Abstract
This report describes a modulatory action of lithium and glutamate on the activity of serine/threonine kinase Akt-1. Lithium is most commonly used to treat bipolar disorder, but the mechanism of its therapeutic action remains unknown. We have recently demonstrated that lithium protects against glutamate-induced excitotoxicity in cultured brain neurons and in an animal model of cerebral ischemia. This study was undertaken to investigate the role of Akt-1, activated by the phosphatidylinositol 3-kinase (PI 3-K) signaling pathway, in mediating glutamate excitotoxicity and lithium protection in cerebellar granule cells. High levels of phosphorylation and activity of Akt-1 were detected in cerebellar neurons cultured in the presence of serum. Protracted treatment with selective PI 3-K inhibitors, wortmannin and LY294002, abolished Akt-1 activity and induced neuronal death that could be reduced by long-term lithium pretreatment. Exposure of cells to glutamate induced a rapid and reversible loss of Akt-1 phosphorylation and kinase activity. These effects were closely correlated with excitotoxicity and caspase 3 activation and were prevented by phosphatase inhibitors, okadaic acid and caliculin A. Long-term lithium pretreatment suppressed glutamate-induced loss of Akt-1 activity and accelerated its recovery toward the control levels. Lithium treatment alone induced rapid increase in PI 3-K activity, and Akt-1 phosphorylation with accompanying kinase activation, which was blocked by PI 3-K inhibitors. Lithium also increased the phosphorylation of glycogen synthase kinase-3 (GSK-3), a downstream physiological target of Akt. Thus, modulation of Akt-1 activity appears to play a key role in the mechanism of glutamate excitotoxicity and lithium neuroprotection.
Regulation of cell survival is crucial to the normal physiology of multicellular organisms. Perturbation of cell survival mechanisms can lead to either excessive or insufficient cell death which may result in pathological conditions. Apoptosis, also referred to as programmed cell death, is an evolutionarily conserved form of cell death critical for tissue homeostasis. Neurotrophins and growth factors have been shown to inhibit apoptosis and promote cell survival by signal transduction mediated through the phosphatidylinositol 3-kinase (PI 3-K)/Akt cascade (1, 2). The PI 3-K/Akt pathway is preferentially activated by insulin and growth factors such as insulin-like growth factor 1 (IGF-1) and platelet-derived growth factor (PDGF) (2–5). Akt, also known as PKB or RAC, is a multi-isoform serine/threonine kinase and downstream target of PI 3-K (3). Activation of Akt requires phosphorylation by upstream PI-dependent kinases, which is preceded by binding of PI 3-K products, PI-3,4,5-trisphosphate (PI-3,4,5-P3) and/or PI-3,4,-bisphosphate (PI-3,4-P2), to the pleckstrin homology domain of Akt (6, 7). PI-dependent kinases activate Akt-1, the most frequently studied isoform of Akt, by phosphorylation on Ser473 and Thr308 (8). This reversible phosphorylation is negatively regulated by protein phosphatase 2A (9).
Excitotoxic neuronal death induced by glutamate has been shown to occur through both necrosis and apoptosis, with apoptosis being predominant when the glutamate insult is relatively mild (10). Although excitotoxicity is triggered by an exaggerated and prolonged rise in intracellular Ca2+, little is known about the subsequent events that ultimately lead to cell death. During cerebral ischemia, neurodegeneration is associated with a massive efflux of glutamate (11), which contributes to neuronal death by overstimulating glutamate receptors. IGF-1 has been reported to reduce brain damage induced by hypoxic-ischemic injury (12) and to rescue rat cerebral cortical neurons from N-methyl-d-aspartate (NMDA) receptor-mediated apoptosis (13) in a PI 3-K-dependent manner. The characteristics of neuronal apoptosis caused by growth factor withdrawal (1, 2) and glutamate treatment (10, 14) in cultured neurons are strikingly similar, suggesting that glutamate-induced apoptosis and growth factor-elicited neuroprotection share a common target.
Our recent studies demonstrate that long-term lithium treatment robustly protects cultured neurons of the central nervous system against glutamate-induced apoptosis mediated by NMDA receptors (14), and in vivo it protects rats against focal ischemia-induced brain damage (15). In light of the similarity of the protective actions elicited by IGF-1 and lithium, the aims of this study are to elucidate the role of the PI 3-K/Akt signaling pathway in glutamate excitotoxicity and in lithium-induced neuroprotection in cerebellar granule cells (CGCs), which represent the most abundant neuronal phenotype in the mammalian brain and are a nearly homogenous glutamatergic neuronal population. CGCs are particularly useful in studying the role of the PI 3-K/Akt pathway, because it has been shown that Akt is a critical mediator of growth factor-induced survival in these neurons (2).
MATERIALS AND METHODS
Cell Culture.
Primary cultures of CGCs were prepared from 8-day-old Sprague–Dawley rats as described (14). The cells were maintained in basal modified Eagle’s medium containing 10% FCS, 2 mM glutamine, 50 μg/ml gentamicin, and 25 mM KCl. Cytosine β-d-arabinofuranoside (10 μM) was added 24 h after plating to arrest the growth of nonneuronal cells. Cultures were harvested after 8 days in vitro.
