Abstract
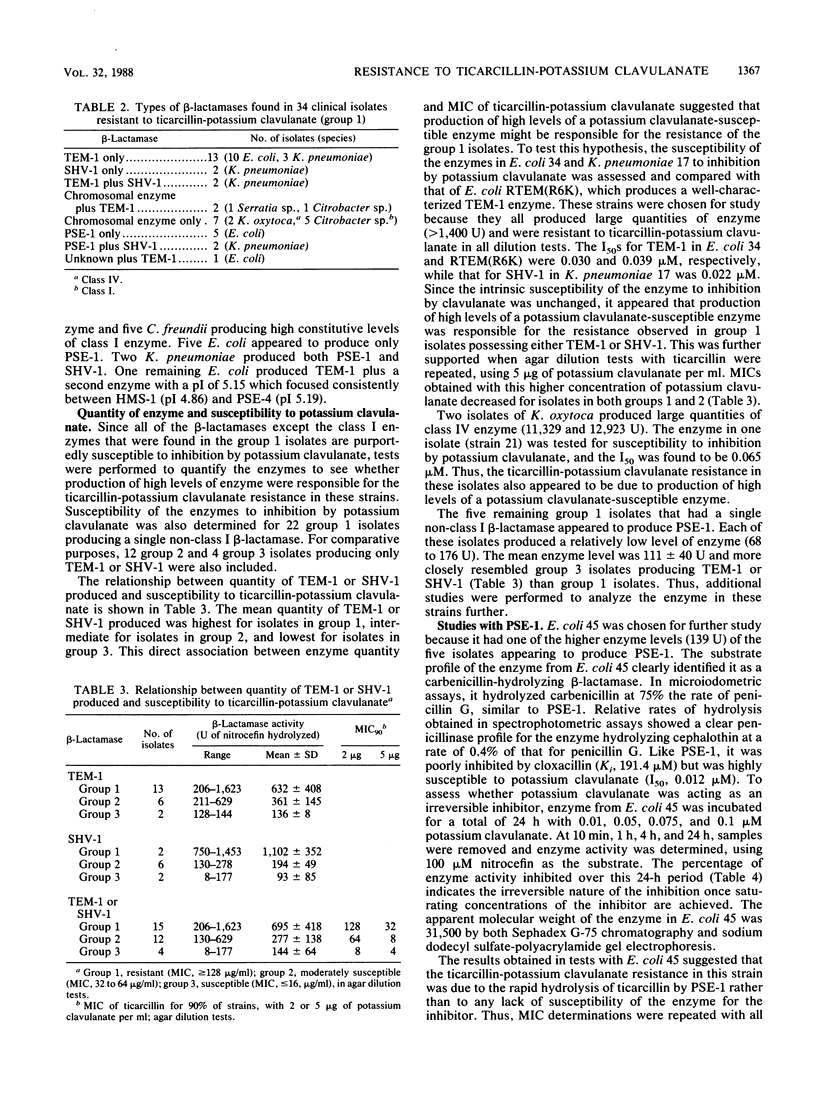
Thirty-four clinical isolates of the family Enterobacteriaceae from the University of Texas M. D. Anderson Cancer Center appeared resistant to ticarcillin-potassium clavulanate in agar dilution and broth macrodilution tests. Among those isolates producing a single non-class I beta-lactamase, resistance was due to production of high levels of TEM-1, SHV-1, or class IV enzymes. In five Escherichia coli isolates, production of low levels of PSE-1 was responsible for resistance which seemed due to rapid hydrolysis of ticarcillin rather than diminished susceptibility of PSE-1 to inhibition by potassium clavulanate. Comparisons of dilution and disk diffusion tests revealed major discrepancies, with 65% false susceptibility in the disk test. Revision of the interpretive criteria used for disk diffusion tests from less than or equal to 11 to less than or equal to 18 mm for resistance is proposed to resolve these discrepancies until clinical data are obtained which can be used to determine which in vitro test is most predictive of therapeutic outcome. These new criteria would diminish false susceptibility without introducing false resistance.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett S., Wise R., Weston D., Dent J. Pharmacokinetics and tissue penetration of ticarcillin combined with clavulanic acid. Antimicrob Agents Chemother. 1983 Jun;23(6):831–834. doi: 10.1128/aac.23.6.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K., Sykes R. B. beta-Lactamase inhibitors in perspective. J Antimicrob Chemother. 1983 Feb;11(2):97–107. doi: 10.1093/jac/11.2.97. [DOI] [PubMed] [Google Scholar]
- Calderwood S. B., Gardella A., Philippon A. M., Jacoby G. A., Moellering R. C., Jr Effects of azlocillin in combination with clavulanic acid, sulbactam, and N-formimidoyl thienamycin against beta-lactamase-producing, carbenicillin-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1982 Aug;22(2):266–271. doi: 10.1128/aac.22.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. C., Barry A. L., Jones R. N. In vitro activity and disk susceptibility of Timentin: current status. Am J Med. 1985 Nov 29;79(5B):25–32. doi: 10.1016/0002-9343(85)90125-1. [DOI] [PubMed] [Google Scholar]
- Gutmann L., Kitzis M. D., Yamabe S., Acar J. F. Comparative evaluation of a new beta-lactamase inhibitor, YTR 830, combined with different beta-lactam antibiotics against bacteria harboring known beta-lactamases. Antimicrob Agents Chemother. 1986 May;29(5):955–957. doi: 10.1128/aac.29.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D., Cockburn A., Cooper D. L., Langley P. F., Tasker T. C., White D. J. Clinical pharmacology and safety evaluation of Timentin. Am J Med. 1985 Nov 29;79(5B):44–55. doi: 10.1016/0002-9343(85)90128-7. [DOI] [PubMed] [Google Scholar]
- Joly B., Chanal M., Sirot D., Cluzel M., Sirot J., Cluzel R. A comparison of agar dilution, identification of beta-lactamases and disc diffusion methods for assessing the sensitivity to ticarcillin-clavulanic acid. J Antimicrob Chemother. 1986 May;17 (Suppl 100):27–33. doi: 10.1093/jac/17.suppl_c.27. [DOI] [PubMed] [Google Scholar]
