Abstract
Homosynaptic long-term depression (LTD) consists of a persistent nonpathological decrease in synaptic transmission, which is induced by low-frequency stimulation. In vivo, low-frequency stimulation (1 Hz, 900 pulses) induces LTD in Wistar but not Hooded Lister rats. In this study, we investigated the influence of behavioral learning and behavioral state on the expression of LTD in both rat strains. Recordings were taken from freely moving animals that had undergone chronic implantation of a recording electrode in the hippocampal CA1 region and a bipolar stimulating electrode in the ipsilateral Schaffer collateral–commissural pathway. Exposure of the rat strains to stress induced a significant elevation in serum corticosterone levels but did not facilitate LTD expression. However, LFS given during exploration of a novel environment resulted in LTD expression in Hooded Lister, and LTD enhancement in Wistar, rats. Reexposure to the same environment did not result in new expression of LTD. Behavioral comparison between the first and second environmental exposure confirmed that the animals had habituated to the novel environment. These observations strongly implicate an association between novelty acquisition and LTD.
It has been postulated that the mechanisms underlying long-term depression (LTD) expression in the cerebral cortex, together with the mechanisms of long-term potentiation (LTP), are responsible for information storage by the hippocampus (1). However, although extensive research has been conducted to establish whether LTP has a physiological basis in the mechanisms underlying learning events in the brain (2–6), very little attention has been paid to whether LTD also plays a role in such phenomena. LTD, rather, has been more widely considered as functioning simply to reverse LTP (7, 8), or alternatively as underlying physiological events that cause forgetting (9). Although homosynaptic LTD has been extensively described in vitro by means of electrophysiological recordings from hippocampal slices, its significance for memory processes has been questioned because of reported failures to induce persistent LTD in vivo (10, 11). On the other hand, LTD has been more recently reported in both anesthetized (12, 13) and freely moving rats (14). Thus, it may be that the conditions during which LTD is induced are critical for robust expression of this form of synaptic plasticity. Furthermore, LTD, when induced in freely moving rats, is persistent and endures for many days—an observation that indicates that synaptic information resulting from LTD induction can be retained long enough to contribute to a hippocampus-dependent memory trace (1).
In preliminary work, we observed that LTD expression induced by low-frequency stimulation (LFS) in the hippocampal CA1 region is strain dependent. This finding prompted the question whether rat strains that do not express LTD after LFS are simply LTD resistant or whether these rats are very sensitive to the induction conditions used. In the present study, we investigated this possibility in two rat strains—Wistar and Hooded Lister rats. Hooded Lister rats demonstrate at best only short-term depression in the CA1 region after LFS, whereas Wistar rats consistently demonstrate robust and persistent LTD after 1 Hz LFS (14). However, in both rat strains it was noticed that during LFS application, a marked increase in exploratory behavior occurred within the recording chamber. This observation provoked the question whether an association exists between exploratory activity and LTD induction. Furthermore, as novelty acquisition occurs during exploration of a new environment, and as novelty acquisition is a hippocampus-dependent phenomenon (15, 16), the question arose whether LTD induction would be facilitated if novel information was available for acquisition during exploratory activity. Therefore, in this study we examined the effect of exposure of the animal to a novel “stimulus-rich” environment on the expression of LTD in the CA1 region. Our results indicate that, under conditions where the animal can learn, LTD is induced even in the rat strain that was apparently LTD-resistant. These data comprise the first evidence that LTD may underlie certain forms of learning.
MATERIALS AND METHODS
Electrode Implantation.
Male (7–8 wk old at the time of surgery) Wistar (Shoenwalde, inbred strain from house stocks) or Hooded Lister rats (Charles River Breeding Laboratories) were chronically implanted with electrodes under pentobarbitone anesthesia (40 mg/kg, i.p.) as described previously (14, 17). Briefly, a recording electrode was lowered into the CA1 region (2.8 mm posterior to bregma, 1.8 mm lateral to the midline), and a bipolar stimulating electrode was placed in the Schaffer collaterals of the dorsal hippocampus (3.1 mm posterior to bregma, 3.1 mm lateral to the midline) via holes drilled through the skull. In some cases (n = 15 for each strain), a second bipolar stimulating electrode was inserted in the commissural pathway of the contralateral side (3.1 mm posterior to bregma, 3.1 mm lateral to the midline). The entire assembly was connected to a rubber socket on the animal’s head and then stabilized by using dental cement. The correct placement of the electrodes into the CA1 region was confirmed via electrophysiological criteria and postmortem histological analysis.
Electrophysiology.