Measurement of Neurotoxicity.
The mitochondrial dehydrogenase activity that reduces 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) in active mitochondria to purple formazan was used to determine cell survival in a colorimetric assay as described (14).
Protein Determination and Normalization.
Equal levels of total Akt, PI 3-K, and GSK-3 proteins were ensured in all phosphorylation and kinase activity assays by using an equal amount of protein for each sample as determined by protein assay (Bio-Rad) and by using immunoblotting with phosphorylation-independent antibodies to determine total Akt or GSK-3 levels.
PI 3-Kinase Activity Assay.
PI 3-K activity was measured as described (5, 16), with modifications. After treatment, CGCs were washed with buffer C (20 mM Tris⋅HCl, pH 7.4/137 mM NaCl/1 mM MgCl2/1 mM CaCl2/0.1 mM Na3VO4) and lysed on ice with 1 ml of buffer C containing 1% Nonidet P-40 and 1 mM PMSF. After removal of insoluble materials, supernatants were incubated at 4°C for 1 h with 5 μl of anti-PI 3-K antiserum, which recognizes the p85 subunit of PI 3-K (Upstate Biotechnology); incubation was followed by precipitation with protein A-agarose (Santa Cruz Biotechnology). The immunoprecipitates were washed with lysis buffer, then with a buffer containing 100 mM Tris⋅HCl, pH 7.4/5 mM NaCl/0.1 mM Na3VO4, and with 10 mM Tris⋅HCl, pH 7.4/150 mM NaCl/5 mM EDTA, containing 0.1 mM Na3VO4. The samples were then incubated for 10 min at 37°C in PI 3-K reaction buffer containing 0.88 mM ATP, 20 mM MgCl2, 30 μCi of [γ-32P]ATP (3,000 Ci/mmol) (DuPont/NEN), and 2 μg/μl phosphatidylinositol (PI) (Sigma) as a substrate. The reaction was terminated by addition of 20 μl of 6 M HCl, and the radiolabeled phospholipids were extracted by the addition of 160 μl of a mixture (1:1) of CHCl3 and MeOH. The lipids in the organic phase were resolved on potassium oxalate-treated TLC plates (Analtech) and developed by chromatography in CHCl3/MeOH/H2O/NH4OH (60:47:11.3:2). Radioactive PI- 3-monophosphate (PI-3-P) products were identified by comparison to the Rf value of unlabeled PI-3-P (Calbiochem), visualized by autoradiography, and quantified.
Immunoblotting.
Cell lysates were prepared by using the same method as for Akt immunocomplex kinase assays (see below). The homogenates were centrifuged and supernatants (20 μg) were used for immunoblotting according to standard procedures. Akt-1 phosphorylated at Ser473 or Thr308 was detected with phospho-specific Akt-1 polyclonal antibodies; total Akt-1 was detected by using phosphorylation-independent antibodies (New England Biolabs). Anti-phospho-Ser21 GSK-3 and poly(ADP-ribose) polymerase (PARP) antibodies were purchased from Upstate Biotechnology and anti-GSK-3 antibody came from Advanced ImmunoChemical. The protein bands were visualized by enhanced chemiluminescence (Amersham).
Akt Immunocomplex in Vitro Kinase Assay.
Cells were lysed for 10 min in ice-cold buffer A (50 mM Tris⋅HCl, pH 7.5/1 mM EDTA/1 mM EGTA/0.5 mM Na3VO4/0.1% 2-mercaptoethanol/1% Triton X-100/50 mM NaF/5 mM sodium pyrophosphate/10 mM sodium β-glycerophosphate/0.1 mM PMSF/1 μM microcystin/1 μg⋅ml−1 each pepstatin, aprotinin, and leupeptin). The lysates were centrifuged and the supernatants were collected. Equal amounts of protein (500 μg) were used for each assay. Akt was immunoprecipitated in buffer A at 4°C for 1 h with isoform-specific Akt-1, Akt-2, or Akt-3 anti-pleckstrin homology domain antibodies (Upstate Biotechnology), precoupled to protein G-agarose (Santa Cruz Biotechnology). The immunoprecipitates were washed with buffer A containing 0.5 M NaCl, then with buffer B (50 mM Tris⋅HCl, pH 7.5/0.03% Brij-35/0.1 mM EGTA/0.1% 2-mercaptoethanol) and finally with assay dilution buffer (100 mM Mops, pH 7.2/125 mM β-glycerophosphate/25 mM EGTA/5 mM Na3VO4/5 mM DTT). In vitro kinase assays were performed in assay dilution buffer by measuring the incorporation of 32P into Akt-specific substrate peptide RPRAATF (Upstate Biotechnology) during a 10-min incubation at 30°C in the presence of 10 μM protein kinase A inhibitor peptide; the latter was used to inhibit the activity of cAMP-dependent protein kinase kinase that may nonspecifically bind to the complex. The sequence of the substrate resembles that surrounding the phosphorylation site of GSK-3 and other Akt substrates (17). The phosphorylated substrate was then separated from the residual [γ-32P]ATP with P81 phosphocellulose (Upstate Biotechnology), and the incorporated radioactivity was quantified. Endogenous phosphorylation of proteins in the cell extract and nonspecific immunoprecipitation were determined by substituting buffer for substrate cocktail and nonspecific sheep polyclonal IgG for Akt antibody, respectively, and were subtracted. The absolute values of the 100% control were 98,500 ± 7,540 dpm.
