Abstract
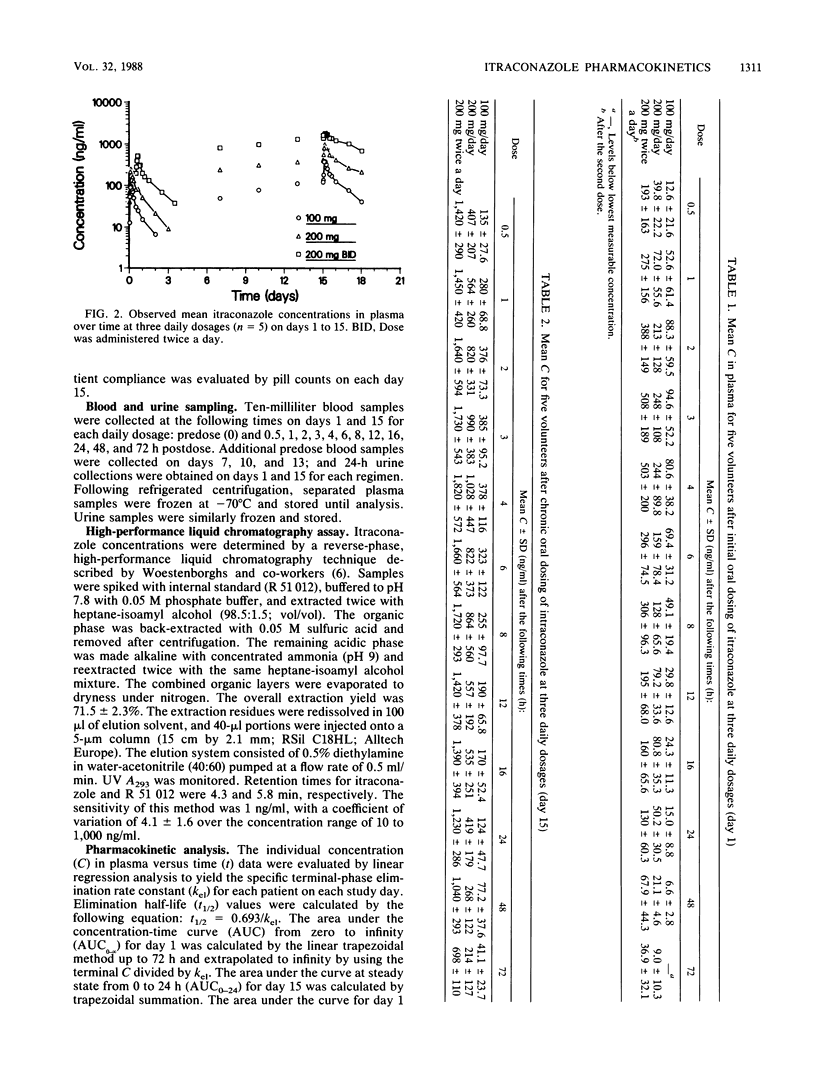
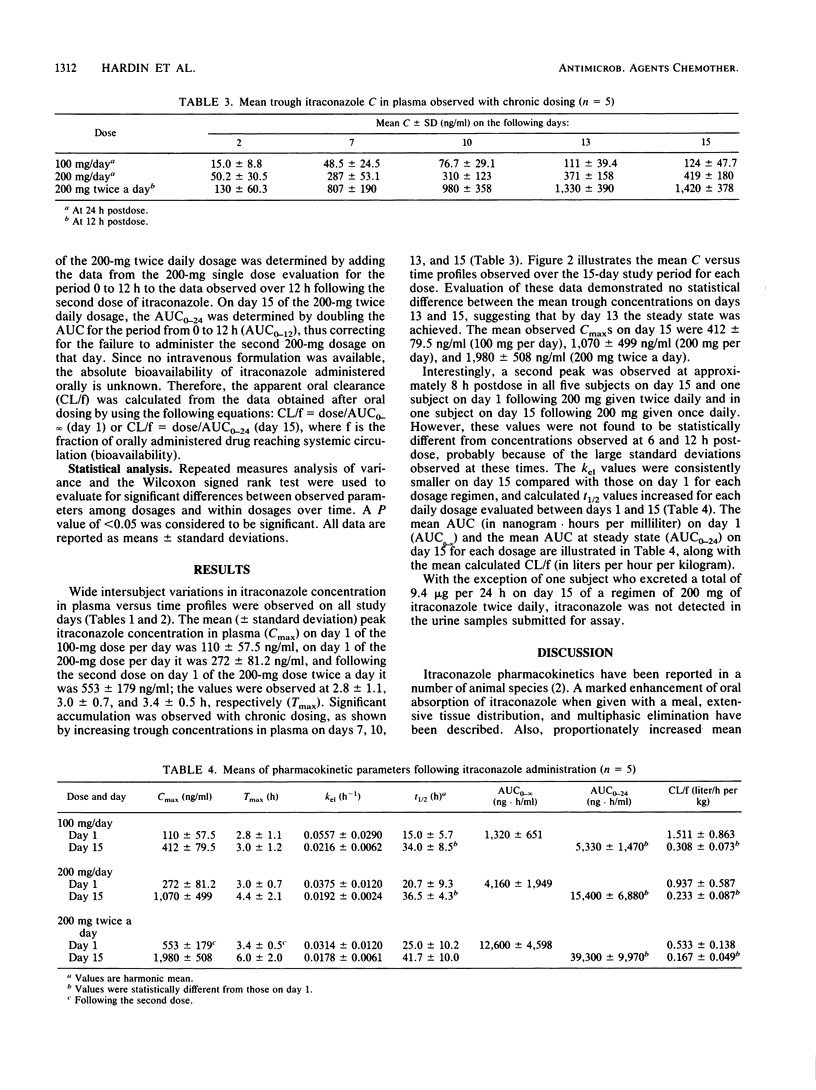
The pharmacokinetics of itraconazole, an orally effective, broad-spectrum, systemic antifungal agent, were evaluated in five healthy male volunteers. Each subject was studied on days 1 and 15 at the following dosages: 100 mg once daily (regimen A), 200 mg once daily (regimen B), and 200 mg twice daily (regimen C). On each study day, itraconazole was administered with a standardized meal. Plasma samples were collected for 72 h postdose, and 24-h urine specimens were obtained. On day 1 of regimen C, plasma samples were collected following the second dose. Samples were assayed for itraconazole by a sensitive, reverse-phase, high-performance liquid chromatography method. Wide intersubject variations in itraconazole concentration in plasma versus time profiles were observed on all study days. Absorption appeared to be slow, with day 1 mean peak itraconazole concentrations in plasma of 110 ng/ml at 2.8 h (regimen A), 272 ng/ml at 3.0 h (regimen B), and 553 ng/ml at 3.4 h (regimen C). Mean peak itraconazole concentrations in plasma on day 15 were 412 ng/ml at 3.0 h (regimen A), 1,070 ng/ml at 4.4 h (regimen B), and 1,980 ng/ml at 6.0 h (regimen C). The steady state was achieved by day 13. Respective elimination half-lives on days 1 and 15 were 15 and 34 h (regimen A), 20.7, and 36.5 h (regimen B), and 25 and 41.7 h (regimen C), respectively. The areas under the plasma concentration versus time curves (0 to infinity) on day 1 were 1,320 (regimen A), 4,160 (regimen B), and 12,600 ng.h/ml (regimen C). With the exception of one patient on day 15 of regimen C, itraconazole was not detected in the urine. All data support dose-dependent pharmacokinetic behavior for itraconazole.