displayed a 10–90% rise time of 9.84 ± 0.50 ms, and their decay was fitted with a single exponential with a τ of 15.66 ± 0.81 ms (n = 7). These results are in accordance with the transporter kinetics described in ref. 10.
The Source of Extracellularly Accumulating Glutamate Is Nonvesicular.
To assess whether glutamate accumulating extracellularly during inhibition of uptake was of vesicular origin, we first examined its dependency on extracellular Ca2+ by blocking voltage-gated Ca2+ channels with Cd2+ (200 μM). This did not change the profile of [glu]o upon application of TBOA (Fig. 4A1 and D; Ctl, 300 ± 49 pA; Cd2+, 252 ± 68 pA; n = 6, P = 0.08). We next inhibited vesicular release of glutamate by treating slice cultures with 500 nM BoNT A or TeNT, which prevent vesicular fusion by cleaving SNAP 25 and synaptobrevin, respectively (11, 12) (Fig. 4B2). Although this treatment was effective in inhibiting vesicular release, as assessed by the disappearance of miniature excitatory postsynaptic currents responses to TBOA were not significantly different from controls (Fig. 4 B1 and D; Ctl, 102 ± 29 pA; TeNT-BoNT A, 121 ± 31 pA; n = 7, P = 0.34).
We also examined whether volume-sensitive Cl− channels, which are permeable to glutamate (13), were responsible for the glutamate efflux by testing the effects of the anion channel blockers NPPB (350 μM) or SITS (2 mM) (14). Although the NMDAR currents were reduced in three of six cells (Fig. 4C), these drugs, on average, did not prevent the rise in glutamate concentrations in response to TBOA (Fig. 4D; Ctl, 564 ± 190 pA; SITS-NPPB, 482 ± 143 pA; n = 6, P = 0.18).
Finally, we assessed whether increasing glial cell glutamate concentration with the specific inhibitor of glutamine synthase MSO (15) would influence the extent of glutamate accumulation. After 2–5 hr of pretreatment with 1.5 mM MSO (16), NMDAR-mediated currents induced by TBOA were ≈4 times larger than currents evoked in control cultures (MSO, 1,464 ± 321 pA; n = 6, P = 0.0009). As was observed in control cultures, DAPV (70 μM) did not induce an inward current after MSO treatment, indicating that baseline [glu]o was not increased despite the increase in the intraglial glutamate concentration (DAPV, 10.7 ± 20.5 pA; n = 6, data not shown).
DISCUSSION
Our experiments demonstrate that acute inhibition of glutamate uptake under resting conditions leads to an immediate and physiologically relevant increase in the extracellular concentration of glutamate, consistent with fast extracellular turnover of this neurotransmitter.
Increases in glutamate concentrations were detected in real-time by using NMDARs as “glutamate sensors” in the presence of TBOA. This potent, nontransportable inhibitor of glutamate uptake shows no appreciable agonist activity at NMDARs and does not release glutamate by heteroexchange. These properties were a prerequisite for this study, because heteroexchange of glutamate with a transportable inhibitor such as THA or tPDC would have obscured the glutamate increase, whereas the nontransportable inhibitor dihydrokainate would have interfered with the glutamate detection system (i.e., NMDARs).
Inhibition of uptake with TBOA thus unmasks a continuous release of glutamate into the extracellular space, implying that even on a short time scale (seconds), a low extracellular concentration can be maintained only if glutamate uptake is operational. This conclusion is substantiated by the observation that TBOA induces an outward shift in the holding current in astrocytes (Fig. 3B), reflecting the inhibition of a tonic inward current associated with the continuous uptake of glutamate (but see ref. 17). A further consequence of this finding is that acute transport failure per se may contribute to increases in [glu]o observed during pathophysiological conditions involving glutamate transporter dysfunction, such as stroke (2).