After surgery, animals were allowed 7–10 d to recover, then acclimatization to the recording chamber (40 × 40 × 40 cm) was permitted for 24 h, except where stress tests (in an unfamiliar chamber) were conducted. The animal could move freely during recordings. Field excitatory postsynaptic potentials (fEPSPs) were evoked by using square-wave stimulation (0.2 ms) at 0.1 Hz. For each time point, the average of five evoked responses was used. At the beginning of each experiment, input/output curves were determined to ascertain the maximum evoked fEPSP slope. For measurement of basal synaptic transmission, a stimulus intensity was used, which evoked a response that was 40% of the maximum. LFS was given by using 900 pulses at 1 Hz. LTP was evoked by using 100 Hz stimulation (10 bursts of 10 stimuli, 0.1-ms stimulus duration, 10-s interburst interval). Data were expressed as mean ± SEM baseline fEPSP. Statistical significance was estimated by using (between-factor) ANOVA with repeated measures and by post hoc Student’s t tests. Throughout the experiments, the electroencephalograms of the animals were monitored.
Stress Induction.
The protocol for stress induction corresponded to methods that were previously reported as being effective in vivo (18, 19). Recording-naive rats were placed in the brightly lit recording chamber. Input–output measurements were taken, and a 15-min baseline recording was then carried out. Thirty minutes after placement in the chamber, LFS was given.
Serum Corticosterone Measurements.
After halothane anesthesia, blood was removed via an incision in the tail vein at a consistent time of day (9:30–11:00 a.m.). Samples were removed immediately after the conclusion of behavioral manipulations and left at room temperature for 2 h, refrigerated for 6 h, and then centrifuged. Corticosterone levels in the serum (50-μl samples in triplicates) were then evaluated by using a radio immunoassay kit (Diagnostic Products, Los Angeles).
RESULTS
LTD Induced by LFS Is Strain Dependent.
In both Wistar (n = 10) and Hooded Lister (n = 8) rat strains, basal synaptic transmission evoked through either the ipsilateral (Fig. 1a) or contralateral pathway (Fig. 1b) was equivalent, in that the profile, amplitude, and stability of responses were consistent across strains.
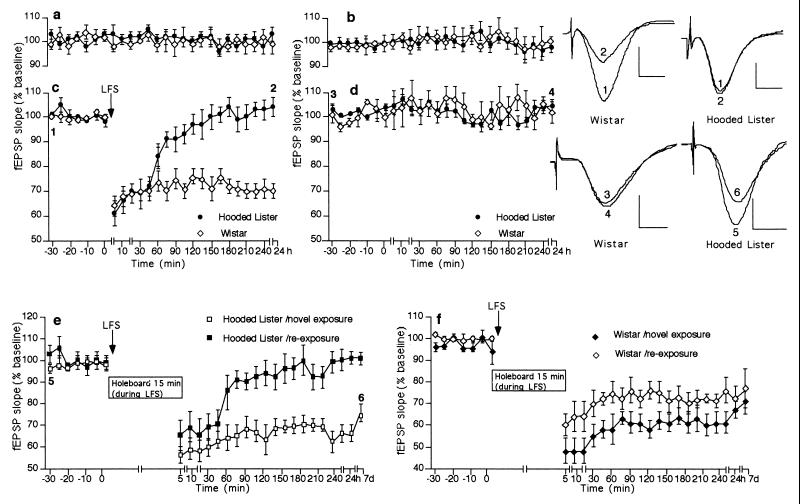
Figure 1.
LTD expression is strain dependent and is facilitated by novelty exploration. Stable basal synaptic transmission was evoked via stimulation of (a) the ipsilateral pathway and (b) the contralateral pathway in Wistar and Hooded Lister rats by using test-pulses. (c) LFS (1 Hz, 900 pulses) induces LTD in Wistar, but not in Hooded Lister, rats. (d) Test-pulses given to the contralateral pathway during LTD expression on the ipsilateral side confirm the input-specificity of LTD obtained. (e) Novel exposure of Hooded Lister rats to a holeboard during LFS induces robust LTD in this rat strain. LTD was still present 7 d after LFS + holeboard was given. Reexposure of the rats to the holeboard 10–14 d after the first exposure, when fEPSP values had returned to basal levels, did not result in LTD induction by LFS. (f) LTD is enhanced in Wistar rats that underwent novel exposure to a holeboard during LFS. LTD was still present 7 d after LFS + holeboard was given. Reexposure of the rats to the holeboard 10–14 d after the first exposure did not result in LTD facilitation by LFS. (Insets) Field potentials (average of five consecutive sweeps) from typical experiments at the times indicated by the numbers. Horizontal bar = 5 ms; vertical bar = 2 mV. Line-breaks indicate change in time-scale.