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- First International Symposium on Itraconazole. Oaxaca, Mexico, October 7-8, 1985. Proceedings. Rev Infect Dis. 1987 Jan-Feb;9 (Suppl 1):S1–152. [PubMed] [Google Scholar]
- Huang Y. C., Colaizzi J. L., Bierman R. H., Woestenborghs R., Heykants J. Pharmacokinetics and dose proportionality of ketoconazole in normal volunteers. Antimicrob Agents Chemother. 1986 Aug;30(2):206–210. doi: 10.1128/aac.30.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larosa E., Cauwenbergh G., Cilli P., Woestenborghs R., Heykants J. Itraconazole pharmacokinetics in the female genital tract: plasma and tissue levels in patients undergoing hysterectomy after a single dose of 200 mg itraconazole. Eur J Obstet Gynecol Reprod Biol. 1986 Oct;23(1-2):85–89. doi: 10.1016/0028-2243(86)90109-7. [DOI] [PubMed] [Google Scholar]
- Van Cutsem J., Van Gerven F., Van de Ven M. A., Borgers M., Janssen P. A. Itraconazole, a new triazole that is orally active in aspergillosis. Antimicrob Agents Chemother. 1984 Oct;26(4):527–534. doi: 10.1128/aac.26.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woestenborghs R., Lorreyne W., Heykants J. Determination of itraconazole in plasma and animal tissues by high-performance liquid chromatography. J Chromatogr. 1987 Jan 23;413:332–337. doi: 10.1016/0378-4347(87)80249-9. [DOI] [PubMed] [Google Scholar]