Glutamate-release mechanisms are classically divided into vesicular and nonvesicular processes (18). Vesicular release of glutamate cannot account for the observed rise in glutamate for several reasons. On the one hand, to avoid epileptiform discharge arising from the increase in glutamate levels induced by TBOA, all neuronal recordings were performed in the presence of TTX, which blocks action-potential-dependent vesicular release. On the other hand, to exclude Ca2+-dependent vesicular release through direct depolarization of the axon terminal, Ca2+ channels were blocked with extracellular Cd2+, which had no effect on glutamate accumulation. Furthermore, experiments using clostridial toxins (11, 12) ruled out all types of synaptic vesicular release as a source of glutamate.
Having established the nonvesicular nature of the glutamate release, a series of potential sources was considered. Ca2+-dependent release of glutamate by astrocytes (19, 20) was examined first. This, however, cannot account for our observations because TeNT prevents this type of release (20). Second, if volume-sensitive Cl− channels, which are permeable to glutamate (13), were activated tonically by the baseline turgescence of neurons or astrocytes, glutamate might exit cells by this route, but our results with SITS and NPPB discount this possibility. Third, Warr and collaborators (21) recently have characterized a mode of glutamate release through cystine/glutamate exchangers. These exchangers are expressed in our system, because application of 300 μM l-cystine, which is not agonist at NMDARs at this concentration (22), induced an outward current blocked by DAPV (55 ± 14 pA, n = 4; not shown). Such a mechanism of release thus may underlie our observation, although lack of established or specific antagonists precludes further experimental evaluation at this time (see ref. 20). Fourth, in view of the large intra- to extracellular concentration gradient of glutamate (18), we also have considered whether transmembrane diffusion would lead to significant increases in extracellular concentration of glutamate during inhibition of uptake. Based on the permeability of lipid bilayers to glutamate of 10−12 cm/s (23), the calculated accumulation in the extracellular space would, however, be negligible over a time course of minutes. Finally, it is unlikely that the accumulation of glutamate observed would be the result of leakage from cells severed during preparation of the slice. Indeed, in contrast to acute hippocampal slices, organotypic hippocampal slices are incubated for several days to weeks after sectioning (8).
Interestingly, in line with our findings, leakage of neurotransmitter also has been reported for acetylcholine at the neuromuscular junction (24) and for γ-aminobutyric acid (GABA) in the central nervous system (25, 26). Whereas the origin of acetylcholine leakage is unknown, GABA escapes through GABA transporters by a voltage-dependent process (25). In our case, because TBOA blocks the major glutamate transporters present in the hippocampus (27), a similar route would be excluded (the ability of TBOA to block EAAT4, which is present in trace amounts in the hippocampus (28), however, is not established.).
We have found that inhibition of glutamine synthase, which multiplies intraglial glutamate concentration by 4 in hippocampal slice cultures (16) greatly enhances glutamate accumulation upon inhibition of uptake. This finding (i) indicates that the rate of extracellular glutamate accumulation depends on the intracellular-to-extracellular glutamate gradient and (ii) suggests that a major source of the rapid turnover of glutamate is glial, because glutamine synthase is confined to this cell type in the brain (29) and TBOA was applied at a saturating concentration (i.e., that completely blocks transporter currents; see Fig. 3). The latter point is of importance, because with a subsaturating TBOA concentration, our observation could be explained by a decrease in the baseline rate of glutamate uptake caused by the rise in intracellular glutamate concentration (30). Indeed, the effects of TBOA would superimpose on the preexisting reduced uptake and result in a higher proportion of glutamate transporters being inhibited without necessarily implying a glial source.
The interstitial glutamate concentration achieved upon inhibition of uptake can be estimated by comparing the increase in membrane conductance at 0 mV evoked by TBOA (200 μM) versus a saturating concentration of NMDA (800 μM) (31–33), measured by brief (400-ms), +5 mV voltage steps. Under these conditions, the increase in conductance evoked by TBOA is ≈13% of the increase caused by NMDA (ΔTBOA-Ctl, +80 ± 10%; ΔNMDA-Ctl, +690 ± 100%; n = 4 for NMDA, not shown), which, based on concentration-response curves for glutamate-induced NMDAR activation (31, 33), corresponds to a glutamate concentration of 200–300 nM. Therefore, after 2 min of uptake inhibition, the interstitial glutamate concentration close to the NMDARs reaches 100–150 times the minimal value maintainable by transporters (2 nM) (30).