In this study, short-term depression (STD) was defined as a reduction of synaptic transmission that endured for up to, but not longer than, 2 h. LTD was defined as a decrease in synaptic transmission that persisted beyond the duration of STD. Hooded Lister rats demonstrated only STD in the CA1 region after LFS (1Hz, 900 pulses; Fig. 1c, n = 9), whereas Wistar rats consistently demonstrated robust LTD after LFS (Fig. 1c, n = 13). Although there was a difference in the persistence of depression obtained, LFS -induced LTD in Wistar rats and STD in Hooded Lister rats was input specific, as no change in basal responses was seen via test-pulse stimulation through the contralateral commissural pathway (Fig. 1 c and d).
LTD Occurs During Exploratory Activity.
To investigate whether exploratory activity can influence the expression of LTD in vivo, a “mini-holeboard” was designed that could be inserted into the base of the recording chamber after measurement of basal synaptic transmission. The mini-holeboard consisted of a dark blue platform (40 × 40 cm), which contained a hole (diameter 5.5 cm, depth 5 cm) in each quadrant. A small object, which differed from its fellow objects in scent and texture, was placed in each hole. Thus a novel “stimulus- rich” environment was offered to the animals. LFS was then immediately given. After LFS, the holeboard was removed. It was found that under these conditions, robust and persistent LTD occurred in the Hooded Lister rats (Fig. 1e, n = 11). Effects became significant from t = 60 min post-LFS (t test, P < 0.01) compared with animals that received LFS only (n = 9; ANOVA, F(1, 26) = 5.78, P < 0.001). After novel exposure to the holeboard, LTD was enhanced in Wistar rats (Fig. 1f, n = 6). A significant enhancement was seen from t = 5 min until t = 150 min post-LFS (t test, P < 0.05) compared with animals that received LFS only (n = 13; ANOVA, F(1, 26) = 1.72, P < 0.05). In both strains, LTD was still present 7 d after LFS had been given.
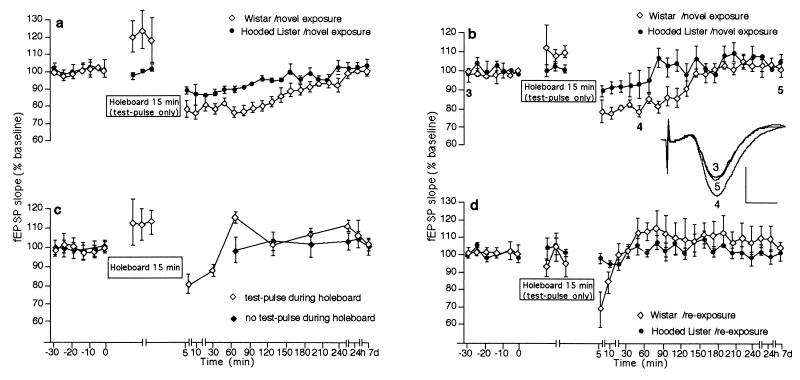
When recordings were elicited via test-pulses (5 × 0.1 Hz every 5 min) given to the ipsilateral pathway during holeboard exposure (Fig. 2a), a reduction in basal synaptic transmission in Hooded Lister rats was seen (n = 8), which was statistically significant for the first 90 min post-LFS (t test, P < 0.05; ANOVA, F(1,14) = 1.8; P < 0.05 compared with controls n = 6). Wistar rats (n = 13) expressed STD after test-pulse stimulation in the presence of the novel holeboard, which lasted for >3 h (t test, P < 0.05; ANOVA, F(1, 26) = 5.0, P < 0.001 compared with controls n = 8, Fig. 2a). This phenomenon also occurred in Wistar rats when basal stimulation was given via the contralateral pathway (n = 5) during novel holeboard exposure (t test, P < 0.05 from t = 05 until t = 120 min postexposure; ANOVA, F(1, 26) = 3.64, P < 0.001 compared with controls, n = 5; Fig. 2b). A transient reduction in basal synaptic transmission that endured for 30 min was also seen in Hooded Lister rats (n = 7; t test, P < 0.05 compared with controls n = 6) when basal stimulation was given via the contralateral pathway during novel holeboard exposure (Fig. 2b). When stimulation after holeboard exploration was reduced to once per hour in Wistar rats (n = 5, Fig. 2c), a transient depression of evoked responses occurred that lasted for 30 min post-LFS (t test, P < 0.05 compared with controls n = 8). No depression occurred, however, in the absence of stimulation during holeboard exposure (n = 5, Fig. 2c). Thus, this phenomenon was input specific and required a threshold level of afferent stimulation. These findings strongly suggest a direct association between novelty exploration and LTD.
Figure 2.