RESULTS
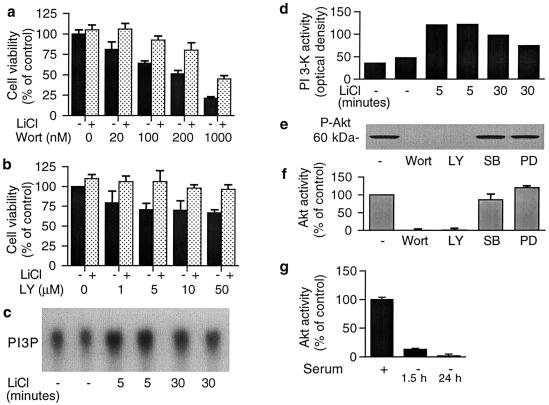
Treatment of CGCs for 4 days with wortmannin or LY294002, two selective PI 3-K inhibitors, resulted in concentration-dependent neuronal death. Protracted pretreatment of cells with 3 mM LiCl markedly reduced the neurotoxicity elicited by these inhibitors (Fig. 1 a and b). Therefore, we examined whether lithium modulates the activity of PI 3-K by measuring the synthesis of PI-3-P from PI by immunoprecipitated PI 3-K. Treatment of cells with 3 mM LiCl increased PI 3-K activity above serum-elevated control levels more than 2-fold at 5 min, and the increase was still observed at 30 min (Fig. 1 c and d). In contrast, glutamate treatment did not alter PI 3-K activity (data not shown), indicating that this enzyme is not a convergent site of action of lithium and glutamate. Because the PI 3-K cascade leads to Akt activation, which is involved in protecting CGCs from apoptosis induced by serum withdrawal (2), we assessed Akt-1 activation by measuring either the levels of Ser473-phosphorylated Akt-1 or the kinase activity after its immunoprecipitation. Both methods detected a relatively high level of basal Akt-1 activity that was completely blocked by wortmannin or LY294002, but was virtually unaffected by a mitogen-activated protein kinase kinase (MEK) inhibitor, PD 98059, and a p38 kinase inhibitor, SB 203580 (Fig. 1 e and f). These results confirmed that Ser473 phosphorylation is critically dependent on PI 3-K activity. The high levels of basal Akt-1 activity are most likely caused by the presence of growth factors in the culture medium, because the removal of serum induced a rapid depletion of Akt-1 activity (Fig. 1g).
Figure 1.
PI 3-K activity is essential for the survival of CGCs and maintenance of basal Akt-1 phosphorylation and kinase activity. (a and b) Effects of PI 3-K inhibitors without or with lithium pretreatment on neuronal survival: CGCs were treated with 3 mM LiCl at day 1 in vitro and then exposed to indicated concentrations of wortmannin (Wort) (a) or LY294002 (LY) (b) at day 4 in vitro. Wortmannin administration was repeated every 12 h because this inhibitor is unstable in water. Cell viability was determined by MTT assay at day 8 in vitro. (c and d) Effects of lithium on PI 3-K activity: Cells were treated with 3 mM LiCl for 5 or 30 min. Activity of immunoprecipitated PI 3-K was determined by using PI as a substrate. Radioactive PI 3-P products were visualized by autoradiography (c) and quantified (d). The experiment was repeated three times with similar results. (e) Immunoblot of Akt-1 phosphorylation at Ser473 in CGCs treated with various kinase inhibitors: Cells were treated for 30 min with 100 nM Wort, 50 μM LY, 10 μM SB 230580 (SB) or 10 μM PD 98059 (PD). Immunoblotting with phospho-specific Akt-1 antibody was performed 3 times with similar results. (f) Immunocomplex Akt-1 kinase assay of CGCs treated with various kinase inhibitors: Cells were treated for 30 min with 100 nM Wort, 50 μM LY, 10 μM SB or 10 μM PD, and cell lysates were examined for Akt-1 kinase activity. (g) Effects of serum deprivation on immunocomplex Akt-1 activity: CGCs were cultured in a medium containing 10% FCS for 8 days. The medium was then switched to a medium without serum at indicated times (1.5 or 24 h) before harvest. Results presented in a, b, f, and g are means ± SEM in three to six independent experiments.