It is noteworthy that the extent of glutamate accumulation is likely to be lower under our experimental conditions as compared with the in vivo situation. The flow of the perfusion fluid and the subphysiological temperature of 30°C serve to limit the rate as well as the extent of extracellular buildup of glutamate. Thus, the [glu]o attained during impaired uptake in vivo may rapidly disrupt synaptic transmission and promote excitotoxic cell death.
In conclusion, we have established that TBOA is an appropriate tool to study glutamate transporter function in the central nervous system. Our data demonstrate that the low [glu]o present in the brain is the result of a dynamic equilibrium, in which glutamate is constantly being released from the intracellular compartment by a nonvesicular mechanism and is continuously being taken up by the membrane transporters.
Acknowledgments
We thank Luc Pellerin for suggesting the use of TBOA, Christian Heuss for reading the manuscript, and L. Heeb, R. Kägi, H. Kasper, and L. Rietschin for technical assistance. This work was supported by the Swiss Academy of Medical Sciences (D.J.), the Prof. Dr. Max Cloëtta Foundation (U.G.), and Swiss National Science Foundation Grant 31–45547.95 (U.G).
ABBREVIATIONS
- TBOA
dl-threo-β-benzyloxyaspartate
- THA
threo-β-hydroxy-aspartic acid
- NMDAR
N-methyl-d-aspartate receptor
- [glu]o
extracellular glutamate concentration
- BoNT A
botulinum toxin A
- TeNT
tetanus toxin
- DAPV
d-2-amino-5-phosphonovaleric acid
- Ctl
control
- NPPB
5-nitro-2-(3-phenylpropylamino) benzoic acid
- SITS
4-acetamido-4′-isothiocyanatostilbene-2,2′-disulfonic acid sodium salt
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Rothstein J D, Dykes-Hoberg M, Pardo C A, Bristol L A, Jin L, Kuncl R W, Kanai Y, Hediger M A, Wang Y, Schielke J P, Welty D F. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi M, Billups B, Rossi D, Sarantis M, Hamann M, Attwell D. J Exp Biol. 1997;200:401–409. doi: 10.1242/jeb.200.2.401. [DOI] [PubMed] [Google Scholar]
- 3.Bridges R J, Stanley M S, Anderson M W, Cotman C W, Chamberlin A R. J Med Chem. 1991;34:717–725. doi: 10.1021/jm00106a037. [DOI] [PubMed] [Google Scholar]
- 4.Johnston G A, Lodge D, Bornstein J C, Curtis D R. J Neurochem. 1980;34:241–243. doi: 10.1111/j.1471-4159.1980.tb04650.x. [DOI] [PubMed] [Google Scholar]
- 5.Volterra A, Bezzi P, Rizzini B L, Trotti D, Ullensvang K, Danbolt N C, Racagni G. Eur J Neurosci. 1996;8:2019–2028. doi: 10.1111/j.1460-9568.1996.tb01345.x. [DOI] [PubMed] [Google Scholar]
- 6.Muñoz M D, Herreras O, Herranz A S, Solis J M, Martin del Rio R, Lerma J. Neuropharmacology. 1987;26:1–8. doi: 10.1016/0028-3908(87)90037-2. [DOI] [PubMed] [Google Scholar]
- 7.Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, Nakajima T. Mol Pharmacol. 1998;53:195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- 8.Gähwiler B H. J Neurosci Methods. 1981;4:329–342. doi: 10.1016/0165-0270(81)90003-0. [DOI] [PubMed] [Google Scholar]
- 9.Sah P, Hestrin S, Nicoll R A. Science. 1989;246:815–818. doi: 10.1126/science.2573153. [DOI] [PubMed] [Google Scholar]
- 10.Bergles D E, Jahr C E. Neuron. 1997;19:1297–1308. doi: 10.1016/s0896-6273(00)80420-1. [DOI] [PubMed] [Google Scholar]