LTD facilitation by novelty exploration requires a threshold level of afferent stimulation. (a) Basal synaptic transmission was depressed in the first 90 min in Hooded Lister animals that did not receive LFS but received test-pulse stimulation during novel exposure to the holeboard, whereas Wistar rats exhibited STD that lasted for >3 h. (b) Test-pulse stimulation via the contralateral commissural pathway during novel exposure to the holeboard resulted in STD that lasted 90 min in Hooded Lister rats and <3 h in Wistar rats. (c) A reduction in test-pulse number (to approximately one sweep per hour) after exposure to the holeboard in the presence of basal stimulation curtailed STD in Wistar rats. STD was completely prevented when holeboard exposure was carried out in the absence of test-pulse stimulation (test-pulses were first given 60 min after the holeboard was removed). (d) Test-pulse stimulation given during reexposure to the holeboard 10 −14 d after the first exposure did not result in STD expression in either the Wistar or Hooded Lister strains. (Insets) Field potentials (average of five consecutive sweeps) from typical experiments at the times indicated by the numbers. Horizontal bar = 5 ms; vertical bar = 2 mV. Line breaks indicate change in time scale.
The transient increase in fEPSP slope that occurred during Wistar rat exposure to the holeboard (Fig. 2 a–c) was not significant from animals where test-pulse stimulation was carried out in the absence of holeboard exposure.
Novelty Acquisition Occurred During Exploration.
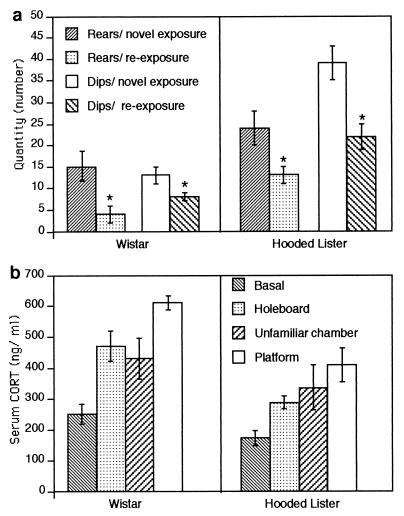
The exploratory behavior of the animals during exposure to the mini-holeboard comprised increased movement throughout the recording chamber, rearing and head-dipping to examine the interior of, or the objects within, the four holeboard holes. This type of exploratory behavior has been described as representing a form of information storage (15, 16). Further proof that learning has taken place is evidenced by habituation on reexposure to the holeboard (20, 21). To evaluate whether learning occurred in our rats, the numbers of rears and head-dips were counted during the 15-min exposure period of the rats to the holeboard (Fig. 3a). It was found that a significant difference in both rearing and head-dipping occurred for both groups when novel exposure to the holeboard was compared with reexposure 10–14 d later (Wistars, n = 6; t test, rears: P < 0.05; dips, P < 0.05; Hooded Listers, n = 6, rears, P < 0.05; dips, P < 0.05).
Figure 3.
(a) Habituation occurs after reexposure to the holeboard in both Wistar and Hooded Lister rats, and exploratory behavior decreases when novel exposure to the holeboard is followed by reexposure to the same holeboard 10–14 d later. A significant decrease in both rearing (P < 0.05 for both strains) and head-dipping (P < 0.05 for both strains) was seen. A significant difference between the exploratory activity of Wistar compared with Hooded Lister rat strains was also found with regard to rearing (P < 0.05 for both novel exposure and reexposure) and head-dipping (P < 0.01, for both novel exposure and reexposure) (P < 0.05). (b) Serum CORT levels are altered after exposure to different behavioral challenges. A significant increase in serum CORT levels, compared with basal values, was seen when Wistar rats were exposed to either a novel holeboard (P < 0.05), an unfamiliar environment (P < 0.05), or an elevated platform (P < 0.001). Similarly, a significant increase in CORT, compared with basal values, was seen when Hooded Lister rats were exposed to either a novel holeboard (P < 0.05), an unfamiliar environment (P < 0.05), or an elevated platform (P < 0.01). The platform-evoked CORT elevation was significantly different from holeboard-induced levels in both strains (P < 0.01 Wistar, P < 0.05 Hooded Lister). Hooded Lister rats had significantly lower basal levels of CORT than Wistar rats (P < 0.05).
A difference in the exploratory behavior of the two animal strains was also found. Hooded Lister rats showed a significantly greater number of head-dips (t test, P < 0.01) during both novel exposure and reexposure to the holeboard, signifying a lower level of stress (Fig. 3a). Similarly, Hooded Lister rats reared more (t test, P < 0.05) during both tests, compared with the Wistar rat strain.
However, the percentage decrease in exploratory behavior was not significantly different between the two strains. On reexposure to the holeboard, the number of rears carried out by Wistar rats was 31.3 ± 5% of novel holeboard levels, compared with 52.2 ± 9% in Hooded Lister rats. The number of head-dips carried out by Wistar rats on reexposure to the holeboard was 64.0 ± 9% of novel holeboard levels, compared with 54.6 ± 4% in Hooded Lister rats. Thus, an equivalent level of habituation appeared to occur within both rat strains.