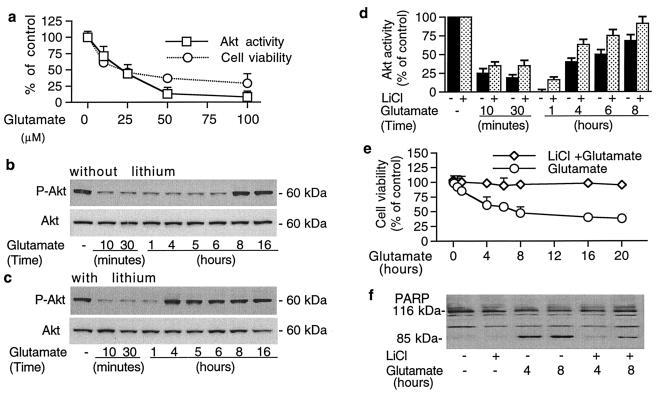
Next, we examined the role of Akt-1 in mediating glutamate excitotoxicity and lithium neuroprotection. Treatment of CGCs with glutamate for 30 min induced a concentration-dependent decrease in Akt-1 kinase activity with a 90% loss at 50 μM (Fig. 2a). The concentration-dependence of this inhibition of Akt-1 activity was parallel with the glutamate-induced death of CGCs at 20 h, as measured by MTT assay. All other experiments were performed by using a relatively low dose of glutamate (50 μM) that is prone to kill cells through apoptotic mechanisms, unlike high doses of glutamate that induce necrotic death (10, 14). Moreover, the levels of Akt-1 phosphorylated at Ser473 (Fig. 2b Upper blot) and Thr308 (results not shown) were rapidly and reversibly decreased between 10 min and 8 h after glutamate exposure. However, levels of total Akt-1 protein were unchanged by glutamate treatment for up to 16 h (Fig. 2b Lower blot). Treatment for more than 20 h caused a rapid decrease in levels of both phosphorylated and total Akt protein, probably because of the progression of apoptotic death (results not shown). Interestingly, pretreatment of CGCs with LiCl (3 mM) for 7 days markedly accelerated the recovery of phosphorylated Akt-1 to untreated levels (Fig. 2c Upper blot). Thus, the recovery was observed at 4 and 8 h in the presence and absence, respectively, of lithium pretreatment. In aliquots of the same samples we found that Akt-1 kinase activity was also drastically inhibited by glutamate treatment (Fig. 2d). The effect was detected at 10 min, reached a nadir around 1 h, and then gradually recovered to 70% of the control at 8 h. Lithium pretreatment prevented the complete inhibition of Akt-1 kinase activity and facilitated its recovery toward the control level.
Figure 2.
Glutamate rapidly inhibits Akt-1 phosphorylation and kinase activity, with concomitant loss of cell viability and induction of PARP cleavage. These effects are suppressed by long-term lithium treatment. (a) Concentration-dependent effects of glutamate on Akt-1 activity and cell viability: CGCs were treated with indicated concentrations of glutamate for 30 min. Akt-1 was immunoprecipitated from cell lysates and the kinase activity was measured. Sister cultures were treated with the same concentrations of glutamate for 20 h and tested for cell viability by MTT assay. (b and c) Time-course of glutamate-induced inhibition of Akt-1 phosphorylation and total Akt protein: Cells were pretreated without (b) or with (c) 3 mM LiCl for 7 days and then treated with 50 μM glutamate for immunoblotting with phospho-specific (Ser473) Akt-1 (Upper blots) and Akt antibodies recognizing Akt-1 independent of its phosphorylation state (Lower blots). (d) Akt-1 kinase activity in CGCs treated with glutamate in the absence or presence of lithium; aliquots (500 μg of protein) from some samples used in the experiments described in b and c were used. Akt-1 was immunoprecipitated from cells pretreated without or with 3 mM LiCl for 7 days and then exposed to 50 μM glutamate for the indicated times and harvested for Akt-1 kinase activity assay. (e) Time-course of glutamate-induced cell loss in the absence or presence of lithium: CGCs were pretreated without or with 3 mM LiCl for 7 days and then exposed to 50 μM glutamate for the times indicated before cell viability measurement by MTT assay. (f) Glutamate-induced caspase 3 activation is prevented by lithium pretreatment: Cells were treated with 3 mM LiCl for 7 days and/or 50 μM glutamate and subjected to immunoblotting with PARP-specific antibodies. Representative immunoblots from at least three experiments are shown in b, c, and f. Results shown in a, d, and e are means ± SEM from three independent experiments.
To examine whether the effects of glutamate on Akt phosphorylation and activity are related to excitotoxicity, we performed in-parallel time-course studies of glutamate-induced CGCs loss. Glutamate induced a rapid, time-dependent decline in cell viability with a loss of about 40% at 4 h and more than 50% between 8 and 20 h (Fig. 2e). These effects were almost completely blocked by lithium pretreatment. Because glutamate excitotoxicity in CGCs involves activation of caspase 3 (10), we examined the effects on the degradation of PARP, a substrate of caspase 3. At 4 and 8 h after glutamate exposure, the conversion of the 116-kDa PARP to its 85-kDa degradation product was markedly increased, and this effect was effectively suppressed by lithium pretreatment (Fig. 2f).