- 11.Capogna M, McKinney R A, O’Connor V, Gähwiler B H, Thompson S M. J Neurosci. 1997;17:7190–7202. doi: 10.1523/JNEUROSCI.17-19-07190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niemann H, Blasi J, Jahn R. Trends Cell Biol. 1994;4:179–185. doi: 10.1016/0962-8924(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 13.Strange K, Emma F, Jackson P S. Am J Physiol. 1996;270:711–730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- 14.Crépel V, Panenka W, Kelly M E, MacVicar B A. J Neurosci. 1998;18:1196–1206. doi: 10.1523/JNEUROSCI.18-04-01196.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Purich D L. Adv Enzymol. 1998;72:9–42. doi: 10.1002/9780470123188.ch2. [DOI] [PubMed] [Google Scholar]
- 16.Laake J H, Slyngstad T A, Smejda Haug F M, Otterson O P. J Neurochem. 1995;65:871–881. doi: 10.1046/j.1471-4159.1995.65020871.x. [DOI] [PubMed] [Google Scholar]
- 17.Otis T S, Jahr C E. J Neurosci. 1998;18:7099–7110. doi: 10.1523/JNEUROSCI.18-18-07099.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Attwell D, Barbour B, Szatkowski M. Neuron. 1993;11:401–407. doi: 10.1016/0896-6273(93)90145-h. [DOI] [PubMed] [Google Scholar]
- 19.Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini B L, Pozzan T, Volterra A. Nature (London) 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- 20.Parpura V, Basarsky T A, Liu F, Jeftinija K, Jeftinija S, Haydon P G. Nature (London) 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 21.Warr O, Takahashi M, Attwell D. J Physiol (London) 1999;514:783–793. doi: 10.1111/j.1469-7793.1999.783ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pullan L M, Olney J W, Price M T, Compton R P, Hood W F, Michel J, Monahan J B. J Neurochem. 1987;49:1301–1307. doi: 10.1111/j.1471-4159.1987.tb10024.x. [DOI] [PubMed] [Google Scholar]
- 23.Chakrabarti A C, Deamer D W. Biochim Biophys Acta. 1992;1111:171–177. doi: 10.1016/0005-2736(92)90308-9. [DOI] [PubMed] [Google Scholar]
- 24.Katz B, Miledi R. Proc R Soc London. 1977;196:59–72. doi: 10.1098/rspb.1977.0029. [DOI] [PubMed] [Google Scholar]
- 25.Brown D. Trends Neurosci. 1979;2:271–273. [Google Scholar]
- 26.Schwarz E A. Science. 1987;238:351–355. [Google Scholar]
- 27.Rothstein J D, Martin L, Levey A I, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl R W. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 28.Furuta A, Martin L J, Lin C L, Dykes-Hoberg M, Rothstein J D. Neuroscience. 1997;81:1031–1042. doi: 10.1016/s0306-4522(97)00252-2. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Hernandez A, Bell K P, Norenberg M D. Science. 1977;195:1356–1358. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- 30.Billups B, Rossi D, Oshima T, Warr O, Takahashi M, Sarantis M, Szatkowski M, Attwell D. Prog Brain Res. 1998;116:45–57. doi: 10.1016/s0079-6123(08)60429-x. [DOI] [PubMed] [Google Scholar]
- 31.Nakanishi N, Axel R, Shneider N A. Proc Natl Acad Sci USA. 1992;89:8552–8556. doi: 10.1073/pnas.89.18.8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verdoorn T A, Dingledine R. Mol Pharmacol. 1988;34:298–307. [PubMed] [Google Scholar]
- 33.Sather W, Dieudonne S, MacDonald J F, Ascher P. J Physiol (London) 1992;450:643–672. doi: 10.1113/jphysiol.1992.sp019148. [DOI] [PMC free article] [PubMed] [Google Scholar]