Novelty Acquisition Is Essential for LTD Facilitation.
To determine whether information storage, or merely increased movement throughout the recording chamber in the form of exploratory activity, was the key factor in the LTD facilitation seen in both strains, the animals underwent a reexposure to the “novel” environment 10–14 d after the first exposure. If exploratory activity, in itself, facilitates LTD induction, then reexposure to the holeboard would enable a further induction of LTD, whereas if novelty acquisition lowers the threshold for LTD induction, then no further LTD should occur on reexposure to the now familiar holeboard. Most significantly, LFS given in conjunction with holeboard reexposure 10–14 d after the first exposure did not result in LTD expression in the LTD-resistant Hooded Lister rats or LTD enhancement in Wistar rats (Fig. 1 e and f). Furthermore, reexposure to the holeboard also did not cause STD in Wistar or Hooded Lister rats during basal stimulation (Fig. 2d). As described earlier, we had found that a significant decrease in exploratory behavior had occurred in both strains on reexposure to the holeboard, consistent with novelty acquisition having occurred.
LTP Is Reversed by Novelty Exploration.
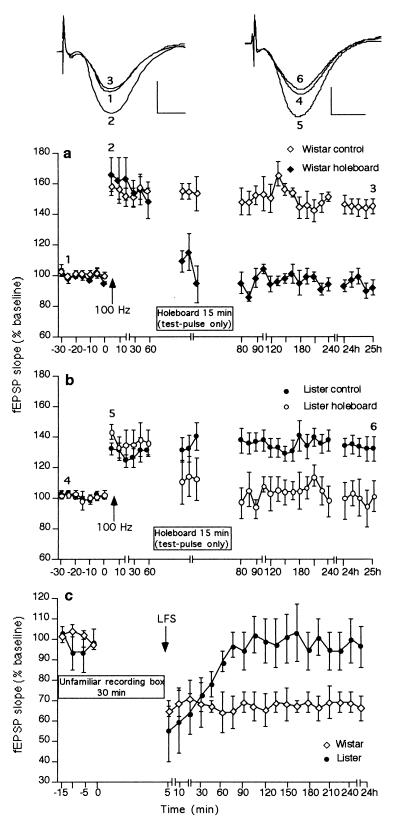
Inhibition of LTP after exposure to novel environments has been described (22, 23). We therefore investigated the effect of exposure to our holeboard paradigm on the expression of LTP in the two rat strains. In control animals, robust LTP was elicited via high-frequency stimulation (HFS) at 100 Hz (Fig. 4 a and b). Twenty-four hours after HFS, the fEPSP was 138 ± 5% of pre-HFS values in Wistar rats (n = 6; t test, P < 0.01; ANOVA, F(1, 33) = 13.19; P < 0.001 compared with non-HFS controls, n = 6) and 132 ± 8% of pre-HFS values in Lister rats (n = 8; t test, P < 0.05; ANOVA, F(1, 33) = 7.27; P < 0.001 compared with non-HFS controls n = 4). If the animals were exposed to the holeboard 60 min after LTP was induced, depotentiation of LTP occurred (Fig. 4 a and b; Hooded Lister n = 5; ANOVA, F(1, 33) = 3.80; P < 0.001; Wistar n = 5; ANOVA, F(1, 33) = 11.39, P < 0.001 compared with HFS animals).
Figure 4.
LTP is reversed by novelty exploration. High-frequency stimulation at 100 Hz induces long-term potentiation in (a) Wistar (n = 6) and (b) Hooded Lister rat strains. Exposure of the animals to a novel holeboard 60 min after induction of LTP resulted in reversal of LTP to basal values in both Wistar (a, P < 0.001) and Hooded Lister Wistar (b, P < 0.001) rats. (c) LTD is not facilitated by stress. Placement of recording-naive Hooded Lister or Wistar rats in an unfamiliar brightly lit recording chamber, followed 30 min later by LFS, does not facilitate LTD induction. (Insets) Field potentials (average of five consecutive sweeps) from typical experiments at the times indicated by the numbers. Horizontal bar = 5 ms; vertical bar = 2 mV. Line-breaks indicate change in time scale.
Corticosterone (CORT) Elevation Does Not Facilitate LTD Expression.
Elevations in CORT levels associated with stress may play a role in LTD induction (19). To determine whether exposure of the LTD-resistant animals to stress could facilitate LTD induction, a comparison was made between the responses to LFS of the LTD-resistant (Hooded Lister) and LTD-expressing (Wistar) rats that had been exposed to an unfamiliar recording chamber in the absence of a holeboard. When placed for the first time in the brightly lit recording chamber, the animals showed behavioral signs of stress, e.g., defecation, urination, and immobility. LFS was given within 30 min of placement in the chamber (Fig. 4c), to correspond with stress-induced peak elevations in cort (19). No significant change in the response of either strain to LFS occurred, compared with control animals that received LFS but were not exposed to the unfamiliar environment (Hooded Lister n = 4; ANOVA, F(1, 25) = 0.47, compared with controls, n = 9; Wistar n = 4, ANOVA, F(1, 25) = 1.02, compared with controls, n = 10). Furthermore, no change in basal synaptic transmission after test-pulse stimulation for 4 h (n = 6 for each strain, not shown) occurred compared with control animals (n = 6 for each strain).