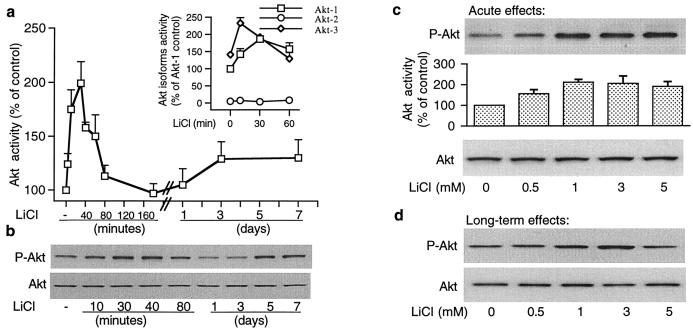
We then examined the actions of lithium alone on Akt-1 kinase activity and Akt-1 phosphorylation in CGCs under culturing conditions in which the enzyme was already extensively phosphorylated. LiCl (3 mM) induced an additional rapid increase in Akt-1 activity, which peaked 30 min after treatment and declined to basal level by 3 h (Fig. 3a). Acute lithium exposure also induced a transient increase in isoform Akt-3 activity, whereas isoform Akt-2 activity was virtually undetectable and not regulated by lithium treatment (Inset in Fig. 3a). A slight increase in Akt-1 activity was detected after lithium treatment for 3 days and persisted for at least 4 days. Measurements of Akt-1 phosphorylation at Ser473 (Fig. 3b Upper blot) and Thr308 (data not shown) confirmed the biphasic short- and long-term effects of lithium on Akt-1 activation, whereas levels of total Akt protein were unaffected (Fig. 3b Lower blot). Short-term (30-min) treatment of CGCs with lithium showed that therapeutically relevant concentrations of lithium increased Akt-1 phosphorylation and kinase activity with a maximal effect at 1 mM, but levels of total Akt protein remained unchanged (Fig. 3c). Similarly, long-term (7-day) treatment with LiCl elicited a dose-dependent increase in Akt-1 phosphorylation with a marked effect at 3 mM and reduced effect at 5 mM (Fig. 3d). We also examined the effects of lithium and glutamate on Akt-1 activity in a cell-free system. Incubation of immunoprecipitated Akt-1 with 3 mM LiCl or 50 μM glutamate for 30 min failed to affect Akt-1 kinase activity, thus excluding the possibility of a direct action of lithium or glutamate on Akt-1 (results not shown).
Figure 3.
Lithium increases Akt kinase activity and phosphorylation in a time- and concentration-dependent manner. (a) Effects of lithium on Akt-1 activity: Cells were treated with 3 mM LiCl for the indicated times and Akt-1 kinase activity was measured in cell lysates by immunocomplex kinase assay. (Inset) The same kinase activity assay was performed at the indicated times after LiCl treatment by using antibodies selective to Akt-1, Akt-2, and Akt-3 isoforms. (b) Effects of lithium on Akt-1 (Ser473) phosphorylation and total Akt protein levels: Aliquots of the samples prepared for experiment presented in a were used for immunoblotting with phospho-specific (Ser473) Akt-1 antibodies (Upper) and Akt antibodies (Lower). (c) Concentration-dependence of acute lithium-induced Akt-1 (Ser473) phosphorylation and Akt-1 kinase activity: Cells were treated with LiCl for 30 min and harvested for immunoblotting with phospho-specific (Ser473) Akt-1 antibodies (Top), for Akt-1 kinase-activity measurement (Middle), and for immunoblotting with phosphorylation-independent Akt antibodies (Bottom). (d) Concentration-dependence of prolonged lithium-induced Akt-1 (Ser473) phosphorylation and total Akt protein levels: Cells were treated with LiCl for 7 days and then immunoblotting was performed with phospho-specific (Ser473) Akt-1 (Upper blot) and Akt antibodies (Lower blot). Akt activity results in a and c are means ± SEM from at least four independent experiments. Representative immunoblots from experiments repeated three times are shown in b, c, and d.