- Kliebe C., Nies B. A., Meyer J. F., Tolxdorff-Neutzling R. M., Wiedemann B. Evolution of plasmid-coded resistance to broad-spectrum cephalosporins. Antimicrob Agents Chemother. 1985 Aug;28(2):302–307. doi: 10.1128/aac.28.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labia R., Morand A., Péduzzi J. Timentin and beta-lactamases. J Antimicrob Chemother. 1986 May;17 (Suppl 100):17–26. doi: 10.1093/jac/17.suppl_c.17. [DOI] [PubMed] [Google Scholar]
- Li J. T., Moosdeen F., Williams J. D. The effect of inhibitors of beta-lactamases on beta-lactamase extracts and on intact cells. J Antimicrob Chemother. 1982 Apr;9(4):287–296. doi: 10.1093/jac/9.4.287. [DOI] [PubMed] [Google Scholar]
- Matthew M. Plasmid-mediated beta-lactamases of Gram-negative bacteria: properties and distribution. J Antimicrob Chemother. 1979 Jul;5(4):349–358. doi: 10.1093/jac/5.4.349. [DOI] [PubMed] [Google Scholar]
- Medeiros A. A., Hedges R. W., Jacoby G. A. Spread of a "Pseudomonas-specific" beta-lactamase to plasmids of enterobacteria. J Bacteriol. 1982 Feb;149(2):700–707. doi: 10.1128/jb.149.2.700-707.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C. The role of beta-lactamase inhibitors in chemotherapy. Pharmacol Ther. 1985;30(1):1–18. doi: 10.1016/0163-7258(85)90044-0. [DOI] [PubMed] [Google Scholar]
- Pulverer G., Peters G., Kunstmann G. In-vitro activity of ticarcillin with and without clavulanic acid against clinical isolates of gram-positive and gram-negative bacteria. J Antimicrob Chemother. 1986 May;17 (Suppl 100):1–5. doi: 10.1093/jac/17.suppl_c.1. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Moland E. S. Characterization of beta-lactamases in situ on polyacrylamide gels. Antimicrob Agents Chemother. 1986 Dec;30(6):951–952. doi: 10.1128/aac.30.6.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Type I beta-lactamases of gram-negative bacteria: interactions with beta-lactam antibiotics. J Infect Dis. 1986 Nov;154(5):792–800. doi: 10.1093/infdis/154.5.792. [DOI] [PubMed] [Google Scholar]
- Sargent M. G. Rapid fixed-time assay for penicillinase. J Bacteriol. 1968 Apr;95(4):1493–1494. doi: 10.1128/jb.95.4.1493-1494.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland R., Beale A. S., Boon R. J., Griffin K. E., Slocombe B., Stokes D. H., White A. R. Antibacterial activity of ticarcillin in the presence of clavulanate potassium. Am J Med. 1985 Nov 29;79(5B):13–24. doi: 10.1016/0002-9343(85)90124-x. [DOI] [PubMed] [Google Scholar]
- Verbist L., Verhaegen J. Susceptibility of ticarcillin-resistant gram-negative bacilli to different combinations of ticarcillin and clavulanic acid. J Antimicrob Chemother. 1986 May;17 (Suppl 100):7–15. doi: 10.1093/jac/17.suppl_c.7. [DOI] [PubMed] [Google Scholar]
- Waley S. G. A spectrophotometric assay of beta-lactamase action on penicillins. Biochem J. 1974 Jun;139(3):789–790. doi: 10.1042/bj1390789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner V., Sanders C. C., Sanders W. E., Jr, Goering R. V. Role of beta-lactamases and outer membrane proteins in multiple beta-lactam resistance of Enterobacter cloacae. Antimicrob Agents Chemother. 1985 Apr;27(4):455–459. doi: 10.1128/aac.27.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]