To compare the relative state of stress of the animals, serum CORT levels were examined after measurement of basal synaptic plasticity in acclimatized rats, after exposure to an unfamiliar recording chamber or to the holeboard for 15 min (Fig. 3b). A moderate increase in CORT (significant at P < 0.05 from basal levels for both strains, n = 9 for both strains) occurred after exposure to the holeboard (Wistar, n = 9; Hooded Lister, n = 9). Placement of animals in the unfamiliar chamber caused an increase in CORT (Wistar, n = 9; Hooded Lister, n = 9) that was not significantly different from holeboard-induced levels but did not facilitate LTD induction by LFS (Fig. 4c). For comparative reasons, we assessed CORT levels after exposure to an elevated platform for 30 min—a protocol that has been reported to induce marked stress in rats (refs. 18, 19; Fig. 3b). CORT levels were significantly different from basal and holeboard values for both Wistar (n = 9; t test, P < 0.001 basal; P < 0.01 holeboard) and Hooded Lister rats (n = 9; P < 0.01 basal; P < 0.05 holeboard) after this experience. Thus the elevation of CORT seen after exposure to the holeboard or unfamiliar environment would appear to be within the range of mild stress.
Basal CORT levels for Wistar rats (n = 9) were significantly higher than those obtained in Hooded Lister rats (n = 9; P < 0.05). Similarly, holeboard- (P < 0.01) and platform-evoked (P < 0.01) CORT levels were significantly higher in Wistar compared with Hooded Lister rats. There was no significant difference, however, between CORT levels in the two rat strains after exposure to the unfamiliar chamber. On the other hand, the percentage change (from basal levels) in CORT after exposure to holeboard or platform was not significantly different between the two strains. On exposure to the holeboard, CORT levels in Wistar rats were 187 ± 25% of basal levels, compared with 166 ± 19% in Hooded Lister rats. After exposure to the elevated platform, CORT levels in Wistar rats were 243 ± 24% of basal levels, compared with 236 ± 37% in Hooded Lister rats.
DISCUSSION
The findings of this study demonstrate that LTD in the CA1 region of freely moving rats can be facilitated by novelty acquisition. Thus, a rat strain that normally does not express LTD after LFS showed robust LTD when LFS was given during novel holeboard exploration. Furthermore, a rat strain that normally expresses LTD after LFS showed enhanced LTD after novelty acquisition and additionally expressed LTD with less afferent stimulation than in controls. The finding that LTD facilitation occurred only during the first exposure to the holeboard but not on reexposure, combined with the behavioral data to support that habituation had occurred after holeboard exposure, offers a strong link between the learning phenomenon of novelty acquisition and hippocampal LTD expression.
It has been reported that LTP can be reversed (or depotentiated) after exposure to novel environments (22, 23). In the present study, a similar phenomenon was observed in that depotentiation of LTP occurred if either rat strain was exposed to the holeboard 60 min after LTP was induced. Based on the observations of the present study, it may in fact be the case that a reversal of LTP was seen during exposure to a novel environment in the previously reported cases, because LTD induction mechanisms were activated. Thus, LTP was not reversed because LTP consolidation was disrupted per se; rather, exposure to the novel environment and the subsequent priming for LTD that occurred resulted in a resetting of the basal levels of synaptic activity. Thus, as in the case of the weakly stimulated Wistar rats where LTD of basal synaptic transmission was induced by holeboard exposure, LTD of basal synaptic transmission was also induced by holeboard exposure in the rats that had undergone LTP induction, resulting in an apparent “depotentiation.”
Behavioral analysis of the rat strains during holeboard exploration demonstrated that habituation occurred when the animals were exposed to the holeboard for a second time 10–14 d after the first exposure. Exploratory behavior may correspond to a form of information acquisition (15, 16), whereas evidence of habituation is believed to represent proof that learning has taken place (20, 21). The observation that LTD facilitation occurred only during the initial exposure to the holeboard, whereas reexposure had no facilitatory effect on LTD, supports the intriguing possibility that exploratory learning may be associated with LTD expression. Interestingly, on exposure to the holeboard, the Hooded Lister rat strain explored significantly more than the Wistar strain. However, there was no apparent difference in the degree of LTD expressed by either strain after novel holeboard exposure. The poorer exploratory performance of the Wistar rats did not correlate with a poorer ability to learn. Despite evidence of significantly less exploration on both novel exposure and reexposure to the holeboard, there was no significant difference between Wistar and Hooded Lister rats in the percentage reduction of rears and head dips when reexposure was compared with novel exposure to the holeboard. Thus the degree of habituation was equivalent in both rat strains.