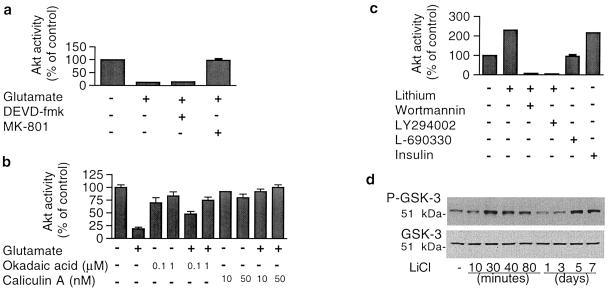
The effects of glutamate and lithium on Akt-1 activity were characterized further. The inhibition of Akt-1 activity by glutamate was completely blocked by MK-801 (Fig. 4a), again supporting our previous report that glutamate-induced excitotoxicity is entirely mediated by NMDA receptors (14). However, DEVD-fmk, a selective inhibitor of caspase 3, had no effect on glutamate inhibition of Akt-1 activity (Fig. 4a), suggesting that caspase 3 activation is a downstream event of Akt-1 inhibition. It has been reported that protein phosphatases, particularly the 2A subtype of protein phosphatase, are prominent regulators of Akt activity (9). To explore whether glutamate–induced loss of Akt activity is because of the activation of phosphatases and therefore enhancement of dephosphorylation, we examined the effects of phosphatase inhibitors. Okadaic acid at 0.1 and 1 μM did not further increase serum-elevated Akt-1 activity in untreated cells, but treatment with okadaic acid did suppress glutamate-induced Akt-1 activity loss in a dose-dependent manner (Fig. 4b). Similarly, caliculin A at 10 and 50 nM completely prevented glutamate-induced effects on Akt-1.
Figure 4.
Pharmacological and biochemical characterization of glutamate-decreased and lithium–increased Akt-1 activity. (a) MK-801, an NMDA receptor antagonist, prevented glutamate-induced inhibition of Akt-1 activity, whereas the fluoromethyl ketone peptide analog Z-DEVD-fmk, a caspase 3 inhibitor, did not. Cells at day 8 in vitro were treated with 10 μM MK-801 or 200 μM Z-DEVD-fmk for 40 min and then treated with 50 μM glutamate for 30 min for the Akt-1 kinase activity assay. (b) Effects of protein phosphatase inhibitors on glutamate-inhibited Akt-1 activity: Cells were pretreated with the indicated concentrations of okadaic acid or caliculin A for 60 min before exposure to 50 μM glutamate for 15 min and assayed for Akt-1 kinase activity. (c) Characterization of lithium-induced Akt-1 activation: Cells at day 8 in vitro were treated with 3 mM LiCl for 20 min without or with 30-min pretreatment with 100 nM wortmannin or 10 μM LY294002. When indicated, cells were also treated with 200 μM L-690330 (an inositol monophosphatase inhibitor) or 2 μM insulin for 20 min and assayed for Akt-1 activity. (d) Time-course of effects of lithium on GSK-3 α (Ser21) phosphorylation and total GSK-3: CGCs were treated with 3 mM LiCl for indicated times and immunoblotting was performed with phospho-specific (Ser21) antibody to GSK-3 α (Upper blot) and total GSK-3 antibody (Lower blot). Representative immunoblots from two experiments are shown. Quantified results in a, b, and c are means ± SEM from at least four independent experiments.
Akt-1 activation induced by short-term lithium treatment was abolished by wortmannin (100 nM) or LY294002 (10 μM) (Fig. 4c), suggesting that lithium-induced Akt activation is mediated through PI 3-K signaling. Lithium’s effect was not mimicked by L-690,330, a potent inhibitor of inositol monophosphatase (18). However, insulin increased Akt-1 activity by more than 2-fold, suggesting a convergent site of action with lithium in CGCs. To determine whether the signals generated by lithium-induced activation of Akt can be correlated with modulation of its physiological targets, we examined the effects of lithium on phosphorylation of GSK-3. Phosphorylation of GSK-3α at Ser21 and GSK-3β at Ser9 via Akt has been shown to mediate insulin-induced inhibition of GSK-3 activity (19). Similar to the action on Akt-1, lithium induced a biphasic, reversible increase in GSK-3α phosphorylation (Ser21) (Fig. 4d). The levels of total GSK-3 protein determined from the same samples remained unchanged. Moreover, the enhanced phosphorylation elicited by 30-min exposure to LiCl was blocked by either LY294002 or wortmannin, suggesting the involvement of the PI 3-K/Akt cascade (data not shown).
DISCUSSION
Our results robustly demonstrate that glutamate and lithium modulate Akt-1 phosphorylation state and kinase activity. In the case of glutamate-induced loss of Akt-1 phosphorylation and activity, we have provided evidence that these effects are most likely caused by the activation of protein phosphatase 2A that is involved in the dephosphorylation of Akt-1 (9), because protein phosphatase inhibitors robustly antagonize glutamate-induced Akt-1 activity loss. Mechanisms underlying the phosphatase activation could be related to the glutamate-induced influx of calcium and sodium ions mediated by NMDA receptors in CGCs (14). In contrast, lithium treatment activates Akt-1 by enhancing phosphorylation triggered by PI 3-K signaling. This conclusion is based on our findings that the enhanced Akt-1 activity is blocked by selective PI 3-K inhibitors and that lithium treatment rapidly increases PI 3-K activity. The activation of PI 3-K under our experimental conditions suggests that lipid second messengers such as PI-3,4,5-P3 or PI-3,4-P2, or both, are generated in response to lithium treatment. The mechanisms by which lithium activates PI 3-K are unclear. However, the similarity in the protective actions of lithium and IGF-1 (2, 5) raises the possibility that lithium may regulate the synthesis or release, or both, of IGF-1 and other growth factors from CGCs.