The observations of this study may correspond to metaplasticity (24, 25) on a behavioral level. Metaplasticity comprises a subtle change in synaptic strength, which is not immediately detectable, but which occurs as a result of prior afferent activity, pharmacological priming of neurotransmitter receptors, or alterations in behavioral state. This alteration in synaptic strength manifests itself when the same synapses are subsequently activated and is expressed as an alteration in synaptic efficacy that differs from the control state. Thus, as was seen in Wistar rats, novelty acquisition facilitates LFS-induced LTD induction (e.g., via the ipsilateral pathway), but also increases the likelihood of STD induction via a weakly stimulated (e.g., the contralateral) pathway. Clearly a threshold level of afferent activation is required for LTD to occur during novelty acquisition, as holeboard exposure in the absence of stimulation does not result in LTD expression. This finding not only demonstrates that the form of hippocampal LTD observed is input specific but also suggests that novelty acquisition strongly lowers the threshold level of stimulation required for LTD induction in either strain. In the case of the Hooded Lister rats, this meant that LFS (during novelty exploration) became sufficient to induce robust LTD; in the case of the Wistar rats, test-pulse stimulation of, for example, the ipsilateral afferent pathway became sufficient to induce LTD that endured for over 3 h.
Alternatively, LTD occurs as a direct consequence of novelty acquisition and is involved in the encoding of this information. This behavioral gating of LTD is likely to be multifactorial, requiring events such as behavioral arousal, activation of the noradrenergic system (26–28), and activation of CORT receptors that mediate actions such as modulation of beta-adrenoreceptors (29), enhancement of calcium entry through voltage-gated calcium channels (30), and modulation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (31). However, whereas elevations of CORT may contribute to the expression of LTD, acute CORT elevations alone do not appear to be sufficient to facilitate LTD in resistant (Hooded Lister) rats or to enhance LTD in LTD-expressing (Wistar) rats. This conclusion is supported by the observation that both the holeboard and unfamiliar chamber caused similar elevations in CORT, but LTD was facilitated only when the animals explored the novel “stimulus-rich” holeboard environment. Thus, the LTD facilitation observed in this study was experience dependent and appeared to occur only when the animals could explore the novel environment. This finding may correlate, however, with previous reports of experience-dependent CORT facilitation of learning (32).
It has been suggested that the mechanisms of LTD, working in concert with the mechanisms of LTP, are responsible for the storage of information by the hippocampus (1). The present study offers substantial evidence that this indeed may be the case. Thus, the findings of this study indicate that LTD occurs during novelty acquisition (i) after priming of basal synaptic transmission with LFS, (ii) after resetting of basal synaptic transmission by LTP induction, and (iii) in the absence of priming stimulation. The association of LTD induction, exploratory learning, and novelty acquisition that in itself corresponds to a learning event has been confirmed in two rat strains. This form of LTD is input specific, robust, and persistent, and strongly provokes a revision of current thinking with regard to LTD: in other words, LTD may underlie certain forms of learning in the mammalian brain.
Acknowledgments
We thank Ms. Silvia Vieweg for expert technical assistance. This work was supported by a Deutsche Forschungsgemeinschaft grant (SFB 515/Teilprojekt B8) to D.M.V.
ABBREVIATIONS
- LTP
long-term potentiation
- LTD
long-term depression
- STD
short-term depression
- CORT
corticosterone
- LFS
low-frequency stimulation
- fEPSP
field excitatory postsynaptic potential
- HFS
high-frequency stimulation
References
- 1.Bear M F. Proc Natl Acad Sci USA. 1996;93:13453–13459. doi: 10.1073/pnas.93.24.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris R G M, Frey U. Philos Trans R Soc London B. 1997;352:1489–1503. doi: 10.1098/rstb.1997.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez J, Derrick B. Annu Rev Psychol. 1996;47:173–203. doi: 10.1146/annurev.psych.47.1.173. [DOI] [PubMed] [Google Scholar]
- 4.Moser E I. Behav Brain Res. 1995;71:11–18. doi: 10.1016/0166-4328(95)00051-8. [DOI] [PubMed] [Google Scholar]
- 5.Barnes C A. Neuron. 1995;15:751–754. doi: 10.1016/0896-6273(95)90166-3. [DOI] [PubMed] [Google Scholar]
- 6.Castro C A, Silbert L H, McNaughton B L, Barnes C A. Nature (London) 1989;342:545–548. doi: 10.1038/342545a0. [DOI] [PubMed] [Google Scholar]
- 7.Wagner J J, Alger B E. Hippocampus. 1996;6:24–29. doi: 10.1002/(SICI)1098-1063(1996)6:1<24::AID-HIPO5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 8.Stanton P K. Hippocampus. 1996;6:35–42. doi: 10.1002/(SICI)1098-1063(1996)6:1<35::AID-HIPO7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 9.Tsumoto T. Neurosci Res. 1993;16:263. doi: 10.1016/0168-0102(93)90036-p. [DOI] [PubMed] [Google Scholar]
- 10.Doyle C A, Cullen W K, Rowan M J, Anwyl R A. Neuroscience. 1997;77:75–85. doi: 10.1016/s0306-4522(96)00427-7. [DOI] [PubMed] [Google Scholar]
- 11.Errington M L, Bliss T V P, Richter-Levin G, Yenk K, Doyere V, Laroche S. J Neurophysiol. 1992;74:1793–1799. doi: 10.1152/jn.1995.74.4.1793. [DOI] [PubMed] [Google Scholar]
- 12.Heynen A J, Abraham W J, Bear M F. Nature (London) 1996;381:163–166. doi: 10.1038/381163a0. [DOI] [PubMed] [Google Scholar]
- 13.Thiels E, Xie X, Zeckel M F, Barrionuevo G, Berger T. Hippocampus. 1996;6:43–51. doi: 10.1002/(SICI)1098-1063(1996)6:1<43::AID-HIPO8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 14.Manahan-Vaughan D. J Neurosci. 1997;17:3303–3311. doi: 10.1523/JNEUROSCI.17-09-03303.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eichenbaum H. Curr Opin Neurobiol. 1996;6:187–195. doi: 10.1016/s0959-4388(96)80072-9. [DOI] [PubMed] [Google Scholar]
- 16.O’Keefe J, Nadel L M. The Hippocampus as a Cognitive Map. Oxford: Clarendon; 1978. [Google Scholar]
- 17.Manahan-Vaughan D, Reymann K G. Neuropharmacology. 1995;34:99–1001. doi: 10.1016/0028-3908(95)00081-g. [DOI] [PubMed] [Google Scholar]
- 18.Balfour D J K, Reid A. Arch Int Pharmacodyn Ther. 1979;237:67–74. [PubMed] [Google Scholar]
- 19.Xu L, Anwyl R, Rowan M J. Nature (London) 1997;387:497–500. doi: 10.1038/387497a0. [DOI] [PubMed] [Google Scholar]
- 20.File S E. Psychopharmacology. 1975;44:53–59. [Google Scholar]
- 21.Platel A, Porsolt R D. Psychopharmacology. 1982;78:346–352. doi: 10.1007/BF00433739. [DOI] [PubMed] [Google Scholar]
- 22.Diamond D M, Fleshner M, Rose G M. Behav Brain Res. 1994;62:1–9. doi: 10.1016/0166-4328(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 23.Xu L, Anwyl R, Rowan M J. Nature (London) 1998;394:891–894. doi: 10.1038/29783. [DOI] [PubMed] [Google Scholar]
- 24.Abraham W C, Bear M F. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- 25.Abraham W C, Tate W P. Prog Neurobiol. 1997;52:303–323. doi: 10.1016/s0301-0082(97)00018-x. [DOI] [PubMed] [Google Scholar]
- 26.Sara S J, Dyon-Laurent C, Herve A. Cognit Brain Res. 1995;2:181–187. doi: 10.1016/0926-6410(95)90007-1. [DOI] [PubMed] [Google Scholar]
- 27.Sara S J, Vankov A, Herve A. Brain Res Bull. 1994;35:457–465. doi: 10.1016/0361-9230(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 28.Vankov A, Herve-Minielle A, Sara S J. Eur J Neurosci. 1995;7:1180–1187. doi: 10.1111/j.1460-9568.1995.tb01108.x. [DOI] [PubMed] [Google Scholar]
- 29.Joels M, de Kloet E R. Trends Neurosci. 1992;15:25–30. doi: 10.1016/0166-2236(92)90345-9. [DOI] [PubMed] [Google Scholar]
- 30.Coussens C M, Kerr D S, Abraham W C. J Neurophysiol. 1997;78:1–9. doi: 10.1152/jn.1997.78.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Venero C, Sandi C. Eur J Neurosci. 1997;9:1923–1928. doi: 10.1111/j.1460-9568.1997.tb00759.x. [DOI] [PubMed] [Google Scholar]
- 32.Sandi C, Loscertales M, Guaza C. Eur J Neurosci. 1997;9:637–642. doi: 10.1111/j.1460-9568.1997.tb01412.x. [DOI] [PubMed] [Google Scholar]