Glutamate-induced loss of Akt-1 phosphorylation and activity is rapid, reversible, dose- and time-dependent, and correlated with the death of CGCs. Cell death and caspase 3 activation are already profound at 4 h after glutamate exposure. Because caspase activation occurs by proteolytic processing and is considered to be irreversible (10), the recovery of Akt-1 activity beyond this time point is not expected to reverse the “death commitment” process. Long-term lithium pretreatment prevents the complete loss of Akt-1 activity and accelerates the recovery of Akt-1 phosphorylation and activity. These effects of lithium may therefore contribute to suppression of caspase activation and prevent cells from entering the death commitment phase. Interestingly, it has been shown that Akt induces phosphorylation and inactivation of BAD, a proapoptotic member of the Bcl-2 family (20), and enhances the expression of Bcl-2, an antiapoptotic protein (21). Indeed, we found that lithium treatment promotes the expression of Bcl-2, but inhibits the expression of p53 and Bax in CGCs, resulting in inhibition of caspase activation (22).
Glutamate and lithium, through their effects on Akt, may also regulate survival by several other mechanisms, because there are additional downstream targets of this kinase that are involved in cell-fate determination. These include caspase 9 (23), 4E-BP1 protein (24), CREB (25), L-type calcium channels (26), and GSK-3 (19, 27). Our results show that lithium induces GSK-3 phosphorylation as a result of Akt activation mediated through the PI 3-K signaling cascade, in addition to the direct inhibitory effects of lithium on this enzyme observed in vitro (28). The Akt-mediated effects of lithium on GSK-3 could be another mechanism underlying lithium-induced transcriptional activation in CGCs (29) and inhibition of τ-protein hyperphosphorylation in other cell types (30).
We have reported that NMDA also protects CGCs from glutamate excitotoxicity (31). It is interesting that NMDA activates PI 3-K (16) and Akt-1 activity (data not shown) in CGCs, suggesting that NMDA and lithium have a convergent mechanism in their neuroprotective actions. In neuroblastoma cells NMDA-induced activation of Akt occurs by phosphorylation at Thr308, but not Ser478, through the activation of a Ca2+/calmodulin-dependent protein kinase kinase in a PI 3-K independent manner (32). Thus, Akt appears to be a common target of the neuroprotective effects of NMDA and lithium, although distinct mechanisms are involved. It is well accepted that a modest increase in intracellular Ca2+ concentrations induced by NMDA treatment is beneficial and promotes survival (26, 32). In contrast, an excessive activation of NMDA receptors induced by glutamate treatment or cerebral ischemia disrupts calcium homeostasis and results in either apoptotic or necrotic neuronal death or both (33, 34). Our results suggest that differential effects on Akt activity may underlie these opposite actions of glutamate and NMDA on cell-fate.
It is unclear whether the effects of lithium on Akt activity are related to its therapeutic efficacy for the treatment of bipolar disorder. However, it is likely that Akt activation is related to some side effects of lithium. For example, the PI 3-K/Akt signaling cascade has been linked to the pathogenesis of certain forms of leukemia (21). Lithium treatment is known to cause leukocytosis and has been used to suppress leukopenia in patients undergoing radiotherapy or chemotherapy (for review, see ref. 35). Lithium has also been known to overcome leukocyte loss induced by carbamazepine (36), another drug used to treat bipolar disorder. Activation of Akt may underlie this aspect of lithium’s clinical efficacy. Our results provide insight into how lithium and glutamate mediate their effects in neurons, and these findings could be used to develop a novel intervention for neurodegenerative disorders.
Acknowledgments
We thank Ms. Emily Earle for her excellent technical assistance.
ABBREVIATIONS
- Akt-1
a serine/threonine kinase
- CGCs
cerebellar granule cells
- IGF-1
insulin-like growth factor 1
- GSK-3
glycogen synthase kinase-3
- NMDA
N-methyl-d-aspartate
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PARP
poly(ADP-ribose) polymerase
- PI
phosphatidylinositol
- PI 3-K
PI 3-kinase
- PI-3-P
PI 3-monophosphate
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Yao R, Cooper G M. Science. 1995;267:2003–2006. doi: 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]
- 2.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 3.Franke T F, Yang S-I, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 4.Kohn A D, Kovacina K S, Roth R A. EMBO J. 1995;14:4288–4295. doi: 10.1002/j.1460-2075.1995.tb00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Mello S R, Borodezt K, Soltoff S P. J Neurosci. 1997;17:1548–1560. doi: 10.1523/JNEUROSCI.17-05-01548.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stokoe D, Stephes L R, Copeland T, Gaffney P R J, Reese C B, Painter G F, Holmes A B, McCormick F, Hawkins P T. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 7.Franke T F, Kaplan D R, Cantley L C, Toker A. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 8.Alessi D R, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings B A. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 9.Andjelkovic M, Jakubowicz T, Cron P, Ming X-F, Han J-W, Hemmings B A. Proc Natl Acad Sci USA. 1996;93:5699–5704. doi: 10.1073/pnas.93.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du Y, Bales K R, Dodel R C, Hamilton-Byrd E, Horn J W, Czilli D L, Simmons L K, Ni B, Paul S M. Proc Natl Acad Sci USA. 1997;94:11657–11662. doi: 10.1073/pnas.94.21.11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szatkowski M, Attwell D. Trends Neurosci. 1994;17:359–365. doi: 10.1016/0166-2236(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 12.Gluckman P, Klempt N, Guan J, Mallard C, Sirimanne E, Dragunow M, Klempt M, Singh K, Williams C, Nikolics K. Biochem Biophys Res Commun. 1992;182:593–599. doi: 10.1016/0006-291x(92)91774-k. [DOI] [PubMed] [Google Scholar]
- 13.Tagami M, Yamagata K, Nara Y, Fujino H, Kubota A, Numano F, Yamori Y. Lab Invest. 1997;76:603–612. [PubMed] [Google Scholar]
- 14.Nonaka S, Hough C J, Chuang D-M. Proc Natl Acad Sci USA. 1998;95:2642–2647. doi: 10.1073/pnas.95.5.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nonaka S, Chuang D-M. NeuroReport. 1998;9:2081–2084. doi: 10.1097/00001756-199806220-00031. [DOI] [PubMed] [Google Scholar]
- 16.Zhang F X, Rubin R, Rooney T A. J Biol Chem. 1998;273:26596–26602. doi: 10.1074/jbc.273.41.26596. [DOI] [PubMed] [Google Scholar]
- 17.Alessi D R, Caudwell F B, Andjelkovic M, Hemmings B A, Cohen P. FEBS Lett. 1996;399:333–338. doi: 10.1016/s0014-5793(96)01370-1. [DOI] [PubMed] [Google Scholar]
- 18.Atack J R, Cook S M, Watt A P, Fletcher S R, Ragan C I. J Neurochem. 1993;60:652–658. doi: 10.1111/j.1471-4159.1993.tb03197.x. [DOI] [PubMed] [Google Scholar]
- 19.van Weeren P C, de Bruyn K M T, de Vries-Smith A M M, van Lint J, Burgering B M T. J Biol Chem. 1998;273:13150–13156. doi: 10.1074/jbc.273.21.13150. [DOI] [PubMed] [Google Scholar]
- 20.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 21.Skorski T, Bellacosa A, Nieborowska-Skorska M, Majewski M, Martinez R, Choi J K, Trotta R, Wlodarski P, Perrotti D, Chan T O, et al. EMBO J. 1997;16:6151–6161. doi: 10.1093/emboj/16.20.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen R-W, Chuang D-M. J Biol Chem. 1999;274:6039–6042. doi: 10.1074/jbc.274.10.6039. [DOI] [PubMed] [Google Scholar]
- 23.Cardone M H, Roy N, Stennicke H R, Salvesen G S, Franke T F, Stanbridge E, Frisch S, Reed J C. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 24.Gingras A-C, Kennedy S G, O’Leary M A, Sonenberg N, Hay N. Genes Dev. 1998;12:502–513. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du K, Montminy M. J Biol Chem. 1998;273:32377–32379. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- 26.Blair L A C, Bence-Hanulec K K, Mehta S, Franke T, Kaplan D, Marshall J. J Neurosci. 1999;19:1940–1951. doi: 10.1523/JNEUROSCI.19-06-01940.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cross D A E, Alessi D R, Cohen P, Andjelkovich M, Hemmings B A. Nature (London) 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 28.Klein P S, Melton D A. Proc Natl Acad Sci USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozaki N, Chuang D-M. J Neurochem. 1997;69:2336–2344. doi: 10.1046/j.1471-4159.1997.69062336.x. [DOI] [PubMed] [Google Scholar]
- 30.Hong M, Chen D C R, Klein P S, Lee V M-Y. J Biol Chem. 1997;272:25326–25332. doi: 10.1074/jbc.272.40.25326. [DOI] [PubMed] [Google Scholar]
- 31.Chuang D-M, Gao X-M, Paul S M. Mol Pharmacol. 1992;42:210–216. [PubMed] [Google Scholar]
- 32.Yano S, Tokamitsu H, Soderling T R. Nature (London) 1998;396:584–587. doi: 10.1038/25147. [DOI] [PubMed] [Google Scholar]
- 33.Choi D W. Trends Neurosci. 1995;18:58–60. [PubMed] [Google Scholar]
- 34.Mattson M P. Neurosci Biobehav Rev. 1997;21:193–206. doi: 10.1016/s0149-7634(96)00010-3. [DOI] [PubMed] [Google Scholar]
- 35.Gallicchio V S. In: Lithium and the Cell. Birch N J, editor. San Diego: Academic; 1991. pp. 185–198. [Google Scholar]
- 36.Kramlinger K G, Post R M. Am J Psychiatry. 1990;147:615–620. doi: 10.1176/ajp.147.5.615. [DOI] [PubMed] [Google Scholar]